Abstract
Linezolid has been associated with reversible myelosuppression. Clinical trial data were evaluated for anemia, thrombocytopenia, and neutropenia. Thrombocytopenia and a slight increased risk for anemia were evident at ≥2 weeks of linezolid treatment. Hematologic abnormalities were consistent with mild, reversible, duration-dependent myelosuppression. Appropriate monitoring is warranted with linezolid use.
Myelosuppression is associated with antibiotics (2-4, 6, 7, 9, 10, 12, 13). Similarly, linezolid, which is effective against gram-positive bacteria (5, 16, 17-19, 21), has been associated with reversible myelosuppression (11, 14; K. Attassi, E. Hershberger, R. Alam, and M. Zervos, Ann. Meet. Infect. Dis. Soc. Am., abstr. 466, 2001; K. Hicks, R. Y. Hachem, A. Huen, and I. I. Raad, Ann. Meet. Infect. Dis. Soc. Am., abstr. 467, 2001; J. S. Lewis and J. E. Patterson, Ann. Meet. Infect. Dis. Soc. Am., abstr. 470, 2001; and J. Peterson, Letter, Pharmacia Corp., 2001). In preclinical studies (up to 3 months), linezolid produced time- and dose-dependent reversible myelosuppression with doses up to 1,000 mg/kg of body weight/day. At lower doses (10 to 40 mg/kg/day), hematologic findings involved erythrocytes and reticulocytes, but mild effects on white blood cells (WBC) and platelet counts (PLTC) were observed (data on file; Pharmacia Corp.). Thus, controlled clinical trials were assessed prospectively for anemia, thrombocytopenia, and neutropenia.
Data from 2,046 linezolid- and 2,001 comparator (i.e., vancomycin, ceftriaxone, cefpodoxime, clarithromycin, or oxacillin-dicloxacillin)-treated adult patients in seven comparator-controlled studies (pneumonia, skin and/or soft tissue, or methicillin-resistant Staphylococcus aureus infections) were assessed for risk of myelosuppression. Linezolid was administered in 600-mg doses twice daily (5 studies) or 400-mg doses twice daily (2 studies). Hematology assays included hemoglobin, hematocrit, red blood cell count, WBC count, WBC differential, PLTC, and absolute neutrophil count (ANC).
Mean hematology values were determined for the intent-to-treat populations. Shifts from baseline to the lowest hematologic value were evaluated using the AIDS Clinical Trial Group criteria (1). Reticulocyte indices (RI) were assessed using standard methods (20) at baseline and follow-up (600-mg-dose group only), and changes were assessed using adjusted difference analysis (8).
For patients with normal baseline values, substantially abnormal values were prospectively defined as follows: hemoglobin and PLTC, <75% of the lower limit of the normal range (LLN), and ANC, <50% of LLN. For patients with abnormal baseline values, substantially abnormal values were prospectively defined as follows: hemoglobin, <75% of baseline; PLTC, <75% of baseline; and ANC, <50% of baseline. Adjusted difference analyses, accounting for differences of study design and sample size, were used to compare proportions in each treatment group with those from substantially abnormal laboratory assays (8). The cumulative percentage of patients with substantially abnormal values was evaluated using the Kaplan-Meier method (15). Adverse events related to anemia, neutropenia, or thrombocytopenia in conjunction with substantially abnormal laboratory values were examined.
Mean hemoglobin, PLTC, and ANC values, which were available for linezolid and comparator groups (for comparisons of hemoglobin values, data were compared for 1,997 versus 1,952 patients, respectively; for platelet comparisons, 1,987 versus 1,944 patients, respectively; and for neutrophil comparisons, 1,931 versus 1,887 patients, respectively) were above LLN at baseline, end of treatment, and follow-up.
Shift analyses (Table 1) showed downward shifts for hemoglobin (6.6% versus 6.4%), PLTC (2.9% versus 1.6%), and ANC (3.3% versus 3.4%) values in linezolid- and comparator-treated patients, respectively. Most shifts were mild (grade 1 or 2) regardless of treatment, with no clinically significant differences in severe shifts (grade 3 or 4). Importantly, most shifts occurred in patients treated ≥2 weeks. RI data (Table 2) were evaluated in 637 linezolid- and 611 comparator-treated patients. In linezolid-treated patients, mean RI at end of treatment decreased significantly from baseline (change, −0.52; P < 0.01) and returned to values significantly above baseline at follow-up (change, 1.29; P < 0.01). In comparator-treated patients, mean RI at end of treatment significantly increased from baseline (change, 0.80; P < 0.01), with an additional increase at follow-up (change, 1.03; P < 0.01). Mean RI were significantly different between groups at end of treatment (P < 0.01) but were not different at follow-up.
TABLE 1.
Shifts from baseline to worst value during study for hemoglobin, platelets, and neutrophils in linezolid phase III comparator-controlled trialsa
| Group (shift category) | % (no. shifted/total no.) for:
|
|||
|---|---|---|---|---|
| All treatment durations in:
|
Treatment durations greater than 2 weeks in:
|
|||
| Linezolid group | Comparator group | Linezolid group | Comparator group | |
| Hemoglobin | ||||
| Any shift | 6.6 (118/1785) | 6.4 (110/1726) | 9.0 (64/708) | 6.5 (43/662) |
| Shift of 1 grade | 5.3 (95/1785) | 5.3 (91/1726) | 7.8 (55/708) | 5.3 (35/662) |
| Shift of 2 grades | 0.8 (15/1785) | 0.6 (11/1726) | 0.7 (5/708) | 0.9 (6/662) |
| Shift of 3 grades | 0.2 (4/1785) | 0.2 (4/1726) | 0.1 (1/708) | 0.3 (2/662) |
| Shift of 4 grades | 0.2 (4/1785) | 0.2 (4/1726) | 0.4 (3/708) | 0 |
| Platelet | ||||
| Any shift | 2.9 (36/1243) | 1.6 (19/1174) | 4.1 (19/461) | 1.0 (4/403) |
| Shift of 1 grade | 1.4 (18/1243) | 0.9 (10/1174) | 2.0 (9/461) | 0.7 (3/403) |
| Shift of 2 grades | 1.1 (14/1243) | 0.5 (6/1174) | 1.5 (7/461) | 0.2 (1/403) |
| Shift of 3 grades | 0.3 (4/1243) | 0.3 (3/1174) | 0.7 (3/461) | 0 |
| Shift of 4 grades | 0 | 0 | 0 | 0 |
| Neutrophil | ||||
| Any shift | 3.3 (55/1673) | 3.4 (54/1612) | 4.7 (31/664) | 3.7 (23/622) |
| Shift of 1 grade | 2.5 (41/1673) | 2.3 (37/1612) | 3.5 (23/664) | 3.1 (19/622) |
| Shift of 2 grades | 0.4 (7/1673) | 0.7 (11/1612) | 0.6 (4/664) | 0.3 (2/622) |
| Shift of 3 grades | 0.3 (5/1673) | 0.2 (3/1612) | 0.5 (3/664) | 0 |
| Shift of 4 grades | 0.1 (2/1673) | 0.2 (3/1612) | 0.2 (1/664) | 0.3 (2/622) |
AIDS Clinical Trial Group criteria (15).
TABLE 2.
Mean RI and change from baseline (± standard deviation)
| Timepoint (parameter) | Linezolid (n = 637) | Comparators (n = 611) | Adjusted difference | 95% CI |
|---|---|---|---|---|
| Baseline | ||||
| Mean index | 4.54 ± 2.38 | 4.62 ± 2.64 | −0.06 | −0.33 to 0.21 |
| End of treatment | ||||
| Mean index | 4.02 ± 2.28 | 5.42 ± 2.45 | −1.43 | −1.68 to −1.17 |
| Change from baseline | −0.52 ± 2.52a | 0.80 ± 2.54a | −1.36 | −1.63 to −1.09 |
| Follow-up | ||||
| Mean index | 5.84 ± 2.89 | 5.65 ± 2.64 | 0.25 | −0.045 to 0.55 |
| Change from baseline | 1.29 ± 2.58a | 1.03 ± 2.64a | 0.31 | 0.031 to 0.60 |
Change from baseline within treatment group (P < 0.01).
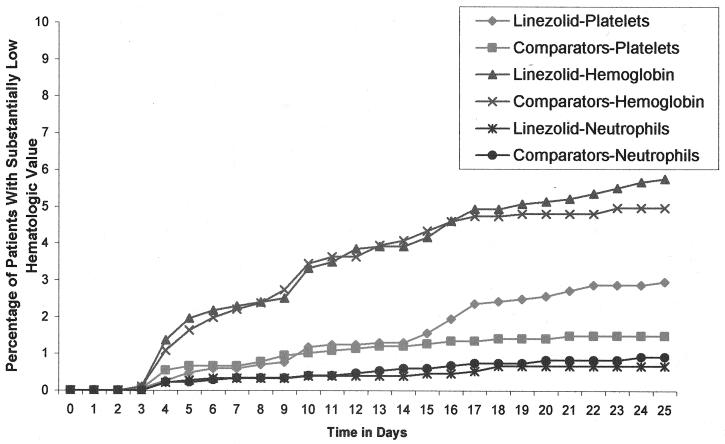
Adjusted difference (AD) analyses showed that the percentages of patients with substantially low hemoglobin (5.5% versus 4.9%; AD, 0.66; 95% confidence interval [CI], −0.02 to 1.35), PLTC (2.4% versus 1.5%; AD, 0.40; 95% CI, −0.25 to 1.04]), or ANC (0.8% versus 0.8%; AD, −0.22; 95% CI, −0.69 to 0.26]) values were not statistically different between treatment groups. The cumulative percentages of patients with at least one substantially low hematologic value were similar between groups (Fig. 1), with a nonstatistically significant difference noted in PLTC levels at day 14 (2.4% versus 1.5%; P, not significant). The mean (± standard deviation) times to onset of these substantially low values were more rapid with the comparator group than with the linezolid group (hemoglobin group, 10.5 ± 8.8 versus 12.1 ± 10.7 days; platelet group, 11.3 ± 10.1 versus 12.0 ± 6.2 days; neutrophil group, 12.4 ± 8.9 versus 14.3 ± 11.3 days), respectively. Overall, substantially abnormal hematologic values, regardless of treatment group, occurred most often in patients with lower baseline values (Table 3).
FIG. 1.
Patients with at least one substantially low PLTC, hemoglobin value, or neutrophil count in linezolid and comparator groups—cumulative percentage over time.
TABLE 3.
Baseline mean hematology assay values for patients with or without subsequent substantially abnormal values for that assaya
| Assay (patient group) | Results for linezolid treatment
|
Results for comparator treatment
|
||
|---|---|---|---|---|
| No. (%) | Baseline meanb | No. (%) | Baseline meanb | |
| Hemoglobin | ||||
| With substantially abnormal values | 85 (4.8) | 10.5 ± 1.4 | 76 (4.5) | 10.6 ± 1.5 |
| Without substantially abnormal values | 1,686 (95.2) | 13.0 ± 2.1 | 1,631 (95.5) | 13.0 ± 2.2 |
| Total | 1,771 | 1,707 | ||
| Platelet | ||||
| With substantially abnormal values | 35 (2.2) | 173 ± 86 | 19 (1.2) | 151 ± 50 |
| Without substantially abnormal values | 1,557 (97.8) | 258 ± 99 | 1,502 (98.8) | 263 ± 111 |
| Total | 1,592 | 1,521 | ||
| Neutrophil | ||||
| With substantially abnormal values | 13 (0.8) | 6.0 ± 6.3 | 13 (0.8) | 4.2 ± 5.0 |
| Without substantially abnormal values | 1,584 (99.2) | 7.8 ± 5.3 | 1,522 (99.2) | 7.9 ± 5.1 |
| Total | 1,597 | 1,535 | ||
For patients with paired baseline and on-treatment assays.
Hemoglobin assay results are given in grams per deciliter; platelet and neutrophil assay results are given in 103/mm3.
Of the 5% of patients with substantially low hemoglobin levels, only 0.7 and 0.2% of the linezolid- and comparator-treated patients, respectively, had a substantially low hemoglobin value and an anemia-related adverse event. These events were not drug related; one report of postoperative anemia resulting in transfusion was considered serious. Three linezolid-treated patients (0.2%), who were receiving concomitant anticoagulation therapy, had a bleeding-related adverse event (epistaxis, purpura, or ecchymosis) and a substantially low PLTC. These events were considered mild and not serious or drug related. No events of neutropenia were reported.
These findings support preclinical data suggesting that linezolid causes mild, reversible, time-dependent myelosuppression (Zyvox package insert, Pharmacia & Upjohn Company, Kalamazoo, Mich.); however, a pronounced effect was not evident in clinical trials with a treatment of <28 days. Thrombocytopenia and anemia occurred in some patients, specifically those treated for ≥2 weeks, with no increased risk of neutropenia. Nonetheless, patients with underlying hematologic abnormalities or lower baseline values were more likely to develop substantially low hematology values, regardless of treatment group.
Substantially low PLTC occurred in more linezolid- than comparator-treated patients; however, the importance of this divergence is unclear, given the small numbers of patients. Overall, a mild, reversible thrombocytopenia may occur in some patients with linezolid treatment at ≥2 weeks. The mechanism of linezolid-associated thrombocytopenia is unknown, but there is no evidence for anti-platelet or anti-linezolid antibodies or interference with platelet function (data on file; Pharmacia Corp.).
Mild effects on hemoglobin were evident, but no appreciable differences between treatment groups were noted. Decreased reticulocytes may be associated with anemia. The RI analysis did not control for other variables possibly associated with RI changes; however, a short-lived and reversible effect on erythropoiesis was detected in linezolid-treated patients. Furthermore, linezolid is not genotoxic in standard in vitro and in vivo genetic toxicology studies, including chromosome aberration assays in human peripheral lymphocytes, and there is no clinical evidence of aplastic anemia (data on file; Pharmacia Corp.).
Reversible myelosuppression is manageable and common with antibiotics (4, 10, 12). Current recommendations suggest monitoring of complete blood counts in predisposed patients (Zyvox package insert, Pharmacia & Upjohn Company; Peterson, letter). Given linezolid's efficacy for treating serious gram-positive infections, activity against resistant pathogens, and equivalent intravenous-to-oral formulations, the benefits of linezolid treatment may outweigh the potential risk of reversible myelosuppression.
Acknowledgments
This work was supported by Pharmacia Corp.
REFERENCES
- 1.Adult AIDS Clinical Trial Group (AACTG). 1992. Table for grading severity of adult adverse experiences. National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, Md.
- 2.Alanis, A., and A. J. Weinstein. 1983. Adverse reactions associated with the use of oral penicillins and cephalosporins. Med. Clin. N. Am. 67:113-129. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, D. P., M. E. Russo, D. E. Fohrman, and G. Rothstein. 1983. Nafcillin-induced platelet dysfunction and bleeding. Antimicrob. Agents Chemother. 23:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beris, P., and P. A. Miescher. 1988. Hematological complications of antiinfectious agents. Sem. Hematol. 25:123-139. [PubMed] [Google Scholar]
- 5.Brickner, S. J., D. K. Hutchinson, M. R. Barbachyn, R. R. Mannienen, D. A. Ulanowicz, K. C. Grega, S. K. Hendes, D. S. Toops, C. W. Ford, and G. E. Zurenko. 1996. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 39:673-679. [DOI] [PubMed] [Google Scholar]
- 6.Ehmann, W. C. 1992. Cephalosporin-induced hemolysis: a case report and review of the literature. Am. J. Hematol. 40:121-125. [DOI] [PubMed] [Google Scholar]
- 7.Fass, R. J., E. A. Copelan, J. T. Brandt, M. L. Moeschberger, and J. J. Ashton. 1987. Platelet-mediated bleeding caused by broad-spectrum penicillins. J. Infect. Dis. 155:1242-1248. [DOI] [PubMed] [Google Scholar]
- 8.Fleiss, J. L. 1972. Statistical methods for rates and proportions, 2nd ed., p. 160-165. John Wiley, New York, N.Y.
- 9.George, J. N., G. E. Raskob, S. R. Shah, M. A. Rizvi, S. A. Hamilton, S. Osborne, and T. Vondracek. 1998. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann. Intern. Med. 129:886-890. [DOI] [PubMed] [Google Scholar]
- 10.Girdwood, R. H. 1976. Drug-induced anaemias. Drugs 11:394-404. [DOI] [PubMed] [Google Scholar]
- 11.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291.. [DOI] [PubMed] [Google Scholar]
- 12.Kueh, Y. K. 1991. Haematological adverse drug reactions in hospital practice. Ann. Acad. Med. Singap. 20:106-113. [PubMed] [Google Scholar]
- 13.Kuruppu, J. C., T. P. Le, and C. Tuazon. 1999. Vancomycin-associated thrombocytopenia: case report and review of the literature. Am. J. Hematol. 60:249-250. [DOI] [PubMed] [Google Scholar]
- 14.Kuter, D. J., and G. S. Tillotson. 2001. Hematologic effects of antimicrobials: focus on the oxazolidinone linezolid. Pharmacotherapy 21:1010-1013. [DOI] [PubMed] [Google Scholar]
- 15.Lee, E. T. 1980. Statistical methods for survival data analysis. Lifetime Learning Publications, Belmont, Calif.
- 16.Noskin, G. A., F. Siddiqui, V. Stosor, D. Hacek, and L. R. Peterson. 1999. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein, E., S. K. Cammarata, T. H. Oliphant, R. G. Wunderink, and the Linezolid Nosocomial Pneumonia Study Group. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32: 402-412. [DOI] [PubMed] [Google Scholar]
- 18.Stevens, D. L., D. Herr, H. Lampiris, J. L. Hunt, D. H. Batts, and B. Hafkin. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Clin. Infect. Dis. 34:1481-1490. [DOI] [PubMed] [Google Scholar]
- 19.Stevens, D. L., L. G. Smith, J. B. Bruss, et al. 2000. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 44:3408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallach, J. 1992. Interpretation of diagnostic tests: a synopsis of laboratory medicine, 5th ed. Little, Brown & Co., Boston, Mass.
- 21.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilurn, S. E. Glickman, D. K. Hutchinson, and M. R. Barbachyn. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]