Abstract
In this study, we propose a simple and reproducible host-cell-based assay for the screening of antimycobacterial drugs that is suitable for drug discovery. The method evaluates both antimycobacterial activity of the drugs and their cytotoxicity to host cells. The basis of this simple fibroblast-based assay (SFA) is that cells of human lung fibroblast cell line MRC-5, which are highly sensitive to mycobacterial cytotoxicity, are killed by virulent Mycobacterium tuberculosis strain H37Rv bacilli in response to the viability of bacilli. Clinically used antimycobacterial drugs inhibited the mycobacterial cytotoxicity to MRC-5 cells in a dose-dependent manner. MICs of isoniazid, streptomycin, rifampin, and ethambutol determined by this SFA (0.428, 1.816, 0.013, and 3.465 μg/ml, respectively) were within 1 log of MICs determined by the broth dilution test (BDT) using Middlebrook 7H9 medium. The MIC of pyrazinamide, which exhibits bactericidal activity only at a high dose by BDT (1,231 μg/ml at pH 6.6 and 492 μg/ml at pH 5.8), was 3.847 μg/ml in the modified method of SFA. On the other hand, sodium azide, a toxic agent for both mammalian cells and bacteria, exhibited cytotoxicity to fibroblasts at a dose lower than that required to inhibit mycobacterial growth. Thus, this fibroblast-based method enabled us to evaluate both antibacterial activity of drugs and their cytotoxicity to human cells within a short period of time.
Tuberculosis is among the most severe of infectious diseases in the world. One-third of the world population is estimated to be latently infected with this pathogen. By current estimates, nearly 2 billion people in the world have been exposed to the bacillus (17, 20). An increase in the number of people having double infections with Mycobacterium tuberculosis and human immunodeficiency virus warns us about the consequences of such and therefore emphasizes the importance of controlling tuberculosis infection (18). Furthermore, the emergence of multidrug-resistant strains of M. tuberculosis has led to the expansion of this disease. The World Health Organization has declared as a priority the need to immediately control tuberculosis infection to prevent the spread of drug-resistant strains (35). More than 14 antimycobacterial drugs are currently available for tuberculosis patients and are indispensable to preventing progression of the disease. Uncontrolled usage or abuses of these strong medicines generates drug-resistant strains (35). Therefore, the need to develop new antibiotics for tuberculosis is inevitable.
As a first step towards the development of new antibiotics, the bactericidal activities of a large number of candidates have to be tested. Currently, candidates are tested by inhibition of the growth of bacteria in a liquid or solid media. However, there are difficulties in the assay because of the slow growth rate of M. tuberculosis. To address the problem associated with current methods of antimycobacterial drug screening, new screening methods need to be developed. The radiometric BACTEC 460 system contributed indeed to determine the susceptibility of mycobacteria to tested drugs more rapidly for the last few decades (3, 13, 16), but the requirement of a large number of bacteria, lack of high-throughput format, and requirement for radioisotope disposal all limit its usefulness for mass screening. Recently, there have been reports of a number of mycobacterial drug susceptibility assays using reporter genes, such as luciferase, β-galactosidase, and green fluorescent protein, and an oxygen-quenched fluorescent indicator (1, 2, 5, 15, 19, 25, 29, 30, 31, 36). Microplate Alamar Blue Assay and Mycobacterium Growth Indicator Tube, MB/BacT, and EPS II are also sensitive and rapid methods for screening antimycobacterial drugs against slow-growing mycobacteria (6, 24, 26, 34). However, antimycobacterial activity of prodrug, which is activated within host cells, is not detectable in the direct growth-inhibitory assay. Moreover, easily metabolized compounds are not effective within host cells. Another problem that the drug implementers encounter is the issue of toxicity and efficacy of these candidates in an animal model, which usually takes more than 6 weeks to determine. Here we propose a simple and rapid fibroblast-based method to evaluate new drug candidates by taking into consideration both antimycobacterial activity to M. tuberculosis and cytotoxicity to human cells.
MATERIALS AND METHODS
Reagents.
Fetal bovine serum (FBS) was purchased from JRH Biosciences (Lenexa, Kans.). Isoniazid (INH), streptomycin (STR), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA) were purchased from Sigma (St. Louis, Mo.). Unless specifically indicated, all other reagents were obtained from Sigma.
Cell culture.
Human embryonic lung fibroblast cell line MRC-5 (ATCC CCL171) was maintained in RPMI 1640 medium containing 15 mM HEPES and 10% heat-inactivated FBS.
Organisms.
M. tuberculosis strain H37Rv (ATCC 25618) was purchased from American Type Culture Collection (ATCC; Manassas, Va.). Bacteria were grown in Middlebrook 7H9 medium supplemented with 0.05% Tween 80 and 10% ADC (Difco, Detroit, Mich.) at 37°C under biosafety level 3 conditions. Bacterial culture medium was prepared with endotoxin-free materials. The viability of the bacilli was determined by colony assay on Middlebrook 7H11 agar plates. Killed bacilli were prepared by treatment with INH (10 μg/ml) and STR (100 μg/ml) for 24 h at 37°C.
Measurement of LDH release from MRC-5 cells.
Briefly, MRC-5 cells were cultured with live M. tuberculosis H37Rv bacilli for 3 days, and then the culture supernatants were collected and filtered through a 0.22-μm-pore-size filter. Then, lactate dehydrogenase (LDH) in the supernatant was measured by the cytotoxicity detection kit (Roche, Mannheim, Germany). Percent cytotoxicity was calculated by following the instructions for the kit.
Measurement of both bactericidal activity and cytotoxicity of drugs to MRC-5 cells.
Cells were plated in 96-well flat-bottom microtiter wells, at 2 × 104 cells per well in 100 μl of the culture medium, and then 50 μl of the culture medium containing drugs was added. Four hours later, 50 μl of the RPMI medium containing a suspension of M. tuberculosis H37Rv (0 to 100 organisms/cell) was added. Three days after the addition of mycobacteria, the supernatant was removed. The cells were fixed in methanol for 1 min, stained with 0.75% crystal violet for 5 min, and rinsed with water. Then, 100 μl of 1% SDS was added to each well to dissolve the dye, and the optical density (OD) at 595 nm was determined using an enzyme-linked immunosorbent assay reader. Error bars indicate standard deviation (SD).
Percent cytotoxicity was calculated as follows: percent cytotoxicity = [100 − (OD of sample/OD of control)] × 100%. The wells without bacteria were used as control. Relative percentage was calculated as follows: relative percentage = (OD of sample/OD of control) × 100%.
The wells without both bacteria and antituberculosis drugs were used as control. The crystal violet staining is conventionally used for measuring host cell viability of adherent type tissue culture cells (11). All of the detached cells were dead as determined by trypan blue dye exclusion, indicating that the crystal violet staining method detected cytotoxicity.
In vitro broth dilution method to measure MICs of antimycobacterial drugs.
The broth dilution method for the measurement of MICs was carried out according to the method described by Wallace et al. (33). Briefly, 100 μl of the drug solution twofold serially diluted with Middlebrook 7H9 broth medium supplemented with 0.05% Tween 80 and 10% ADC were prepared in a 96-well plate, and then 100 μl of the broth medium containing 1 × 104 to 2 × 104 organisms was added. Then, the plate was incubated at 37°C for 2 weeks.
Definition of MIC in SFA and BDT.
In the simple fibroblast-based assay (SFA), MIC is defined as the minimal dose of drugs exhibiting s statistically significant inhibitory effect on the bacterial cytotoxicity as examined by the fibroblast cell viability. In the broth dilution test (BDT), MIC is defined as the lowest concentration of agents that inhibit visible growth of the bacteria. The partial reduction in turbidity was scored as negative.
Colony assay from tissue culture medium and MRC-5 cell lysate after infection with M. tuberculosis H37Rv.
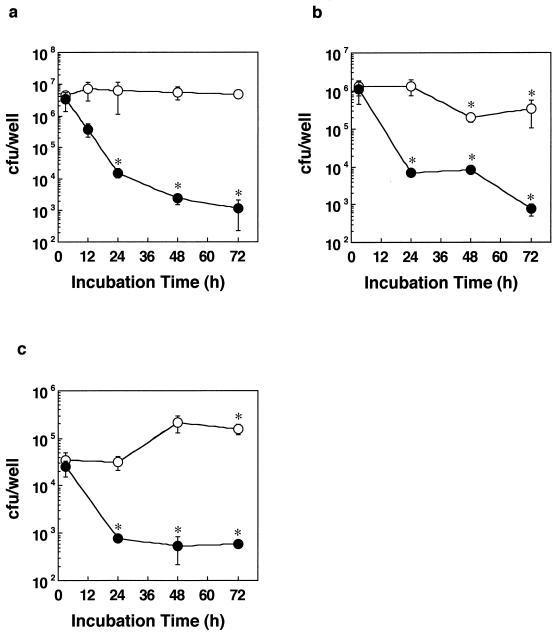
M. tuberculosis H37Rv (106 organisms/ml) bacilli were cultured in 200 μl of tissue culture medium (RPMI 1640 containing 10% FBS) in a 96-well plate for the indicated period (see Fig. 4a) in the presence or absence of INH (10 μg/ml). The medium containing bacilli was collected at each time point and inoculated on Middlebrook 7H11 agar plates.
FIG. 4.
Time-dependent effect of INH on mycobacterial viability in MRC fibroblasts. (a) Colony assay of the bacilli in tissue culture medium alone. M. tuberculosis H37Rv bacilli (106 organisms/ml) were cultured in 200 μl of tissue culture medium (RPMI 1640 containing 10% FBS) in a 96-well plate for the indicated period in the presence or absence of 10 μg of INH/ml for the indicated period. (b) Colony assay of bacilli in the culture supernatant. (c) Cell lysate. MRC-5 cells (4 × 104 cells/well in a six-well plate) were cultured with H37Rv (MOI, 50:1) in the presence (•) or absence (○) of 10 μg of INH/ml for the indicated period. Culture supernatant and cell lysate were obtained at each time point, and then the number of viable bacilli was determined by the colony assay. The results are the means ± SD of six wells (a) or three independent experiments (b and c). Data were analyzed by t test compared with control cells cultured without INH at 3 h. ∗, statistical significance (P < 0.01) versus the cells without bacteria.
In other experiments (see Fig. 4b and c), MRC-5 cells (4 × 104 cells/well in a six-well plate) were cultured with H37Rv (multiplicity of infection [MOI], 50:1) in the presence or absence of INH (10 μg/ml) for the indicated period. As shown (see Fig. 5), MRC-5 cells were infected with the bacilli for 24 h, then the host cells were washed three times with phosphate-buffered saline (PBS), and then fresh tissue culture medium with or without antimycobacterial drugs (INH or PZA) was added. After 3 days of culture, the cells were washed three times with PBS and then lysed with lysis buffer (0.1% NP-40, 150 mM NaCl, 100 mM HEPES [pH 7.5]). The lysates were inoculated on Middlebrook 7H11 agar plates after dilution. The colonies on the plate were counted after 2 weeks of incubation at 37°C.
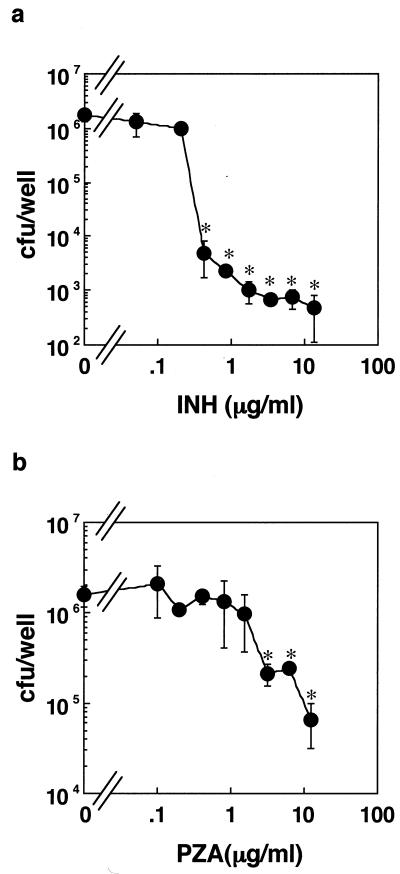
FIG. 5.
Dose effect of INH or PZA on mycobacterial viability within MRC-5 cells. MRC-5 cells were cultured with M. tuberculosis H37Rv bacilli at an MOI of 50:1 for 24 h. After washing out the remaining bacilli in culture medium, the cells were incubated with various concentrations of INH (a) or PZA (b) for 3 days. The number of bacilli within MRC-5 cells was determined by the colony assay. The results were the mean ± SD of three independent experiments. Data were analyzed by t test compared with control cells containing no drugs. ∗, statistical significance (P < 0.01) versus the cells without bacteria.
RESULTS
Live virulent cells of M. tuberculosis strain H37Rv exhibit cytotoxicity to human fibroblast cell line MRC-5.
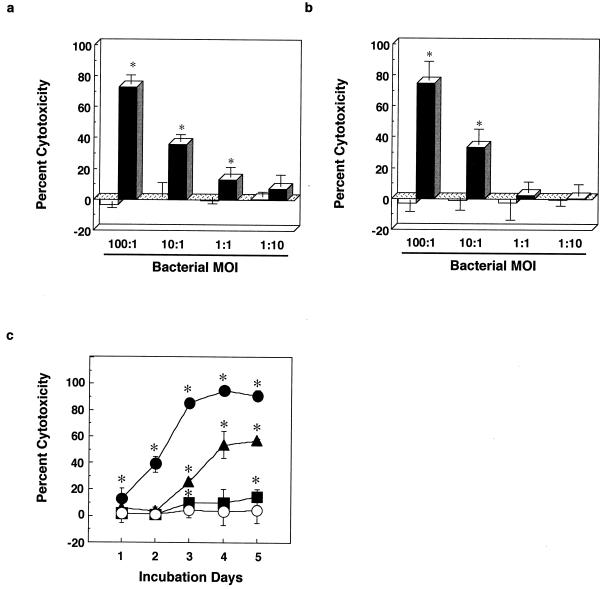
We have previously reported that among human cell lines, including U937 (monocyte), THP-1 (monocyte), HL-60 (myeloid), and A549 (lung epithelium), human lung fibroblast cell line MRC-5 is most susceptible to the cytotoxicity of virulent M. tuberculosis strain H37Rv, and this cytotoxicity is observed in live, but not dead, bacilli (32). When MRC-5 cells were cultured with live H37Rv bacilli, the cells were killed in a dose-dependent manner (Fig. 1). In contrast, the bacilli that were treated with INH and STR exhibited no cytotoxicity. Likewise, the live bacilli induced the release of LDH from infected cells, whereas drug-treated bacilli did not release any LDH (Fig. 1b), confirming the cytotoxic effect of the live bacilli. This bacterial cytotoxicity was observed according to the MOI (Fig. 1a). A time course experiment showed that the mycobacterial cytotoxicity became obvious on the second day after infection (Fig. 1c). During the assay period, control MRC-5 cells did not proliferate because the cell growth was inhibited by contact inhibition. The net absorbances at 595 nm of control cells stained with crystal violet at the time points from day 1 to 5 were 0.74 ± 0.02, 0.77 ± 0.04, 0.79 ± 0.02, 0.76 ± 0.09, and 0.79 ± 0.09.
FIG. 1.
Live virulent bacilli of M. tuberculosis strain H37Rv exhibit cytotoxicity to human lung fibroblast MRC-5 cells. MRC-5 cells were cultured with live (closed column) or killed (open column) bacilli of M. tuberculosis strain H37Rv. (a) Crystal violet stain. The viability of MRC-5 cells was measured by staining with crystal violet on day 3. (b) LDH release. Mycobacterial cytotoxicity was also determined by LDH release from MRC-5 cells on day 3. (c) Time course. MRC-5 cells were cultured with live M. tuberculosis H37Rv bacilli for up to 5 days. •, MOI of 100:1; ▴, MOI of 10:1; ▪, MOI of 1:1; ○, MOI of 1:10. The assay procedure and method of computing percent cytotoxicity are described in Materials and Methods. The results are the means ± SD of six wells. Experiments were carried out more than three times, and representative data are shown. Data were analyzed by paired t test compared with control cells containing no bacilli. ∗, statistical significance (P < 0.01) versus the cells without bacteria.
Antimycobacterial drugs inhibit mycobacterial cytotoxicity to MRC-5 cells.
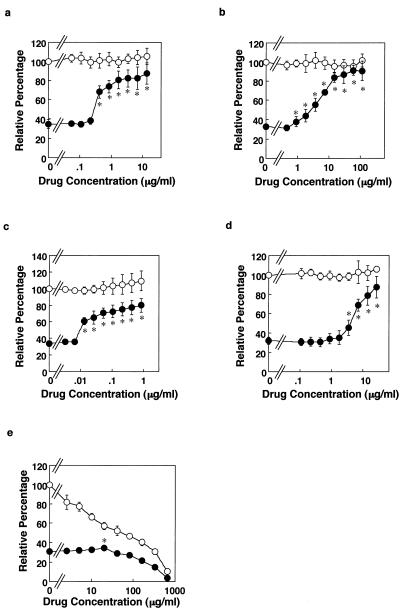
As the bacterial cytotoxicity correlates with bacterial viability, we next investigated whether clinically used typical antimycobacterial drugs can inhibit the cytotoxicity. We utilized MOI (50:1) because it yielded a reproducible semimaximal cytotoxic effect. When MRC-5 cells were cultured with or without live M. tuberculosis H37Rv in the presence of antimycobacterial drugs INH, STR, RIF, or EMB, the cytotoxicity was inhibited in a manner depending on the dose of drugs (Fig. 2). MICs determined by SFA and BDT are shown in Table 1. The MIC determined by SFA was defined as the minimal dose of agents exhibiting an inhibitory effect on bacterial cytotoxicity as measured by the viability of fibroblasts, while the MIC determined by BDT was defined as the lowest concentration of agents that inhibited visible growth of the bacteria. MICs of INH, STR, and EMB by SFA were 0.428, 1.816, and 3.465 μg/ml, respectively. The BDT/SFA MIC ratios were within 1 log for INH, STR, RIF, and EMB. These results indicate that this host cell-based assay can evaluate the antimycobacterial activities of drugs within host cells. Next, we investigated the effect of a typical toxic compound on the host cells under this assay system. Sodium azide (SA), an energy uncoupler, killed bacilli at a concentration of 40.63 μg/ml (Table 1). SA not only inhibited bacterial growth but also exhibited cytotoxicity to host cells at lower concentrations (Fig. 2e). First-line antituberculosis drugs, such as INH, STR, RIF, and EMB, did not exhibit any cytotoxicity to host cells at the indicated concentrations. These results indicate that SFA can evaluate both bactericidal activity and cytotoxicity to host cells.
FIG. 2.
Evaluation of both bactericidal activity and cytotoxicity to MRC-5 cells of antimycobacterial drugs. MRC-5 cells were cultured for 3 days without (○) or with (•) live M. tuberculosis H37Rv bacilli at an MOI 50:1 in the presence of antimycobacterial drugs INH (a), STR (b), RIF (c), EMB (d), and SA (e) at the indicated concentrations of each. Relative percentage demonstrates the viability of MRC-5 cells. The absorbance of control cells, which were cultured in the medium containing no drugs and no bacilli, was set as 100%. The assay procedure and method of computing relative percentage are described in Materials and Methods. The results are the means ± SD of six wells. Experiments were carried out more than three times, and representative data are shown. Data were analyzed by t test compared with control cells containing no bacteria and no drug. ∗, statistical significance (P < 0.01) versus the cells without bacteria.
TABLE 1.
Comparison of MIC of antimycobacterial agents as determined by BDT and SFA
| Reagents | MIC (μg/ml)
|
MIC of SFA/ MIC of BDT | |
|---|---|---|---|
| BDTa | SFAb | ||
| Antibiotics | |||
| INH | 0.107 | 0.428 | 4 |
| STR | 0.227 | 1.816 | 8 |
| RIF | 0.026 | 0.013 | 1/2 |
| EMB | 1.733 | 3.465 | 2 |
| PZA | 1,231 (pH 6.6)c | >123.1 | |
| 492 (pH 5.8)d | 3.847 | 1/128 | |
| Other agent | |||
| SA | 40.63 | 20.32 | 1/2 |
MIC was obtained by the BDT by using Middlebrook 7H9 medium supplemented with 0.05% Tween 80 and 10% ADC. MIC determined by the BDT is defined as the lowest concentration of agents that inhibited visible growth of the bacteria. The partial reduction in turbidity was scored as negative.
MIC was obtained by SFA. MIC determined by SFA is defined as the minimal dose of drugs exhibiting a statistically significant inhibitory effect on the bacterial cytotoxicity as examined by fibroblast viability. The values demonstrate statistically significant inhibition (P < 0.01) of the mycobacterial cytotoxicity. These assay procedures are described in Materials and Methods. The values are means from six wells.
M. tuberculosis H37Rv bacilli were cultured in 7H9 broth at pH 6.6.
The bacilli were cultured in 7H9 broth at pH 5.8.
MRC-5 cells were cultured with bacilli in the presence of PZA for 3 days.
MRC-5 cells were infected with bacilli for 12 h at an MOI of 50:1. The culture medium was replaced by fresh medium without bacilli, and then the cells were cultured with PZA for 2 days.
Evaluation of inhibitory effect of PZA on mycobacterial cytotoxicity to MRC-5 cells.
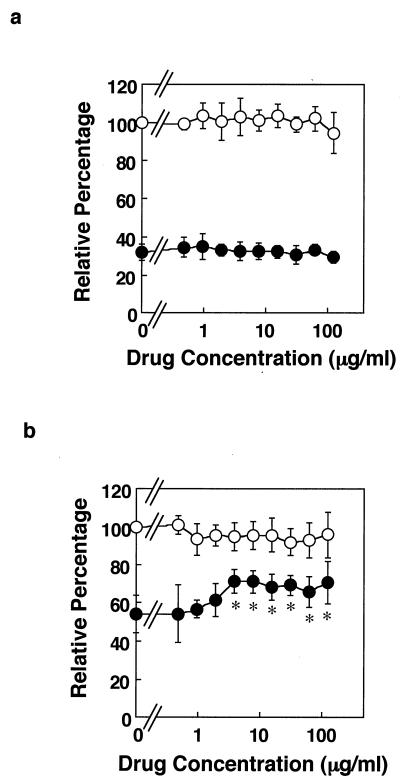
We also investigated antimycobacterial effect of PZA by SFA. In a preliminary experiment, MRC-5 cells were cocultured with the bacilli and PZA for 3 days; however, inhibitory effect of PZA was not observed (Fig. 3a). Measuring the bactericidal activity of PZA in vitro is difficult and controversial (9, 12). Acidic condition in phagolysosome is required for PZA to exhibit its antibacterial activity (27, 37). Indeed, PZA exhibited antibacterial effect at pH 5.8 but not at pH 6.6 in 7H9 broth medium (Table 1). Therefore, it may not be surprising that PZA did not inhibit mycobacterial cytotoxicity by this method (Fig. 3a) because the pH of the tissue culture medium was 7.4. We therefore modified the assay. When MRC-5 cells were exposed to M. tuberculosis H37Rv for an initial 6, 12, 24, or 72 h during the entire 72-h culture, the percent cytotoxicity was 26.20% ± 5.7%, 60.40% ± 7.20%, 79.90% ± 3.10%, and 87.10% ± 0.80%, respectively. Therefore, in the modified method, MRC-5 cells were exposed to the live bacilli for 12 h, the extracellular bacilli were washed out, and then the cells were cultured for 3 days in fresh medium containing varyious concentrations of PZA (Fig. 3b). In this culture condition, as expected, the bacterial cytotoxic level was low compared to that depicted in Fig. 3a because MRC-5 cells were exposed to the bacilli for only 12 h. Under these conditions, the inhibitory effect of PZA on mycobacterial cytotoxicity was observed even at low doses (Fig. 3b). The MIC of PZA by this modified method was 3.847 μg/ml, which was 128-fold lower than that obtained by the BDT method at pH 5.8. These results indicate that the modified SFA can evaluate antimycobacterial activity of PZA to M. tuberculosis within host cells.
FIG. 3.
Measurement of inhibitory effect of PZA on mycobacterial cytotoxicity by modified method of SFA. (a) Coculture with bacilli and PZA. MRC-5 cells were cultured without (○) or with (•) M. tuberculosis H37Rv bacilli at an MOI of 50:1 in the presence of PZA at the indicated concentrations for 3 days. (b) Culture with PZA after infection. MRC-5 cells were precultured without (○) or with (•) M. tuberculosis H37Rv bacilli at an MOI of 50:1 for 12 h, and then the cells were washed to remove bacilli remaining in the culture medium. Then, the cells were cultured for 3 days in the presence of PZA at the indicated concentrations. The results are the means ± SD of six wells. Experiments were carried out more than three times, and representative data are shown. Data were analyzed by t test compared with control cells containing no bacteria and no PZA. ∗, statistical significance (P < 0.01) versus the cells without bacteria.
Inhibitory effect of antimycobacterial drugs on bacterial cytotoxicity is dependent on bacterial viability both inside and outside MRC-5 cells.
Next, we investigated whether the inhibitory effect of antimycobacterial drugs on the cytotoxicity to MRC-5 cells is correlated to the viability of the bacilli inside MRC-5 cells. M. tuberculosis H37Rv bacilli were cultured in either the host-cell-free medium or medium containing MRC-5 cells with or without INH, and the number of viable bacilli in the medium, in culture supernatants, or within the host cells was determined. INH reduced bacterial number in both host-cell-free medium and the culture supernatant in a time-dependent manner (Fig. 4a and b). Antimycobacterial effect of INH on internalized bacilli was also observed at 24 h after infection (Fig. 4c). In drug-free conditions, the colony assay indicated that the bacilli scarcely multiplied in the tissue culture medium (Fig. 4a). In the presence of host cells, the bacterial number in the culture supernatant at 48 and 72 h decreased (Fig. 4b). Conversely, the bacterial number in the cell lysate increased at 48 and 72 h (Fig. 4c). These results showed that phagocytosis occurred even at 48 h and there was almost no multiplication of bacilli at the indicated time. Furthermore, we determined whether INH or PZA can reduce the bacterial number inside the infected cells. MRC-5 cells were exposed to the bacilli in the absence of antimycobacterial drugs for 24 h so that the bacilli are phagocytosed. After washing out the bacilli remaining in the medium, the cells were cultured with various concentrations of INH or PZA for 3 days, and then the bacterial number in the cell lysate was determined by colony assay using 7H11 agar plate. Both drugs reduced the bacterial number in a dose-dependent manner (Fig. 5a and b). The MICs of INH or PZA obtained from the colony assay were not statistically different from those obtained by SFA (Table 1). Taken together, the results suggest that the bacterial cytotoxicity is correlated to the viability of bacilli inside MRC-5 cells but not to bacterial growth.
DISCUSSION
There are several methods in use to evaluate the antimycobacterial activity of drugs in vitro and in vivo. Currently used methods for the evaluation of compounds for antimycobacterial activity in vitro require CFU determinations. In vivo activities of these compounds can also be evaluated by CFU determinations in organ homogenates from infected animals. Such experiments generally require an incubation period of 3 to 4 weeks before colonies can be accurately counted. Because these studies are extremely laborious, require multiple serial dilutions, and use large numbers of agar plates or culture tubes, it is difficult to test more than a few compounds in any one experiment. Nowadays, radiometric methods based on the measurement of 14CO2 release from a radiolabeled metabolic substrate, such as 14C-palmitic acid (BACTEC TB 460 system), or measurement of enzymatic activity of a reporter gene as a measure of killing activity inside macrophage cell line (1, 3, 22, 29) contribute to evaluating drugs rapidly, but these are still of limited use. One of the problems associated with these assays is the unavailability of case facilities for isotopic measurement or the high cost and inconsistencies in the ATP assays.
Methods based on reporter genes, such as β-galactosidase (30, 31) and luciferase (1, 2, 5, 14, 25), are rapid and sensitive and the results correlate well with those of culture-based methods. However, these methods require recombinant bacilli, and various factors affect the measurement of the enzymatic activity. To eliminate the procedure for measuring enzymatic activity, a method using green fluorescent protein gene was recently reported (29, 36). However, measurement of both the antimycobacterial effect on the bacilli within host cells and cytotoxic effect of drugs on host cells cannot be performed.
To study the antimycobacterial activities of drugs, here we propose a host-cell-based assay which evaluates both antimycobacterial activity and cytotoxicity to host cells. We have previously shown that human-lung-derived fibroblast MRC-5 cells are highly sensitive to mycobacterial cytotoxicity when compared to other human cell lines (32). The other host-cell-based methods using macrophages of either human or animal origin are documented (4, 7, 23, 28), but the drawbacks of the use of macrophages derived from experimental animals is the heterogeneity of different batches of animal cells and consequent problems of reproducibility (21). The comparative studies of antimycobacterial activities of INH, STR, RIF, and EMB in a macrophage model and BDT have also been reported (7, 8, 10, 21). A good correlation exists between the MICs obtained using SFA and those obtained using the macrophage model. Furthermore, the inhibitory effect of antimycobacterial drugs on the bacterial cytotoxicity is based on the bacterial viability (Fig. 4 and 5). Our study revealed that the degree of bacterial cytotoxicity is correlated with the number of intracellular viable bacilli. The experiment shown in Fig. 4b and c indicated that most of bacilli remained in culture medium. However, bacterial culture supernatant (unpublished data) and dead bacilli did not exhibit cytotoxicity, and cytotoxicity depended on the number of phagocytosed bacilli. Therefore, it is unlikely that extracellular viable bacilli affect the viability of MRC-5 cells. It is important to know whether antimycobacterial agents are bactericidal or bacteriostatic. However, a clear distinction between the two activities cannot be made by SFA.
This cell-based method evaluates antimycobacterial activities of drugs against both extracellular and intracellular bacilli. In order to evaluate antimycobacterial activities of drugs to intracellular bacilli, we used PZA, which was reported to require a low pH condition to exert bactericidal activity (27, 37). Thus, PZA is effective in only phagolysosome and cannot kill the bacilli outside host cells. Indeed, PZA did not inhibit bacterial cytotoxicity in SFA and exhibited bactericidal activity at high doses at acidic pH in BDT. Therefore, we modified SFA by eliminating the bacilli which are not taken up by the cells. One considerable reason why antimycobacterial effect of PZA was not observed in cocultured condition (Fig. 3a) would be that the time taken to phagocytose intact bacilli would be faster than the appearance of antimycobacterial effect of PZA. When PZA was added in culture medium containing no bacilli, the bacilli would come in contact with the drug only in the acidic environment, i.e., in the phagolysosome. This was confirmed by determining the number of extracellular and intracellular viable bacilli. INH exhibited bactericidal activity to the bacilli both outside and inside host cells. In contrast, PZA only exhibited bactericidal activity to the bacilli inside host cells.
The MIC of PZA was 1,231 μg/ml in BDT and 3.847 μg/ml in SFA. These findings are compatible with the report that PZA was effective in the macrophage model at a dose comparable to a clinically effective dose (at 20 μg/ml or higher); however, it was ineffective in BDT even at concentrations as high as 2,560 μg/ml (9). It was also shown that SFA is more sensitive to evaluating drug susceptibility to PZA, which is useful for detecting drugs like PZA. Collectively, it was demonstrated that this modified SFA enabled us to evaluate the bactericidal activity of compounds to intracellular bacilli.
SA, a toxic agent for both mammalian cells and bacteria, exhibited bactericidal activity; however, it also showed cytotoxicity to host cells at lower concentrations. In this study, other drugs exhibited cytotoxicity to host cells only at a high concentration.
The advantages of this SFA system are that it is a high-throughput system, results can be obtained within 3 to 4 days, and expensive reagents and huge facility are not needed for quantitative assessment of antimycobacterial activity. Moreover, SFA provides a simple screening method to evaluate antimycobacterial drugs in terms of both antimycobacterial activity and cytotoxicity to host cells, both of which being parameters that are important for developing new drug candidates. SFA also could be useful to evaluate the drug susceptibility of clinical isolates, including PZA, which is difficult to measure with current methods using broth or solid agar.
Acknowledgments
This work was supported in part by grants from the Japan Health Sciences Foundation “Research on Emerging and Re-emerging Infectious Diseases,” Ministry of Health and Welfare of Japan and the U.S.-Japan Cooperative Medical Sciences Program, Grant in Aid for Research in Nagoya City University, and Kurozumi Medical Foundation.
REFERENCES
- 1.Arain, T. M., A. E. Resconi, D. C. Singh, and C. K. Stover. 1996. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob. Agents Chemother. 40:1542-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arain, T. M., A. E. Resconi, M. J. Hickey, and C. K. Stover. 1996. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob. Agents Chemother. 40:1536-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee, D. K., and B. R. Patel. 1993. Evaluation of the activity of a number of antimicrobial agents against mycobacteria within mouse macrophages by a radiometric method. J. Antimicrob. Chemother. 31:289-302. [DOI] [PubMed] [Google Scholar]
- 4.Carlone, N. A., G. Acocella, A. M. Cuffini, and M. Forno-Pizzoglio. 1985. Killing of macrophage-ingested mycobacteria by rifampicin, pyrazinamide, and pyrazinoic acid alone and in combination. Am. Rev. Respir. Dis. 132:1274-1277. [DOI] [PubMed] [Google Scholar]
- 5.Carrière, C., P. F. Riska, O. Zimhony, J. Kriakov, S. Bardarov, J. Burns, J. Chan, and W. R. Jacobs, Jr. 1997. Conditionally replicating luciferase reporter phages: improved sensitivity for rapid detection and assessment of drug susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 35:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, L., and S. G. Franzblau. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowle, A. J., N. Elkins, and M. H. May. 1988. Effectiveness of ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium, and rifampin against M. tuberculosis in cultured human macrophages. Am. Rev. Respir. Dis. 137:1141-1146. [DOI] [PubMed] [Google Scholar]
- 8.Crowle, A. J., J. A. Sbarbaro, and M. H. May. 1988. Effects of isoniazid and of ceforanide against virulent tubercle bacilli in cultured human macrophages. Tubercle 69:15-25. [DOI] [PubMed] [Google Scholar]
- 9.Crowle, A. J., J. A. Sbaro, and M. H. May. 1986. Inhibition by pyrazinamide of tubercle bacilli within cultured human macrophages. Am. Rev. Respir. Dis. 134:1052-1055. [DOI] [PubMed] [Google Scholar]
- 10.Crowle, A. J., J. A. Sbarbaro, F. N. Judson, G. S. Douvas, and M. H. May. 1984. Inhibition by streptomycin of tubercle bacilli within cultured human macrophages. Am. Rev. Respir. Dis. 130:839-844. [DOI] [PubMed] [Google Scholar]
- 11.Flick, D. A., and G. E. Gifford. 1984. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J. Immunol. Methods 68:167-175. [DOI] [PubMed] [Google Scholar]
- 12.Heifets, L., M. Higgins, and B. Simon. 2000. Pyrazinamide is not active against Mycobacterium tuberculosis residing in cultured human monocyte-derived macrophages. Int. J. Tuber. Lung Dis. 4: 491-495. [PubMed] [Google Scholar]
- 13.Heifets, L. B. 1991. Drug susceptibility tests in the management of chemotherapy of tuberculosis, p. 89-122. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infection. CRC Press, Inc., Boca Raton, Fla.
- 14.Hickey, M. J., T. M. Arain, R. M. Shawar, D. J. Humble, M. H. Langhorne, J. N. Morgenroth, and C. K. Stover. 1996. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob. Agents Chemother. 40:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffner, S., C. Jimenez-Misas, and A. Lundin. 1999. Improved extraction and assay and assay of mycobacterial ATP for rapid drug susceptibility testing. Luminescence 14:255-261. [DOI] [PubMed] [Google Scholar]
- 16.Inderieid, C. B., and M. Salfinger. 1995. Antimicrobial agents and susceptibility tests: mycobacteria, p. 1385-1404. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 17.Maher, D., and M. C. Raviglion. 1999. The global epidemic of tuberculosis: a World Health Organization prospective, p. 104-115. In D. Schlossberg (ed.), Tuberculosis and nontuberculous mycobacterial infections, 4th ed. W. B. Saunders Company, Philadelphia, Pa.
- 18.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 19.Marone, P., L. Bono, E. Carretto, D. Barbarini, and S. Telecco. 1997. Rapid drug susceptibility of Mycobacterium avium complex using a fluorescence quenching method. J. Chemother. 9:247-250. [DOI] [PubMed] [Google Scholar]
- 20.McKinney, J. D. 2000. In vivo veritas: the search for TB drug targets goes live. Nat. Med. 6:1330-1333. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi, N., V. Labrousse, and K. S. Goh. 1996. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr. Microbiol. 33:167-175. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi, N., M-C. Potar, and H. L. David. 1987. Intracellular growth of pathogenic mycobacteria in the continuous murine macrophage cell line J774: ultra-structure and drug-susceptibility studies. Curr. Microbiol. 16:79-92. [Google Scholar]
- 23.Reddy, M. V., S. Srinivasan, B. Andersen, and P. R. Gangadharam. 1994. Rapid assessment of mycobacterial growth inside macrophages and mice, using the radiometric (BACTEC) method. Tuber. Lung Dis. 75:127-131. [DOI] [PubMed] [Google Scholar]
- 24.Reisner, B. S., A. M. Gatson, and G. L. Woods. 1995. Evaluation of mycobacteria growth indicator tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagn. Microbiol. Infect. Dis. 22:325-329. [DOI] [PubMed] [Google Scholar]
- 25.Riska, P. F., Y. Su, S. Bardarov, L. Freundlich, G. Sarkis, G. Hatfull, C. Carriere, V. Kumar, J. Chan, and W. R. Jacobs, Jr. 1999. Rapid film-based determination of antibiotic susceptibilities of Mycobacterium tuberculosis strains by using a luciferase reporter phage and the Bronx Box. J. Clin. Microbiol. 37:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohner, P., B. Ninet, C. Metral, S. Emler, and R. Auckenthaler. 1997. Evaluation of the MB/BacT system and comparison to the BACTEC 460 system and solid media for isolation of mycobacteria from clinical specimens. J. Clin. Microbiol. 35:3127-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salfinger, M., A. J. Crowle, and L. B. Reller. 1990. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J. Infect. Dis. 162:201-207. [DOI] [PubMed] [Google Scholar]
- 28.Sbarbaro, J. A., M. D. Iseman, and A. J. Crowle. 1996. Combined effect of pyrazinamide and ofloxacin within the human macrophage. Tuber. Lung Dis. 77:491-495. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava, R., D. K. Deb, K. K. Srivastava, C. Locht, and B. S. Srivastava. 1998. Green fluorescent protein as a reporter in rapid screening of antituberculosis compounds in vitro and in macrophages. Biochem. Biophys. Res. Commun. 253:431-436. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava, R., D. Kumar, and B. S. Srivastava. 1997. Recombinant Mycobacterium aurum expressing Escherichia coli β-galactosidase in high throughput screening of antituberculosis drugs. Biochem. Biophys. Res. Commun. 240:536-539. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava, R., D. Kumar, P. Subramaniam, and B. S. Srivastava. 1997. β-Galactosidase reporter system in mycobacteria and its application in rapid antimycobacterial drug screening. Biochem. Biophys. Res. Commun. 235:602-605. [DOI] [PubMed] [Google Scholar]
- 32.Takii, T., C. Abe, A. Tamura, S. Ramayah, J. T. Belisle, P. J. Brennan, and K. Onozaki. 2001. Interleukin 1 or tumor necrosis factor α augmented cytotoxic effect of mycobacteria on human fibroblasts: application to evaluation of pathogenesis of clinical isolates of M. tuberculosis and M. avium complex. J. Interferon Cytokine Res. 21:187-196. [DOI] [PubMed] [Google Scholar]
- 33.Wallace, R. J., Jr., D. R. Nash, L. C. Steele, and V. Steingrube. 1986. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J. Clin. Micro. 24:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods, G. L., G. Fish, M. Plaunt, and T. Murphy. 1997. Clinical evaluation of difco ESP culture system II for growth and detection of mycobacteria. J. Clin. Microbiol. 35:121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Anti-tuberculosis drug resistance in the world (World Health Organization, Geneva, 2000). [Online.] http://www.who.int/gtb/publications/dritw/contents.htm.
- 36.Zafer, A. A., Y. E. Taylor, and S. A. Sattar. 2001. Rapid screening method for mycobactericidal activity of chemical germicides that uses Mycobacterium terrae expressing a green fluorescent protein gene. Appl. Environ. Microbiol. 67:1239-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]