Abstract
Use of combinations of antimicrobials that together achieve synergistic activities against targeted microorganisms is one potential strategy for overcoming bacterial resistance. As the incidence of infections caused by multidrug-resistant staphylococci and enterococci increases, the importance of devising additional synergistic drug combinations for these bacteria is magnified. We evaluated a number of antimicrobial combinations, with a focus on quinupristin-dalfopristin (Q-D), cefepime, and linezolid, using a previously described in vitro pharmacodynamic model. The combination of Q-D with either linezolid or vancomycin, as well as the combination of cefepime-vancomycin, resulted in enhanced killing (≥2-log10 increase in killing versus the most-active single agent) against methicillin-resistant Staphylococcus aureus (MRSA) 494. An improved effect (<2 log10 kill increase in kill) against MRSA 494 was noted for cefepime plus either Q-D or linezolid, as well as linezolid-vancomycin. Similar relationships were observed for a methicillin-susceptible S. aureus isolate (isolate 1199). Against methicillin-resistant S. epidermidis R444, enhanced killing was achieved with the combination of cefepime-linezolid, while improvement was noted for vancomycin with either cefepime or linezolid. The combination of cefepime and vancomycin also achieved enhanced killing against a glycopeptide-intermediate-susceptible S. aureus isolate (isolate 992). The combination of linezolid and doxycycline achieved an enhanced effect against vancomycin-resistant Enterococcus faecalis (VREFc) and E. faecium. Q-D plus ampicillin or linezolid resulted in similar enhancement of activity against the VREFc isolate. The results of this study suggest a number of novel antimicrobial combinations that may be useful against staphylococci and enterococci. Combination regimens including cefepime, Q-D, and/or linezolid warrant further investigation for the treatment of refractive infections due to multidrug-resistant gram-positive pathogens.
During the past 15 to 20 years, antimicrobial resistance among gram-positive bacteria (most notably, enterococci, staphylococci, and streptococci) has become increasingly prevalent and problematic (7, 8). At the same time, serious infections caused by gram-positive bacteria have become more widespread. The above trends have warranted an increase in efforts to develop new antimicrobials possessing activity against gram-positive organisms. In addition, optimization of pharmacokinetic and pharmacodynamic properties of new and existing antibiotics may enhance efficacy and prevent the development of resistance. Combination therapy using agents that together achieve synergistic activity is one potential means of achieving these goals.
Quinupristin-dalfopristin (Q-D) is a semisynthetic combination of streptogramins in a 30:70 (Q-D, wt/wt) ratio. The compound possesses bactericidal activity against most staphylococci and streptococci, as well as weakly bactericidal or bacteriostatic activity against most enterococci. Bactericidal activity has been demonstrated in vitro against multidrug-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium (VREF). However, only moderate activity is attained against vancomycin-resistant E. faecalis (VREFc) (6, 9, 16, 18).
Practical applications for the use of Q-D may include the treatment of infections caused by highly resistant gram-positive bacteria, including methicillin-resistant S. aureus (MRSA) and VREF. Use of the agent in combination with other antibiotics possessing activity against gram-positive organisms is of potential interest, since synergistic activity against highly resistant bacteria may be obtained. Such a strategy may be especially useful in the empirical treatment of infections caused by multiply resistant gram-positive organisms, including VREFc. Although the rate of emergence of resistance to Q-D has thus far been fairly low, combination therapy may also help to prevent the development of such resistance, thus preserving the clinical utility of Q-D.
Cefepime is a broad-spectrum cephalosporin with activity against many gram-positive bacteria, including S. aureus. It has poor affinity for inducible chromosomally mediated cephalosporinases such as those of the Bush group 1 type and is resistant to hydrolysis by many common chromosomally and plasmid-mediated enzymes, including the extended-broad-spectrum β-lactamases of the Bush 2b′ classification (14, 30). For these reasons, cefepime has been used in the empirical treatment of febrile neutropenic patients, as well as in a variety of serious infections. Synergism or additivity between cefepime and Q-D would be useful from the standpoint that Q-D could then be investigated as an alternative to vancomycin in regimens containing cefepime. Use of such vancomycin-sparing regimens, as in the case of febrile neutropenia, has been shown to help reduce the development of VRE (31, 32).
Linezolid is a synthetic oxazolidinone that possesses activity against a variety of gram-positive bacteria, including MRSA and both VREF and VREFc (11, 26-28). Linezolid has gained U.S. Food and Drug Administration approval for a variety of indications, including nosocomial and community-acquired pneumonia, skin and skin structure infections, and infections caused by VREF. Again, although resistance appears to be low at present, use in combination with other antimicrobials is desirable in order to maintain the drug's activity.
In this study, we evaluated the in vitro activities of Q-D, cefepime, linezolid, ampicillin, doxycycline, and vancomycin, alone and/or in combination, in order to identify combinations where enhanced activity might be obtained against enterococci and staphylococci.
(A portion of this work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, September 2000.)
MATERIALS AND METHODS
Bacterial strains.
MRSA 494 and methicillin-susceptible S. aureus (MSSA) 1199 were provided by Glenn Kaatz (John D. Dingell VA Medical Center, Detroit, Mich.). Clinical isolates methicillin-resistant S. epidermidis (MRSE) R444 and methicillin-susceptible S. epidermidis (MSSE) R387 were obtained from the Detroit Medical Center microbiology laboratory. VREF 12311 and VREFc SF11848 were provided by Marcus J. Zervos (William Beaumont Hospital, Royal Oak, Mich.). Glycopeptide-intermediate-susceptible S. aureus (GISA) strain 992 (New Jersey strain) was obtained from the Centers for Disease Control and Prevention.
Medium.
All in vitro pharmacodynamic models utilized Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB). Colony counts for all experiments were determined using tryptic soy agar (TSA) (Difco).
Antimicrobial agents.
Cefepime (lot 903; Dura Pharmaceuticals) and linezolid [lot (D2)1500-5148-JLH-48; Pharmacia-Upjohn Laboratories, Kalamazoo, Mich.] were supplied by their respective manufacturers. Quinupristin (lot 9830220) and dalfopristin (lot WSD 3047) were supplied as separate components by Aventis Pharmaceuticals, Collegeville, Pa. Ampicillin (Sigma Chemical Company, St. Louis, Mo.), doxycycline (Sigma), and vancomycin (Sigma) were commercially purchased. Stock solutions of each antibiotic were freshly prepared on the day of use.
In vitro susceptibility testing.
MICs and minimum bactericidal concentrations (MBCs) were determined using microdilution with an inoculum of 5 × 105 CFU/ml according to National Committee for Clinical Laboratory Standards guidelines (25).
Confirmation of methicillin resistance.
A 413-bp internal fragment of the mecA gene was amplified from staphylococcal strains MRSA 494 and MRSE R444 using PCR. Primers employed were 5′-AACCGAAGATAAAAAAGAAAC-3′ (forward) and 5′-GTCCGTAACCTGAATCAGC-3′ (reverse). PCR parameters were 94°C for 1 min, 55°C for 1 min, and 72°C for 30 s for 30 cycles. PCR products were separated in agarose gels and visualized by staining with ethidium bromide (3).
In vitro pharmacodynamic model.
An in vitro pharmacodynamic model consisting of a one-compartment glass chamber with multiple ports for the removal of SMHB, delivery of antibiotics, and collection of bacterial and antimicrobial samples was utilized (2). All model simulations were conducted over 48 h and were performed in duplicate to ensure reproducibility. Prior to each experiment, several colonies from an overnight growth on TSA were added to SMHB to obtain a suspension corresponding to a 0.5 McFarland standard. Next, 2.5 ml of this suspension was added to each of the pharmacodynamic models to produce an initial inoculum of 106 CFU/ml. Each model was placed in a 37°C water bath for the duration of the experiment, with a magnetic stir bar in each model to produce continuous mixing of medium. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to continually replace antibiotic-containing medium with fresh SMHB (at a rate to simulate the half-lives (t1/2s)of respective antibiotics). All antimicrobials were infused over approximately 1 min. Regimen simulations were as follows: Q-D 7.5 mg/kg every 8 h (estimated peak concentration and t1/2 of quinupristin and dalfopristin of 3 and 8 mg/liter every 1 and 0.7 h, respectively); ampicillin, 1 g every 6 h (45 mg/liter; 1 h); cefepime, 2 g every 12 h (130 mg/liter; 2 h); doxycycline, 200 mg every 24 h (4 to 6 mg/liter; 19.5 h); linezolid, 600 mg every 12 h (18 mg/liter; 5 h); and vancomycin, 1 g every 12 h (35 to 40 mg/liter; 6 h). For Q-D models, each component was administered separately in order to facilitate simulation of the respective elimination t1/2s of Q and D. In models utilizing combination regimens, the model elimination rate was set for the antibiotic with the shorter t1/2, and the agent with a longer t1/2 was supplemented (5).
Pharmacokinetic analysis.
Antibiotic concentrations were determined from samples drawn in duplicate from each model at 0, 0.5, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h. Samples were stored at −70°C until analysis. Antibiotic peak and trough concentrations and t1/2 were calculated from concentration-time plots of the model samples, using the PKANALYST program (version 1.10; MicroMath Scientific Software, Salt Lake City, Utah).
Pharmacodynamic analysis.
Samples (approximately 0.5 ml each) from each model were collected at 0, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h and serially diluted in cold 0.9% sodium chloride. Bacterial counts were determined by plating 100-μl aliquots of each diluted sample on TSA, using an automated spiral dispenser (Whitley Automatic Spiral Plater; Don Whitley Scientific Limited, West Yorkshire, England). All samples were diluted 10- to 100-fold before plating in order to minimize antibiotic carryover. Plated samples were incubated at 37°C for 24 h, and colony counts (log10 CFU per milliliter) were determined using a laser colony counter (ProtoCOL [version 2.05.02]; Synbiosis, Cambridge, England). The limit of detection for this method of colony count determination is 2.5 log10 CFU/ml. Time-kill curves were determined by plotting mean colony counts (log10 CFU per milliliter) from each model versus time. Bactericidal activity (99.9% kill) was defined as a ≥3-log10-CFU/ml reduction in colony count from the initial inoculum. Enhancement of activity was defined as an increase in kill of ≥2 log10 CFU/ml by a combination of antimicrobials versus the most-active single agent of that combination. Improvement was defined as a <2-log10 increase in kill in comparison to the most active single agent, while combinations that resulted in ≥1-log10 bacterial growth in comparison to the least-active single agent were considered to represent antagonism. The terms “improvement” and “enhancement” were used because our simulations did not permit the mathematical modeling necessary to consider the standard terms “additivity” and “synergy.” Reductions in colony counts were determined over a 48-h period and compared between regimens. Time to achieve 99.9% killing was determined using linear regression (if r2 ≥ 0.95) or by visual inspection.
Antibiotic assays.
Concentrations of vancomycin were determined using fluorescence polarization immunoassay (TDX assay; Abbott Diagnostics). Linezolid concentrations were determined at the Division of Infectious Diseases at the National Jewish Medical and Research Center (Denver, Colo.) using a validated high-performance liquid chromatography (HPLC) assay that conforms to the guidelines set forth by the College of American Pathologists. Samples were measured using a system consisting of a Waters (Milford, Mass.) 515 HPLC pump with a model 680 gradient controller and a solvent select valve, a Spectra Physics (San Jose, Calif.) model 8875 fixed-volume autosampler, a Waters model 486 UV detector, a Macintosh 7100 computer (Apple Computers Inc., Cupertino, Calif.), and the Rainin (Woburn, Mass.) Dynamax HPLC data management system. The plasma standard curve for linezolid ranged from 0.5 to 30 mg/ml. The absolute recovery of linezolid from plasma was 95%. The within-sample precision (percent coefficient of variation) of validation for a single standard concentration was 0.69%, and the overall validation precision across all standards was 1.04 to 4.39.
Concentrations of all other agents were determined using standard agar diffusion bioassay procedures. Doxycycline was assayed using antibiotic assay medium 8 (Difco) and Bacillus cereus ATCC 11778 as an indicator organism, ampicillin was assayed using antibiotic assay medium 5 (Difco) and Bacillus subtilis spore suspension 6633 (Difco), and cefepime concentrations were determined using antibiotic assay medium 5 and Micrococcus luteus ATCC 9341. Quinupristin concentrations were assayed using S. aureus HBD 511 (resistant to dalfopristin via streptogramin A acetylase) in Mueller-Hinton II agar (Difco) containing dalfopristin at 8 mg/liter, while dalfopristin concentrations were determined using S. epidermidis HBD 523 (resistant to quinupristin via constitutive expression of the erm gene) in antibiotic assay medium 5 impregnated with quinupristin at 8 mg/liter (12). The limits of detection for each of the above assays were 2 mg/liter (vancomycin), 0.5 mg/liter (linezolid), 1.5 mg/liter (doxycycline), 0.5 mg/liter (ampicillin), 0.5 mg/liter (cefepime), 0.1 mg/liter (quinupristin), and 0.5 mg/liter (dalfopristin). Coefficients of variation for all assays were less than 10%.
Detection of resistance.
Samples (100 μl each) from each time point were plated onto TSA containing an antibiotic concentration of four to eight times the MIC for each organism and incubated for 48 h at 37°C to monitor for the development of resistance. Plates were visually inspected for growth of resistant subpopulations after 24, 32, and 48 h of incubation. The MIC for resistant organisms was determined using microdilution as described above.
Statistical analysis.
Differences between regimens in log10 CFU per milliliter at 48 h, time to 99.9% kill, and all pharmacodynamic variables were determined using analysis of variance with Tukey's test for multiple comparisons. For all experiments, a P value of ≤0.05 was considered indicative of statistical significance. All statistical analyses were performed using SPSS (version 10; SPSS, Inc., Chicago, Ill.).
RESULTS
Susceptibility testing.
Microdilution MICs and MBCs for all isolates are shown in Table 1. The VREF isolate was susceptible to Q-D, linezolid, and doxycycline, and resistant to ampicillin and cefepime. VREFc SF11848 was resistant to Q-D but susceptible to ampicillin and linezolid. The pattern of susceptibilities for the staphylococci tested was in accordance with expected values.
TABLE 1.
Susceptibility testing results
| Agent | MIC (mg/liter) (MBC [mg/liter]) for:
|
||||||
|---|---|---|---|---|---|---|---|
| MSSE R387 | MRSE R444 | MSSA 1199 | MRSA 494 | GISA 992 | VREF 12311 | VREFc SF11848 | |
| Q-D | 0.03 (0.125) | 0.06 (0.25) | 0.25 (0.5) | 0.5 (1) | 0.125 (0.25) | 0.25 (0.5) | 4 (4) |
| Cefepime | 0.5 (0.5) | 8 (8) | 2 (2) | 8 (16) | 1.6 (32) | >8,192 (>8,192) | >8,192 (>8,192) |
| Linezolid | 1 (2) | 1 (2) | 2 (4) | 4 (8) | 1 (32) | 2 (32) | 2 (32) |
| Ampicillin | 0.5 (1) | 32 (32) | 0.5 (1) | 512 (1,024) | 512 (1,024) | 128 (1,024) | 1 (32) |
| Doxycycline | 0.06 (2) | 0.5 (4) | 0.06 (2) | 4 (64) | 4 (64) | 0.125 (8) | 0.125 (8) |
| Vancomycin | 1 (1) | 1 (2) | 1 (1) | 0.25 (0.5) | 4 (8) | 512 (>8,192) | 512 (>8,192) |
Confirmation of methicillin resistance.
Staphylococcal strains MRSA 494 and MRSE R444 were both found to be positive for the mecA gene.
Pharmacokinetics.
Observed pharmacokinetic parameters (± standard deviation) for the tested agents were as follows (listed as peak [in milligrams per liter], trough [in milligrams per liter], t1/2 [in hours]): quinupristin: 2.85 ± 0.18, 0.07 ± 0.06, 1.49 ± 0.14; dalfopristin: 8.11 ± 0.24, 0.09 ± 0.07, 1.23 ± 0.56; ampicillin: 42.58 ± 0.45, 0.85 ± 0.11, 1.06 ± 0.37; cefepime: 126.50 ± 2.40, 2.25 ± 0.86, 2.06 ± 0.36; doxycycline: 5.80 ± 0.31, 2.67 ± 0.20, 21.44 ± 1.15; linezolid: 18.42 ± 0.28, 4.55 ± 0.22, 5.94 ± 0.26; vancomycin: 40.24 ± 2.61, 11.15 ± 1.42, 6.48 ± 1.08.
Pharmacodynamics.
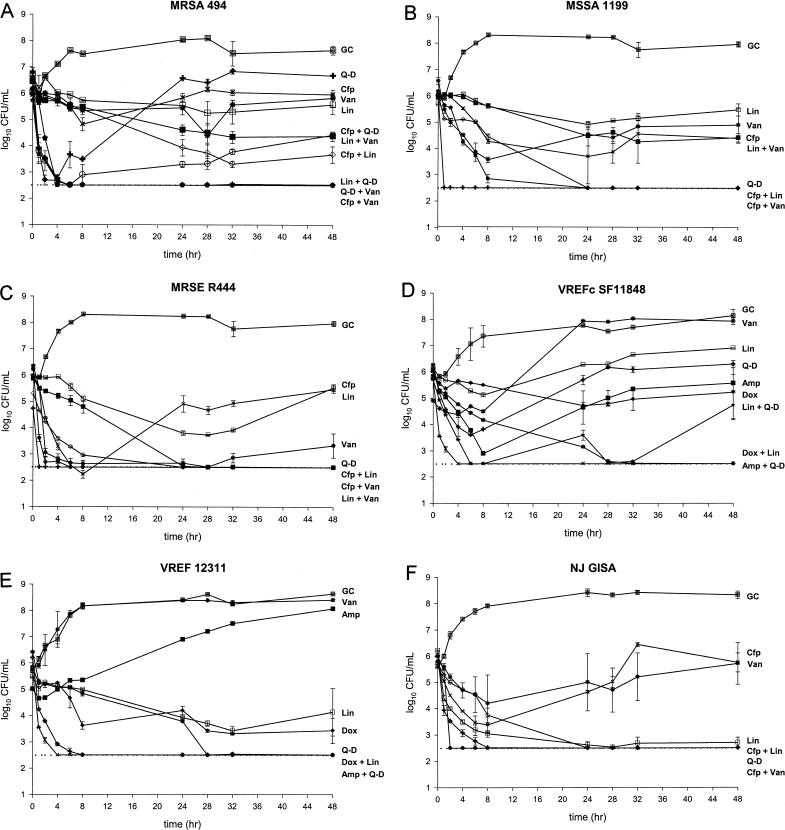
Results of 48-h pharmacodynamic models for the tested strains are shown in Fig. 1. The magnitude of reduction in bacterial inoculum for combination regimens (and relevant monotherapy simulations) is shown in Table 2. Note that negative values indicate regrowth.
FIG. 1.
Activities of tested antimicrobials (alone and in combination) versus MRSA 494 (A), MSSA 1199 (B), MRSE R444 (C), VREFc SF11848 (D), VREF 12311 (E), and GISA 992 (F). The key to symbols for each regimen is as follows: ampicillin (Amp, ▪); cefepime (Cfp, ✖); doxycycline (Dox, ⧫); linezolid (Lin, □); quinupristin-dalfopristin (Q-D, +); vancomycin (Van, ∗); ampicillin + quinupristin-dalfopristin (Amp + Q-D, ✖); cefepime + linezolid (Cfp + Lin, ◊); cefepime + quinupristin-dalfopristin (Cfp + Q-D, ○); cefepime + vancomycin (Cfp + Van,  ); doxycycline + linezolid (Dox + Lin, ∗); linezolid + quinupristin-dalfopristin (Lin + Q-D, ★); linezolid + vancomycin (Lin + Van, ▪); quinupristin-dalfopristin + vancomycin (Q-D + Van, ⧫); growth control (GC, ⊡). The dotted line indicates the lower limit of detection (2.5 log10 CFU/ml) used for bacterial quantification.
); doxycycline + linezolid (Dox + Lin, ∗); linezolid + quinupristin-dalfopristin (Lin + Q-D, ★); linezolid + vancomycin (Lin + Van, ▪); quinupristin-dalfopristin + vancomycin (Q-D + Van, ⧫); growth control (GC, ⊡). The dotted line indicates the lower limit of detection (2.5 log10 CFU/ml) used for bacterial quantification.
TABLE 2.
Inoculum change (over 48 h) obtained in model simulations
| Agent(s) | Inoculum change (log10 CFU/ml) over 48 h in model with:
|
|||||
|---|---|---|---|---|---|---|
| MRSA 494 | MSSA 1199 | MRSE R444 | VREFc SF11848 | VREF 12311 | GISA 992 | |
| Q-D | 0.14 | 3.49 | 2.23 | −0.15 | 3.70 | 3.53 |
| Cefepime | −0.03 | 1.58 | 0.52 | — | — | −0.03 |
| Linezolid | 1.09 | 0.20 | 0.41 | −0.78 | 1.34 | 3.52 |
| Ampicillin | — | — | — | 0.49 | — | — |
| Doxycycline | — | — | — | 0.65 | 2.41 | — |
| Vancomycin | 0.75 | 1.65 | 3.01 | −3.01 | −2.58 | 0.10 |
| Cefepime + linezolid | 3.02a | 3.52a | 2.80b | — | — | 3.73a |
| Cefepime + Q-D | 1.67a | — | — | — | — | — |
| Cefepime + vancomycin | 3.46b | 1.96a | 3.71a | — | — | 3.50b |
| Q-D + ampicillin | — | — | — | 3.04b | 3.23 | — |
| Q-D + linezolid | 3.22b | — | — | 0.98a | — | — |
| Q-D + vancomycin | 3.42b | — | — | — | — | — |
| Linezolid + doxycycline | — | — | — | 3.75b | 3.91a | — |
| Linezolid + vancomycin | 2.08a | 2.28a | 3.38a | — | — | — |
Improvement in kill observed with combination.
Enhancement of kill observed with combination.
—, model not performed.
In model experiments using MRSA 494, Q-D was initially bactericidal at 2 h, but regrowth to initial inoculum levels occurred after 8 h. Cefepime, linezolid, and vancomycin were each bacteriostatic when administered alone. Improvement was noted when cefepime was combined with Q-D or linezolid (1.53 and 1.93 log10 increase in kill at 48 h, respectively), while the combination of Q-D and linezolid achieved enhancement (2.13 log10 increase in kill) and was bactericidal (by 4 h). Q-D and vancomycin together displayed enhancement, with a 2.67-log10 increase in kill with the combination, and maintained bactericidal activity from 2 to 48 h. The combination of cefepime and vancomycin also displayed enhancement (2.65-log10 increase in kill) and was bactericidal from 4 to 48 h. Linezolid and vancomycin together achieved improvement (0.99-log10 increase in kill) (Fig. 1A).
In the case of MSSA 1199, the combination of cefepime and linezolid displayed improvement (1.94-log10 increase in inoculum reduction). Vancomycin and linezolid each were bacteriostatic for this strain, and the combination of the two also exhibited improvement (0.05-log10 increase in kill). Cefepime alone was bacteriostatic versus MSSA 1199. An improved effect was also noted with the combination of cefepime with vancomycin (1.96-log10 increase in kill) (Fig. 1B).
For MRSE R444, cefepime and linezolid each were bacteriostatic, but the combination of the two achieved enhancement and was bactericidal through 48 h. Vancomycin achieved bactericidal activity against this strain, with slight regrowth evident at 48 h. The combination of vancomycin with either cefepime or linezolid achieved bactericidal activity and maintained colony counts at the limit of detection through 48 h. Each of these combinations resulted in an overall improvement in effect. Q-D was bactericidal against this strain (by 1 h) and maintained colony counts at the limit of detection throughout 48 h, and so enhanced effects of Q-D in combination could not be assessed (Fig. 1C).
For VREFc SF11848, ampicillin monotherapy achieved initial bactericidal activity, with rapid regrowth to nearly the original inoculum density. Q-D achieved an initial kill (2.53-log10 reduction in the initial inoculum by 6 h) but was also associated with eventual regrowth from 8 to 48 h. The combination of ampicillin and Q-D was bactericidal by 4 h, displayed enhancement (2.55-log10 increase in kill) by 24 h, and maintained bacterial counts at the limit of detection for the duration of this model. The combination of doxycycline and linezolid also achieved enhancement plus bactericidal activity (3.07-log10 increase in kill) versus VREFc SF11848. Linezolid and Q-D together achieved improvement (1.13-log10 increase in kill) at 48 h. This combination was bactericidal by 6 h, but regrowth occurred after 32 h (Fig. 1D).
Q-D alone was bactericidal by 4 h versus VREF 12311 and maintained inhibition of growth for the duration of the 48-h model experiment. Thus, the presence of enhancement with Q-D could not be assessed. The combination of doxycycline and linezolid achieved improvement (1.50-log10 increase in kill) for VREF 12311, while each agent alone was bacteriostatic (Fig. 1E).
Q-D (at 4 h) and linezolid (after 6 h) each also achieved bactericidal activity against GISA 992, and so the presence of an enhanced effect could not be assessed for these agents. Cefepime and vancomycin monotherapy simulations each achieved initial killing of the GISA strain (maximum kills of 2.35 and 1.60 log10 for cefepime and vancomycin, respectively) but resulted in regrowth to nearly pretreatment levels. The combination of cefepime and vancomycin exhibited enhancement (3.40-log10 increase in kill) and was bactericidal by 2 h (Fig. 1F).
For MSSE R387, the presence of enhanced effects could not be assessed, since all tested agents were bactericidal when administered alone. Q-D and vancomycin each were bactericidal by 4 h, while cefepime and linezolid achieved bactericidal activity against this isolate by 6 h and 24 h, respectively (data not shown).
No tested combinations displayed antagonism for any of the tested strains.
Detection of resistance.
Resistance was not detected in any tested samples from monotherapy regimens. We failed to detect MIC elevations even in those models where significant bacterial regrowth occurred.
DISCUSSION
Q-D and linezolid are important new antimicrobials that serve to expand the available armamentarium of agents available for the treatment of infections caused by multidrug-resistant gram-positive pathogens. Cefepime has also been used successfully in the treatment of a variety of infections caused by gram-positive microorganisms. Despite the utility of these agents, cases of clinical failure accompanied by the development of resistance have been reported (4, 22). In the case of linezolid or Q-D, many of these reports of diminished susceptibility and/or treatment failure have occurred in patients possessing a sequestered site of infection (10; R. D. Gonzales, P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn, Letter, Lancet 357:1179, 2001). For example, among over 2,000 patients enrolled in the linezolid clinical-use trials, E. faecium isolates resistant to linezolid were identified in five patients, each of whom had had longstanding indwelling devices and complicated hospital courses (Gonzales et al., Letter).
Synergy between Q-D and ampicillin or doxycycline has been demonstrated in vitro, and both of the latter agents have been used successfully in combination with Q-D in vivo (1). In vitro synergy has also been observed for the combination of Q-D and rifampin or ciprofloxacin against MRSA (29); Q-D and vancomycin against MRSA and GISA (13, 15) and VREF (16, 17, 23); and Q-D plus vancomycin, ampicillin-sulbactam, or doxycycline versus VREF (21). Aeschlimann et al. (1) also found that the addition of doxycycline to Q-D enhanced killing and prevented the emergence of Q-D resistance in VREF. Interactions of Q-D and other antimicrobials have also been studied in animal models of infection (20, 33, 34).
Cefepime has been noted to display synergy with imipenem against Enterobacter cloacae in an animal model of pneumonia (24), and the combination of cefepime and vancomycin was synergistic against a majority of strains of MSSA and MRSA in vitro (19). A limited number of studies have examined the effect of combinations containing linezolid. Sweeney et al. (M. T. Sweeney and G. E. Zurenko, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2295, 2000) tested linezolid in combination with a variety of antimicrobials against multiple organisms, using an in vitro checkerboard methodology for detection of synergy. Of 557 total combinations, the combination of linezolid and tetracycline was antagonistic for one VREF isolate, whereas synergy was noted with the combination of linezolid and teicoplanin (versus vancomycin-susceptible E. faecalis) and linezolid plus tetracycline (against a single isolate of VREF).
A majority of published studies of in vitro synergistic relationships of antimicrobials are performed using methodologies such as fractional inhibitory concentration testing or other procedures using static antimicrobial concentrations. The disadvantage of such techniques is that they are designed only to evaluate antibiotic effects at a single point in time and at fixed concentrations. Use of in vitro pharmacodynamic models to evaluate the potential for an enhancement of effect between antimicrobials offers the advantage of studying agents in a dynamic system that more closely mimics human pharmacokinetics. However, a potential limitation of our research is the use of therapeutic concentrations only of all tested agents. In contrast, traditional synergy testing is commonly conducted using a range of subinhibitory concentrations of one or both antimicrobials. While this practice typically leads to identification of a wider range of synergistic combinations, we chose to study clinically achievable concentrations in order to allow broader applicability of our results to clinical practice.
We observed enhanced killing with a number of the antimicrobial combinations tested. Of particular interest was our finding of enhanced killing with Q-D and ampicillin for the VREFc isolate. To our knowledge this has not been reported elsewhere. Given Q-D's lack of activity against VREFc, this combination represents a promising avenue for further research. A number of combinations achieved positive results against the MRSA isolate. For this strain, the combination of Q-D with either vancomycin or linezolid achieved enhanced killing, as did the combination of cefepime and vancomycin. An improved effect was noted when cefepime was combined with either Q-D or linezolid and when linezolid was paired with vancomycin. Similar results were obtained for both MRSE and MSSA. Also of note was our finding of enhancement or improvement for the combination of doxycycline and linezolid versus VREFc and VREF, respectively. Q-D and linezolid together also achieved improved killing against the VREFc isolate. To our knowledge, this is the first instance in which a positive effect of combining these agents has been reported. Although the pairing of Q-D and linezolid has not been investigated clinically, this combination may represent a potential therapy for refractory infections caused by multidrug-resistant staphylococci and enterococci.
Although a large number of combination regimens were investigated in our in vitro model, a limitation of the present study is the use of a single isolate only for each strain tested. In addition, we cannot conclude with certainty that our results will hold true with longer treatment durations. Therefore, our results should be applied to clinical practice with caution. However, a majority of the combinations that were found to result in an improved and/or enhanced effect have been reported elsewhere, as described above. Nonetheless, confirmation of our results with further study would be beneficial before adoption of these combinations in the care of patients occurs.
In the present study we were able to show improved or enhanced activity through use of a variety of antimicrobial combinations encompassing cefepime, Q-D, and linezolid. Further investigation of such combinations is warranted, especially in those patient populations at increased risk for the development of infections caused by multiply resistant pathogens.
Acknowledgments
This work was supported by grants from Aventis Pharmaceuticals and Elan Pharmaceuticals.
We thank Glenn W. Kaatz for mecA PCR analysis in the MRSA and MRSE isolates, Charles Peloquin for determination of linezolid concentrations, and Richard Grucz for technical assistance.
REFERENCES
- 1.Aeschlimann, J. R., M. J. Zervos, and M. J. Rybak. 1998. Treatment of vancomycin-resistant Enterococcus faecium with RP 59500 (quinupristin-dalfopristin) administered by intermittent or continuous infusion, alone or in combination with doxycycline, in an in vitro pharmacodynamic infection model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 42:2710-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1998. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 4.Babcock, H. M., D. J. Ritchie, E. Christiansen, R. Starlin, R. Little, and S. Stanley. 2001. Successful treatment of vancomycin-resistant Enterococcus endocarditis with oral linezolid. Clin. Infect. Dis. 32:1373-1375. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 6.Bouanchaud, D. H. 1997. In-vitro and in-vivo antibacterial activity of quinupristin/dalfopristin. J. Antimicrob. Chemother. 39(Suppl. A):15-21. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:813-815. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1993. Nosocomial enterococci resistant to vancomycin—United States, 1989-1993. Morb. Mortal. Wkly. Rep. 42:597-599. [PubMed] [Google Scholar]
- 9.Collins, L. A., G. J. Malanoski, G. M. Eliopoulos, C. B. Wennersten, M. J. Ferraro, and R. C. Moellering. 1993. In vitro activity of RP59500, an injectable streptogramin antibiotic, against vancomycin-resistant gram-positive organisms. Antimicrob. Agents Chemother. 37:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowzicky, M., G. H. Talbot, P. Prokocimer, J. Etienne, and R. Leclercq. 2000. Characterization of isolates associated with emerging resistance to quinupristin/dalfopristin (Synercid®) during a worldwide clinical program. Diagn. Microbiol. Infect. Dis. 37:57-62. [DOI] [PubMed] [Google Scholar]
- 11.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 12.Entenza, J. M., H. Drugeon, M. P. Glauser, and P. Moreillon. 1995. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob. Agents Chemother. 39:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershberger, E., J. R. Aeschlimann, T. Moldovan, and M. J. Rybak. 1999. Evaluation of bactericidal activities of LY333328, vancomycin, teicoplanin, ampicillin-sulbactam, trovafloxacin, and RP59500 alone or in combination with rifampin or gentamicin against different strains of vancomycin-intermediate Staphylococcus aureus by time-kill curve methods. Antimicrob. Agents Chemother. 43:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway, W. J., and D. Palmer. 1996. Clinical applications of a new parenteral antibiotic in the treatment of severe bacterial infections. Am. J. Med. 100(Suppl. 6A):52S-59S. [DOI] [PubMed] [Google Scholar]
- 15.Kang, S. L., and M. J. Rybak. 1995. Pharmacodynamics of RP 59500 alone and in combination with vancomycin against Staphylococcus aureus in an in vitro-infected fibrin clot model. Antimicrob. Agents Chemother. 39:1505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang, S. L., and M. J. Rybak. 1997. In-vitro bactericidal activity of quinupristin/dalfopristin alone and in combination against resistant strains of Enterococcus species and Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):33-39. [DOI] [PubMed] [Google Scholar]
- 17.Lorian, V., and F. Fernandes. 1997. Synergic activity of vancomycin-quinupristin/dalfopristin combination against Enterococcus faecium. J. Antimicrob. Chemother. 39(Suppl. A):63-66. [DOI] [PubMed] [Google Scholar]
- 18.Low, D. E., and H. L. Nadler. 1997. A review of in-vitro antibacterial activity of quinupristin/dalfopristin against methicillin-susceptible and -resistant Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):53-58. [DOI] [PubMed] [Google Scholar]
- 19.Lozniewski, A., C. Lion, F. Mory, and M. Weber. 2001. In vitro synergy between cefepime and vancomycin against methicillin-susceptible and -resistant Staphylococcus aureus and Staphylococcus epidermidis. J. Antimicrob. Chemother. 47:83-86. [DOI] [PubMed] [Google Scholar]
- 20.Matsumara, S., and A. E. Simor. 1998. Treatment of endocarditis due to vancomycin-resistant Enterococcus faecium with quinupristin/dalfopristin, doxycycline, and rifampin: a synergistic drug combination. Clin. Infect. Dis. 27:1554-1556. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura, S. O., L. Louie, M. Louie, and A. E. Simor. 1999. Synergy testing of vancomycin-resistant Enterococcus faecium against quinupristin-dalfopristin in combination with other antimicrobial agents. Antimicrob. Agents Chemother. 43:2776-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil, S. A., N. M. Clark, P. H. Chandrasekar, and C. A. Kauffman. 2000. Successful treatment of vancomycin-resistant Enterococcus faecium bacteremia with linezolid after failure of treatment with Synercid (quinupristin-dalfopristin). Clin. Infect. Dis. 30:403-404. [DOI] [PubMed] [Google Scholar]
- 23.Mercier, R. C., S. R. Penzak, and M. J. Rybak. 1997. In vitro activities of an investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 41:2573-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mimoz, O., A. Jacolot, S. Leotard, N. Hidri, K. Samii, P. Nordmann, and O. Petitjean. 1998. Efficacies of cefepime, ceftazidime, and imipenem alone or in combination with amikacin in rats with experimental pneumonia due to ceftazidime-susceptible or -resistant Enterobacter cloacae strains. Antimicrob. Agents Chemother. 42:3304-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1997. Approved standard. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Noskin, G. A., F. Siddiqui, V. Stosor, D. Hacek, and L. R. Peterson. 1999. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel, R., M. S. Rouse, K. E. Piper, and J. M. Steckelberg. 1999. In vitro activity of linezolid against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 34:119-122. [DOI] [PubMed] [Google Scholar]
- 28.Rybak, M. J., D. M. Cappelletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz. 1998. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambatakou, H., E. J. Giamarellos-Bourboulis, P. Grecka, Z. Chryssouli, and H. Giamarellou. 1998. In-vitro activity and killing effect of quinupristin/dalfopristin (RP59500) on nosocomial Staphylococcus aureus and interactions with rifampicin and ciprofloxacin against methicillin-resistant isolates. J. Antimicrob. Chemother. 41:349-355. [DOI] [PubMed] [Google Scholar]
- 30.Segreti, J., and S. Levin. 1996. Bacteriologic and clinical applications of a new extended-spectrum parenteral cephalosporin. Am. J. Med. 100(Suppl. 6A):45S-51S. [DOI] [PubMed] [Google Scholar]
- 31.Shay, D. K., S. A. Maloney, M. Montecalvo, S. Banerjee, G. P. Wormser, M. J. Arduino, L. A. Bland, and W. R. Jarvis. 1995. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J. Infect. Dis. 172:993-1000. [DOI] [PubMed] [Google Scholar]
- 32.Tornieporth, N. G., R. B. Roberts, J. John, A. Hafner, and L. W. Riley. 1996. Risk factors associated with vancomycin-resistant Enterococcus faecium infection or colonization in 145 matched case patients and control patients. Clin. Infect. Dis. 23:767-772. [DOI] [PubMed] [Google Scholar]
- 33.Vouillamoz, J., J. M. Entenza, C. Féger, M. P. Glauser, and P. Moreilon. 2000. Quinupristin-dalfopristin combined with β-lactams for treatment of experimental endocarditis due to Staphylococcus aureus constitutively resistant to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 44:1789-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarrouk, V., B. Bozdogan, R. Leclercq, L. Garry, C. Feger, C. Carbon, and B. Fantin. 2001. Activities of the combination of quinupristin-dalfopristin with rifampin in vitro and in experimental endocarditis due to Staphylococcus aureus strains with various phenotypes of resistance to macrolide-lincosamide-streptogramin antibiotics. Antimicrob. Agents Chemother. 45:1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]