Abstract
The safety, pharmacokinetics, and pharmacodynamics of cyclodextrin itraconazole (CD-ITRA) oral suspension were investigated in an open sequential dose escalation study with 26 human immunodeficiency virus (HIV)-infected children and adolescents (5 to 18 years old; mean CD4+-cell count, 128/μl) with oropharyngeal candidiasis (OPC). Patients received CD-ITRA at either 2.5 mg/kg of body weight once a day (QD) or 2.5 mg/kg twice a day (BID) for a total of 15 days. Pharmacokinetic sampling was performed after the first dose and for up to 120 h after the last dose, and antifungal efficacy was evaluated by standardized scoring of the oropharynx. Apart from mild to moderate gastrointestinal disturbances in three patients (11.5%), CD-ITRA was well tolerated. Two patients (7.6%) discontinued treatment prematurely due to study drug-related adverse events. After 15 days of treatment, the peak concentration of drug in plasma (Cmax), the area under the plasma concentration-time curve (AUC) from 0 to 24 h (AUC0-24), the concentration in plasma at the end of the dosing interval (predose) (Cmin), and the terminal half-life of itraconazole (ITRA) were (means and standard deviations) 0.604 ± 0.53 μg/ml, 6.80 ± 7.4 μg · h/ml, 0.192 ± 0.06 μg/ml, and 56.48 ± 44 h, respectively, for the QD regimen and 1.340 ± 0.75 μg/ml, 23.04 ± 14.5 μg · h/ml, 0.782 ± 0.19 μg/ml, and 104.22 ± 94 h, respectively, for the BID regimen. The mean AUC-based accumulation factors for ITRA on day 15 were 4.14 ± 0.9 and 3.53 ± 0.6, respectively. A comparison of the dose-normalized median AUC of the two dosage regimens revealed a trend toward nonlinear drug disposition (P = 0.05). The mean metabolic ratios (AUC of hydroxyitraconazole/AUC of ITRA) at day 15 were 1.96 ± 0.1 for the QD regimen and 1.29 ± 0.2 for the BID regimen, respectively (P < 0.05). The OPC score (range, 0 to 13) for all 26 patients decreased from a mean of 7.46 ± 0.8 at baseline to 2.8 ± 0.7 at the end of therapy (P < 0.001), demonstrating antifungal efficacy in this setting. The relationships among Cmax, Cmin, AUC0-12, Cmax/MIC, Cmin/MIC, AUC0-12/MIC, time during the dosing interval when the plasma drug concentrations were above the MIC for the infecting isolate, and the residual OPC score at day 15 for the entire study population fit inhibitory effect pharmacodynamic models (r, 0.595 to 0.421; P, <0.01 to <0.05). All patients with fluconazole-resistant isolates responded to treatment with CD-ITRA; however, there was no clear correlation between the MIC of ITRA and response to therapy. In conclusion, CD-ITRA was well tolerated and efficacious for the treatment of OPC in HIV-infected pediatric patients. Pharmacodynamic modeling revealed significant correlations between plasma drug concentrations and antifungal efficacy. Based on this documented safety and efficacy, a dosage of 2.5 mg/kg BID can be recommended for the treatment of OPC in pediatric patients ≥5 years old.
Oropharyngeal candidiasis (OPC) is the most frequent fungal infection in children and adolescents infected with human immunodeficiency virus (HIV). Its clinical manifestations can range from isolated, asymptomatic mucosal plaques to complete involvement of the oropharynx and painful mucositis. OPC may be complicated by esophageal candidiasis and may lead to impaired food and fluid intake, weight loss, and dehydration, particularly in infants and young children (18).
Topical polyenes and topical imidazoles are not always successful, except for mild forms of OPC, and many patients require systemic therapy with either ketoconazole or fluconazole (18, 34). During the past decade, however, clinical and microbiological resistance of Candida spp. to fluconazole, the most widely used agent, has emerged among patients with advanced stages of HIV disease (32). For infections in these patients, systemic administration of amphotericin B still remains the sole approved therapeutic approach but is fraught by the need for reliable intravenous access, prolonged infusion times, and potentially significant adverse effects (14, 20).
Itraconazole (ITRA), a highly lipophilic antifungal triazole, has potent and broad-spectrum activity against Candida spp. in vitro and in vivo, including many fluconazole-resistant strains (6). However, the conventional capsule formulation of ITRA provides only erratic systemic bioavailability and no topical exposure to the mucosa, and it is difficult to administer to infants and younger children (14). The recently developed oral hydroxypropyl-β-cyclodextrin (CD) solution of ITRA (CD-ITRA) improves the solubility and thereby the gastric absorption of ITRA and provides high topical drug concentrations in the oral cavity and the esophagus (30, 36). Clinical studies with adults have documented acceptable tolerance of oral CD-ITRA, markedly improved bioavailability compared to that of the capsule formulation (1, 2, 33), and clinical efficacy in the primary (12, 21) and secondary (3, 8, 22, 28) treatment of OPC.
CD-ITRA is a potentially useful alternative to conventional therapies in immunocompromised pediatric patients (13). However, little is known about the safety and pharmacokinetic properties of CD-ITRA for the treatment of OPC in this population. We investigated the safety, pharmacokinetics, and pharmacodynamics of this novel compound in HIV-infected children and adolescents with OPC.
MATERIALS AND METHODS
Patients.
The study was performed at the Pediatric Oncology Branch and the HIV- and AIDS-Related Malignancy Branch of the National Cancer Institute and the Warren Grant Magnuson Clinical Center under a protocol approved by the institutional internal review board. Written informed consent was obtained from the parent or legal guardian of the patient prior to study entry. Male and female children and adolescents (5 to 18 years old) with HIV infections and with clinically overt, culture- or smear-proven OPC were eligible for enrollment. Exclusion criteria other than pregnancy, lactation, or a history of hypersensitivity to azole compounds included aspartate aminotransferase or alanine aminotransferase levels exceeding 10 times the upper limit of normal; bilirubin levels exceeding 3 times the upper limit of normal; a serum creatinine level of greater than 2.0 mg/dl; concomitant use of terfenadine, astemizole, cisapride, rifampin, rifabutin, cyclosporine, phenytoin, or phenobarbital; and therapy with ITRA during the 2 weeks prior to enrollment in the study. All antiretroviral and other supportive care medications were permitted, except for concomitant topical or systemic antifungal agents.
Administration of study drug.
The study was designed as an open-label, age-stratified dose escalation trial of oral CD-ITRA for the treatment of HIV-associated OPC. Patients were enrolled in two age categories, 5 to <13 years old and 13 to 18 years old, in order to ensure even enrollment across the selected age range. The first cohort of 14 children (7 per age category) received CD-ITRA at 2.5 mg/kg of body weight once a day (QD) (maximum dose, 200 mg/day) for 15 days. The second cohort of 12 children (6 per age category) received CD-ITRA at 2.5 mg/kg twice a day (BID) (maximum, 300 mg/day) for 15 days. Both cohorts were evaluated for toxicity, pharmacokinetics, and antifungal efficacy. Enrollment of patients into the second cohort was permitted after seven patients had been entered into an age category in the first cohort and at least five had completed therapy within that specific age category in compliance with protocol-defined safety criteria. If treatment was successful and well tolerated through day 15, patients were eligible to receive the study drug for an additional 5 months with scheduled monthly monitoring for safety and efficacy. CD-ITRA was provided by Janssen Research Foundation, Beerse, Belgium, as an oral solution containing 10 mg of ITRA per ml and 400 mg of CD per ml and was administered on an empty stomach at least 1 h after eating or at least 1 h before eating and at least 2 h before or after the administration of antacids or antacid-containing drugs.
Criteria for discontinuation.
Criteria for discontinuation from the study included serious or intolerable adverse experiences (defined as grade III or IV toxicity with special protocol-defined guidelines for liver function tests) related to the study medication; diagnosis after initiation of the study of a fungal infection that is normally not treated with oral CD-ITRA; deterioration during therapy or lack of response at day 15; and refusal of further treatment or follow-up.
Safety assessment.
All patients who received at least one dose of the study drug were included in the analysis of safety and tolerance. Data on adverse events were collected throughout the study. Clinical and laboratory parameters for safety and tolerance were evaluated at baseline, at day 8, at the end of treatment (day 15), and at day 20. Patients who remained on therapy beyond day 20 were evaluated monthly. The evaluation at study visits consisted of history, physical examination, and laboratory tests (i.e., hematology, blood chemistry, liver function tests, and urinalysis). Toxicities were graded according to National Cancer Institute common toxicity criteria, and their relationship to the study drug was determined by the principal investigator (Thomas J. Walsh).
Pharmacokinetics. (i) Pharmacokinetic sampling.
Serial blood specimens (2 ml per sample) were collected for up to 24 h after the 1st dose and for up to 120 h after the last (15th) dose. In the second cohort (twice-daily dosing), patients had the second dose withheld on days 1 and 15 so that 24-h and washout kinetic profiles could be determined. Samples were obtained at the following time points: immediately before the first dose on day 1; at 1, 2, 4, 6, 8, and 24 h after the first dose; immediately before the last dose on day 15; and at 1, 2, 4, 6, 24, 48, 72, and 120 h after the last dose. Blood was collected into heparinized tubes, inverted to mix heparin, and centrifuged for 10 min at 1,000 × g within 2 h after collection. Separated plasma was transferred to clean polypropylene tubes and stored at −70°C until assayed.
(ii) Analytical method.
Concentrations of ITRA and its bioactive metabolite, hydroxyitraconazole (OH-ITRA), in plasma were measured by a modified, validated reversed-phase high-performance liquid chromatographic method originally developed by Gubbins et al. (16).
For extraction, 350 μl of plasma was vortexed with 50 μl of the internal standard, saperconazole (SAP; R 066905; 5 μg/ml in 100% acetonitrile; purchased from Janssen Research Foundation), in clean borosilicate conical test tubes. To each tube, 50 μl of 0.3 N BaOH2 and 50 μl of 0.4 N ZnSO4 · 7H2O (Fisher Scientific, Fair Lawn, N.J.) were added and vortexed for 15 s. Thereafter, 950 μl of 100% acetonitrile was added and vortexed for 1 min. After centrifugation at 4,000 × g for 10 min, 1.2 ml of the supernatant was transferred to a fresh tube, and the organic solvent was dried in an evaporator (Zymark Corp., Hopkinston, Mass.) under a steady stream of nitrogen at 60°C. The sample was reconstituted with 250 μl of the mobile phase, vortexed for 30 s, transferred to a fresh microcentrifuge tube, and centrifuged at 12,500 × g for 10 min prior to injection. Standards and quality control samples were similarly prepared by adding known amounts of the reference standards for ITRA and OH-ITRA (R 051211 and R 063373; purchased from Janssen Research Foundation) and the internal standard to pooled human plasma (Gibco Laboratories, Grand Island, N.Y.).
The mobile phase consisted of acetonitrile-deionized water-methanol (570:350:80 [vol/vol]), adjusted to pH 5 with 0.1 N H3PO4, and was delivered after sonication under vacuum at a rate 1 ml/min. The injection volume was 75 μl. The analysis was performed with a C18 (250 by 4.6 mm; 5-μm particle size) base-deactivated Alltec Alltima analytical column maintained at 37°C and preceded by a C18 (7.5 by 4.6 mm; 5-μm particle size) Alltech Alltima C18 precolumn (both columns from Alltech Associates, Deerfield, Ill.). OH-ITRA, SAP, and ITRA eluted at approximately 9.5, 11, and 21 min without interference from endogenous plasma components.
Quantitation was based on the ratio of peak heights of ITRA and OH-ITRA to that of the internal standard (SAP). Eight-point standard curves (25 to 1,500 ng/ml) were linear, with R2 values of ≥0.998. The lower limits of quantitation were 25 ng/ml for ITRA and 50 ng/ml for OH-ITRA. Accuracies were within ±5.2%, and intraday and interday variabilities (precision) ranged from 3.2 to 6.2% and from 2.6 to 6.4%, respectively, at 200, 600, and 1,200 ng/ml. Over-curve controls (1,800, 2,400, and 5,000 ng/ml) for determining concentrations of ITRA and OH-ITRA that exceeded the upper limit of the standard curves confirmed parallelism when specimens required dilution for analysis.
(iii) Pharmacokinetic data analysis.
Pharmacokinetic parameters for ITRA and OH-ITRA were determined by using model-independent analysis. Based on the plasma concentration-time profiles for individual patients, the following pharmacokinetic parameters were determined: the concentration in plasma at the end of the dosing interval (predose) (Cmin); the peak concentration in plasma (Cmax); the time to Cmax (Tmax); the area under the plasma concentration-time curve (AUC) of dosing interval τ (AUCτ) and the AUC from 0 to 12 h (AUC0-12), calculated by trapezoidal estimation; dose linearity, determined by comparison of the mean dose-normalized AUCτ values for the two dosage regimens; accumulation, assessed for each patient by dividing the AUCτ on day 15 by the respective value on day 1; and metabolic ratio, calculated as the AUCτ for OH-ITRA divided by the AUCτ for ITRA. Plasma drug clearance, volume of distribution, and elimination half-life were calculated following standard equations (10) with the aid of the WinNonlin computer program (Scientific Consulting, Lexington, Ky.).
Assessment of efficacy.
The efficacy of therapy was evaluated clinically by assessment of the extent of OPC at baseline, on day 8, at the end of treatment (day 15), and 5 days after the end of treatment. Oropharyngeal swab samples of oral lesions were simultaneously obtained for species identification and assessment of antifungal susceptibility. Patients who continued medication with the study drug were monitored monthly during the postkinetic phase. Responses to treatment were quantified by a standardized score for OPC (OPC score). The OPC score is based on the division of the entire oropharyngeal mucosa into 13 anatomical areas. The presence or absence of typical lesions is given a point value of 1 or 0, respectively, and the point values for the 13 areas are added. Accordingly, involvement of the entire oropharynx corresponds to an OPC score of 13, and the absence of candidiasis in the oropharynx corresponds to a score of 0. For the evaluation of efficacy, the OPC score at the end of treatment (day 15) was compared to the baseline value. A complete response was defined as complete clearance of all attributable lesions, corresponding to an OPC score of 0 at the end of treatment, and a partial response was defined as any diminution of the OPC score. Patients with identical or higher OPC scores at the end of treatment were classified as experiencing treatment failures.
Pharmacodynamics.
The relationship between pharmacodynamic parameters and antifungal efficacy was evaluated by using inhibitory maximum-effect pharmacodynamic models with the WinNonlin computer program. Pharmacodynamic parameters were computed from ITRA concentration data obtained on day 15 and the MIC of ITRA for the baseline isolate from each patient. The residual OPC score at day 15 served as the end point of antifungal efficacy. Pharmacodynamic parameters studied included Cmin, Cmax, AUC0-12, and the MIC-related parameters Cmin/MIC, Cmax/MIC, AUC0-12/MIC, and Tτ ≥ MIC (time during dosing interval τ when plasma drug concentrations were above the MIC for the infecting isolate). The MICs of the antifungal agents were determined by microdilution assays with the NCCLS reference method for broth dilution antifungal susceptibility testing of yeasts (18) after completion of the study.
Statistics.
All patients who fulfilled the entry criteria and received at least one dose of the study drug were eligible for analysis on an intent-to-treat basis. The primary efficacy end point was clinical response at day 15 as assessed by the OPC score. Statistical comparisons between categorical data were performed by Fisher's exact test. For comparison of continuous data, the Mann-Whitney U test was used. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Patients.
A total of 26 patients were enrolled in the study. Twenty-five patients received CD-ITRA for at least 15 consecutive days; the remaining patient was withdrawn from the study after 14 consecutive days of treatment but was considered eligible for analysis of clinical efficacy at day 15. Adequate pharmacokinetic sampling on days 1 and 15 was completed for 26 and 25 study subjects, respectively. Twenty-three patients continued treatment with CD-ITRA following washout pharmacokinetic sampling; the total mean duration of treatment with CD-ITRA was 142 days (range, 46 to 180 days; cohort 1, 129 [46 to 180] days; cohort 2, 156 [57 to 180] days).
The demographic and baseline characteristics of the 26 patients are shown in Table 1. There were 13 boys and 13 girls, with a mean age of 12 years (range, 5 to 18 years) and a mean body mass index of 16.63 (range, 13 to 26.3). All had serologically documented HIV infection; the mean CD4+-lymphocyte count at study entry was 128 CD4+ cells/μl (range, 0 to 801 cells/μl), and the majority of the patients (73%) were classified as Centers for Disease Control and Prevention (CDC) stage C3. All 26 patients had received prior antifungal therapy for OPC, including six with a history of fluconazole-resistant OPC. Apart from various other concomitant medications, virtually all patients were receiving antibacterial agents at the time of enrollment. Twenty-three patients (88%) were being treated with antiretroviral agents; among these, 14 (54% of all patients) were receiving protease inhibitor-containing regimens (indinavir, 7; ritonavir, 3; nelfinavir, 3; and saquinavir plus ritonavir, 1).
TABLE 1.
Demographic and clinical baseline characteristics of patients receiving CD-ITRA
| Characteristic | Valuea for the following patients:
|
||
|---|---|---|---|
| Cohort receiving 2.5 mg of CD-ITRA/kg
|
All (n = 26) | ||
| QD (n = 14) | BID (n = 12) | ||
| Age | |||
| Mean (yr) | 12.3 | 12.0 | 12.1 |
| Range (yr) | 6.3-18.3 | 5.7-17.3 | 5.7-18.3 |
| Group | |||
| 5-<13 yr | 7 | 6 | 13 |
| 13-18 yr | 7 | 6 | 13 |
| Gender | |||
| Male | 10 | 3 | 13 |
| Female | 4 | 9 | 13 |
| CD4+-cell count (μl) | |||
| Mean | 91 | 170 | 128 |
| Range | 0-668 | 0-801 | 0-801 |
| CDC classificationb | |||
| C3 | 10 | 9 | 19 |
| C2 | 1 | 2 | 3 |
| B3 | 2 | 1 | 3 |
| B2 | 1 | 0 | 1 |
| History of OPC | |||
| Any history | 14 | 12 | 26 |
| Fluconazole resistant | 5 | 1 | 6 |
| History of esophageal candidiasis | 3 | 1 | 4 |
| Concomitant treatment with antibacterial agents | |||
| Any | 14 | 11 | 25 |
| SXTc | 11 | 7 | 18 |
| Other than SXT | 11 | 11 | 22 |
| Concomitant treatment with protease inhibitors | 4 | 10 | 14 |
Values are given as number of patients unless otherwise indicated.
CDC revised classification system (4).
SXT, trimethoprim-sulfamethoxazole.
Candida albicans was the predominant isolate in cultures at baseline; other species included C. glabrata and C. humicola (Table 2). According to current NCCLS breakpoints, 7 Candida isolates were resistant in vitro to fluconazole and 13 were resistant to ITRA. All isolates were susceptible in vitro to amphotericin B.
TABLE 2.
Identification and in vitro susceptibility of baseline isolates from patients receiving CD-ITRA
| Characteristic | Valuea for the following patients:
|
||
|---|---|---|---|
| Cohort receiving 2.5 mg of CD-ITRA/kg
|
All (n = 26) | ||
| QD (n = 14) | BID (n = 12) | ||
| Identified species | |||
| C. albicans | 10 | 9 | 19 |
| C. glabrata | 1 | 1 | 2b |
| C. humicola | 0 | 1 | 1b |
| Yeast-like cells on smear | 3 | 1 | 4 |
| Susceptibilityc to: | |||
| Fluconazole at: | |||
| ≤8 | 4 | 6 | 10 |
| 16-32 | 3 | 2 | 5 |
| ≥64 | 4 | 3 | 7 |
| Itraconazole at: | |||
| ≤0.125 | 0 | 0 | 0 |
| 0.25-0.5 | 4 | 5 | 9 |
| ≥1 | 7 | 6 | 13 |
| 1 | 1 | 2 | 3 |
| 2 | 1 | 0 | 1 |
| 4 | 2 | 1 | 3 |
| 8 | 1 | 0 | 1 |
| >16 | 2 | 3 | 5 |
| Amphotericin B at: | |||
| 0.5 | 5 | 8 | 13 |
| 1.0 | 6 | 3 | 9 |
Values are given as number of patients.
The MICs of fluconazole, ITRA, and amphotericin B for the two C. glabrata isolates were 32, 2, and 1 and >64, >16, and 1 μg/ml, respectively; the MICs of the same drugs for the C. humicola isolate were 32, 1, and 1 μg/ml, respectively.
Drug concentrations are given in micrograms per milliliter.
Safety and tolerance.
Two patients discontinued therapy with CD-ITRA prematurely due to the occurrence of adverse effects likely related to the administration of the study drug. In the first case (2.5 mg/kg BID), CD-ITRA was discontinued on day 14 due to the development of a maculopapular rash and a decrease in visual acuity due to impaired accommodation. In the second case (2.5 mg/kg QD), the patient was withdrawn from the study after washout pharmacokinetics due to persistent diarrhea with onset at day 2 of therapy. In both cases, all abnormal findings completely resolved without intervention upon discontinuation of CD-ITRA. One additional patient (2.5 mg/kg QD) developed vomiting and loose stools during month 5 of therapy. After the study drug was withheld, the vomiting and diarrhea resolved, and CD-ITRA was subsequently discontinued. One patient in each dosage cohort experienced mild and transient nausea or vomiting possibly related to therapy with CD-ITRA without necessitating its discontinuation.
Laboratory toxicity was not observed. Serum electrolyte levels and biochemical parameters of liver and kidney function remained without significant change in all patients. Indeed, there was a net decrease in the levels of serum transaminases from mean aspartate aminotransferase and alanine aminotransferase values of 68 and 52 U/liter at baseline to 55 and 39 U/liter on day 15, respectively.
Pharmacokinetics.
There were no consistent, unidirectional differences between patients who were 5 to <13 years old and those who were 13 to 18 years old. Therefore, pharmacokinetic data for these age categories were combined.
(i) Single-dose pharmacokinetics.
The concentration profiles for ITRA and OH-ITRA in plasma after the administration of the first dose of 2.5 mg of CD-ITRA/kg are depicted in Fig. 1, and the corresponding pharmacokinetic parameters are tabulated in Table 3. Since the second dose was withheld on the first day in patients assigned to the BID regimen, all 26 patients received a single dose of 2.5 mg/kg on that day and were therefore analyzed together. The peak concentration (mean and standard error of the mean [SEM]) of ITRA in plasma was 0.420 ± 0.06 μg/ml and occurred after a mean of 2.35 ± 0.37 h postdosing. The mean AUC0-24 was 3.72 ± 0.65 μg · h/ml, and the half-life was 25.6 ± 5.7 h. The peak level of the metabolite OH-ITRA in plasma was measured after a mean of 7.14 ± 1.69 h and was 0.319 ± 0.04 μg/ml. The AUC0-24 was 5.24 ± 0.81 μg · h/ml, and OH-ITRA was eliminated from plasma with a mean half-life of 26.8 ± 4.0 h.
FIG. 1.
Concentration-time profiles of ITRA (ITC) (top) and its bioactive metabolite, OH-ITRA (bottom), in plasma after administration of the first dose of 2.5 mg of CD-ITRA oral solution/kg. Since the second dose was withheld on the first day in patients assigned to the BID regimen, all 26 patients received a single dose of 2.5 mg/kg on day 1 and were therefore analyzed together. Note the different exposures to the parent compound and the metabolite between patients receiving concomitant therapy with protease inhibitors (PI) and those not receiving PI-containing antiretroviral therapy.
TABLE 3.
Single-dose pharmacokinetic parameters of ITRA and OH-ITRA at 2.5 mg/kg in patients with and without concurrent treatment with protease inhibitors (PIs)
| Parametera | Value for the following drug and patient groupb:
|
|||||
|---|---|---|---|---|---|---|
| ITRA
|
OH-ITRA
|
|||||
| Receiving PIs (n = 14) | All (n = 26) | Not receiving PIs (n = 12) | Receiving PIs (n = 14) | All (n = 26) | Not receiving PIs (n = 12) | |
| Tmax (h) | 2.31 ± 0.36 | 2.35 ± 0.37 | 2.41 ± 0.76 | 10.46 ± 2.64 | 7.14 ± 1.69 | 2.83 ± 0.52* |
| Cmax (μg/ml) | 0.586 ± 0.09 | 0.420 ± 0.06 | 0.226 ± 0.05*** | 0.424 ± 0.07 | 0.319 ± 0.04 | 0.197 ± 0.03* |
| AUC0-24 (μg · h/ml) | 5.350 ± 0.96 | 3.720 ± 0.65 | 1.820 ± 0.49** | 6.550 ± 1.23 | 5.240 ± 0.81 | 3.340 ± 0.50* |
| Vdss (liters/kg) | 7.32 ± 1.44 | 18.90 ± 5.3 | 33.0 ± 10.2**** | NA | NA | NA |
| CL (liters/h/kg) | 0.297 ± 0.05 | 0.660 ± 0.17 | 1.100 ± 0.32** | 0.236 ± 0.05 | 0.339 ± 0.05 | 0.430 ± 0.08 |
| t1/2 (h) | 24.5 ± 0.0 | 25.6 ± 5.7 | 27.2 ± 8.4 | 27.9 ± 5.7 | 26.8 ± 4.0 | 25.8 ± 6.0 |
| Metabolic ratio | NA | NA | NA | 1.51 ± 0.40 | 1.52 ± 0.26 | 1.54 ± 0.32 |
Vdss, volume of distribution at steady state; CL, clearance; t1/2, half-life.
P values, determined by the Mann-Whitney U test, for comparisons between patients with and without concurrent treatment with PIs were ≤0.05 (*), ≤0.01 (**), ≤0.005 (***), and ≤0.001 (****). NA, not applicable.
Comparison of the single-dose pharmacokinetics between patients receiving concomitant protease inhibitor-containing antiretroviral regimens and patients not receiving such regimens revealed higher Cmax and AUC0-24 values among the patients receiving therapy with protease inhibitors. This finding was accompanied by a trend toward slower clearance but an essentially unchanged half-life (Fig. 1 and Table 3).
(ii) Multiple-dose pharmacokinetics.
The concentration profiles for ITRA and OH-ITRA in plasma after the administration of CD-ITRA for 15 days are depicted in Fig. 2, and the corresponding pharmacokinetic parameters are tabulated in Table 4. A comparison of dose-normalized AUCτ and plasma clearance between the two dosage cohorts overall suggested a nonlinear disposition across dosage levels. Independent of the dosage regimen, ITRA and the metabolite OH-ITRA accumulated in plasma 3.5- to 6.2-fold after dosing over 15 days, as measured by a comparison of AUCτ on days 1 and 15. Cmin, Cmax, and AUC0-24 values for both ITRA and OH-ITRA were significantly higher and the mean clearance was significantly slower after BID dosing. Both ITRA and OH-ITRA exhibited a prolonged mean terminal elimination half-life that exceeded 100 h after BID dosing (Fig. 2 and Table 3).
FIG. 2.
Washout concentration-time profiles of ITRA (top) and its bioactive metabolite, OH-ITRA (bottom), in plasma after treatment with CD-ITRA oral solution at 2.5 mg/kg QD or 2.5 mg/kg BID for 15 days. Note the prolonged terminal elimination half-life after BID dosing, in particular.
TABLE 4.
Multiple-dose pharmacokinetic parameters of ITRA and OH-ITRA at 2.5 mg/kg
| Parametera | Value for the following drug and patient groupb:
|
|||
|---|---|---|---|---|
| ITRA
|
OH-ITRA
|
|||
| QD | BID | QD | BID | |
| Cmin (μg/ml) | 0.192 ± 0.06 | 0.782 ± 0.19*** | 0.383 ± 0.10 | 0.997 ± 0.15*** |
| Tmax (h) | 1.9 ± 0.3 | 1.8 ± 0.3 | 5.9 ± 1.5 | 14.7 ± 6.9 |
| Cmax (μg/ml) | 0.623 ± 0.14 | 1.340 ± 0.22** | 0.552 ± 0.08 | 1.170 ± 0.18** |
| AUCτ (μg · h/ml) | 7.05 ± 2.06 | 11.52 ± 2.19* | 11.18 ± 2.82 | 11.89 ± 2.06 |
| AUC0-24 (μg · h/ml) | 7.05 ± 2.06 | 23.04 ± 4.39*** | 11.18 ± 2.82 | 23.75 ± 4.11** |
| Vdss (liters/kg) | 15.52 ± 4.47 | 5.11 ± 1.28** | NA | NA |
| CL (liters/h/kg) | 0.601 ± 0.26 | 0.073 ± 0.029* | 0.160 ± 0.05 | 0.047 ± 0.01* |
| t1/2 (h) | 58.9 ± 13.1 | 104.2 ± 28.3 | 55.6 ± 21.3 | 168.8 ± 81.3 |
| Accumulation factor | 3.84 ± 0.88 | 3.53 ± 0.67 | 5.15 ± 1.97 | 6.28 ± 2.42 |
| AUCτ/dose | 0.101 ± 0.02 | 0.193 ± 0.04* | 0.154 ± 0.02 | 0.188 ± 0.03 |
| Metabolic ratio | NA | NA | 1.88 ± 0.20 | 1.29 ± 0.21* |
See Table 3, footnote a.
P values, determined by the Mann-Whitney U test, for comparisons between QD and BID cohorts were <0.05 (*), ≤0.01 (**), and ≤0.001 (***).
Response to antifungal therapy.
As measured by the OPC score, either dosage regimen of CD-ITRA showed highly significant therapeutic activity. The OPC score (mean and SEM) for all 26 patients decreased from 7.46 ± 0.8 at baseline to 2.88 ± 0.7 at the end of therapy (day 15) (P < 0.001). Twenty-two patients (85%) responded to treatment with CD-ITRA. While four patients had no change in their OPC score, no patients had worsening of their OPC from baseline. More patients in the BID cohort than in the QD cohort had a complete response (Table 5).
TABLE 5.
Responses to treatment after 15 days of therapy with CD-ITRA at 2.5 mg/kg
| Patient group (n) | Mean ± SEM OPC score ata:
|
No. (%) of patients showing the following response:
|
||||
|---|---|---|---|---|---|---|
| Baseline | Day 15 | Total | Complete | Partial | None | |
| QD (14) | 8.43 ± 1.0 | 4.57 ± 1.2 | 12 (86) | 3 (22) | 9 (64) | 2 (14) |
| BID (12) | 6.33 ± 1.4 | 0.91 ± 0.8 | 10 (83) | 7 (58) | 3 (25) | 2 (17) |
| All (26) | 7.46 ± 0.8 | 2.88 ± 0.7 | 22 (85) | 10 (39) | 12 (46) | 4 (15) |
P values for baseline versus day 15 were 0.0186, <0.001, and <0.001 for QD, BID, and all groups, respectively.
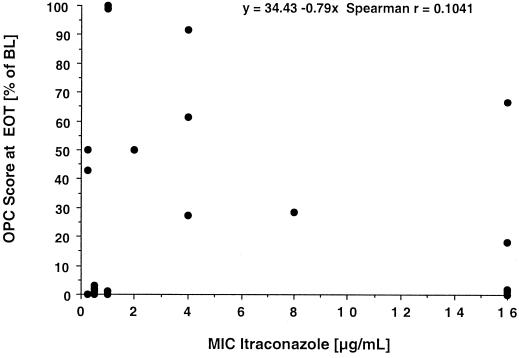
There was no meaningful correlation between the in vitro susceptibility of baseline isolates to triazoles and response to therapy with CD-ITRA (Fig. 3). Eighty-five percent of patients with ITRA-resistant isolates (i.e., an MIC of ≥1 μg/ml) showed clearance or improvement of oropharyngeal lesions, and all patients with fluconazole-resistant isolates (i.e., an MIC of ≥64 μg/ml) responded to therapy with CD-ITRA. A mycological response (sterile cultures at day 15) was achieved in 8 of 22 patients with positive baseline cultures.
FIG. 3.
Plot of the relationship between the MIC at baseline and response to treatment as a function of the residual OPC score at the end of treatment (EOT). The residual OPC score is expressed as a percentage of the baseline value (BL). As calculated with the absolute OPC score at the end of treatment, the Spearman coefficient of correlation was 0.1143.
With the exception of one patient who developed esophageal candidiasis 2.5 months into treatment with 2.5 mg of CD-ITRA/kg, none of the 23 remaining study subjects who continued therapy with CD-ITRA following washout pharmacokinetic sampling had to have therapy discontinued due to deterioration of mucosal candidiasis. The mean OPC score at the time of the ultimate discontinuation of the study in these patients was 1.91 (range, 0 to 13; cohort 1, 2.00 [0 to 13]; cohort 2, 1.82 [0 to 11]). Therapy for the single patient with an OPC score of 13 was discontinued on day 78 due to the patient's inability to take oral medications for reasons unrelated to the study drug.
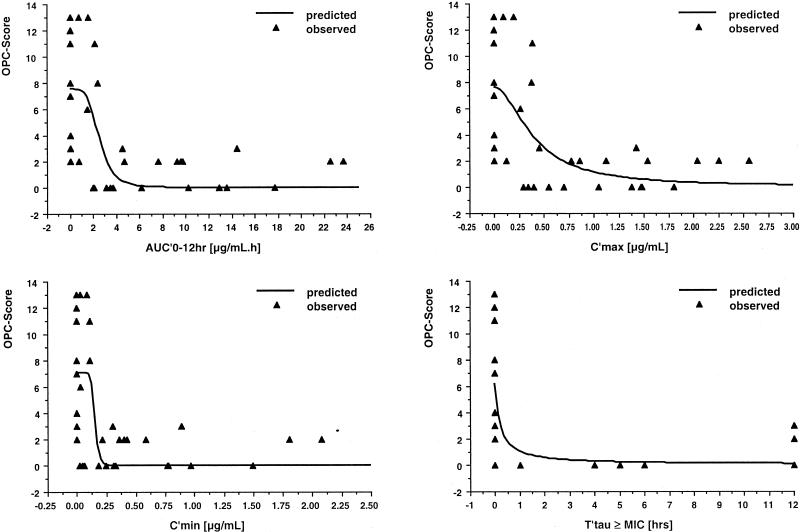
Pharmacodynamics.
The relationships of Cmin, Cmax, AUC0-12, and the MIC-related parameters Cmin/MIC, Cmax/MIC, AUC0-12/MIC, and Tτ ≥ MIC with outcome, as measured by the residual OPC score at day 15, fit inhibitory effect pharmacodynamic models (Table 6 and Fig. 4). Best fits were found for AUC0-12, Cmax, and Cmin; the MIC-related parameters fit less well, reflecting the poor correlation between in vitro susceptibility and residual OPC score. The model-estimated 50% effective trough concentration of ITRA at the end of the dosing interval was 0.156 ± 0.09 μg/ml, and the 50% effective peak concentration was 0.401 ± 0.15 μg/ml (Table 6).
TABLE 6.
Relationships between pharmacodynamic parameters and residual OPC score at day 15a
| Parameter | Emax | EP50 | Gamma | r (observed/ predicted) | P |
|---|---|---|---|---|---|
| AUC0-12b | 7.53 ± 0.77 | 2.57 ± 0.58 | 4.56 ± 4.01 | 0.595 | 0.01 |
| AUC0-12/MICc | 7.15 ± 0.85 | 0.76 ± 0.65 | NA | 0.483 | 0.02 |
| Cmaxb | 7.61 ± 0.79 | 0.401 ± 0.15 | 1.91 ± 1.32 | 0.584 | 0.01 |
| Cmax/MICc | 7.19 ± 0.81 | 0.214 ± 0.18 | NA | 0.530 | 0.01 |
| Cminb | 7.06 ± 0.72 | 0.156 ± 0.09 | 9.90 ± 32.1 | 0.571 | 0.01 |
| Cmin/MICc | 6.94 ± 0.80 | 0.086 ± 0.07 | NA | 0.505 | 0.02 |
| Tτ ≥ MICc | 6.21 ± 0.73 | 0.202 ± 0.92 | NA | 0.419 | 0.05 |
Emax, maximum effect; EP50, 50% effect parameter value; gamma, slope of the central part of the curve; NA, not applicable. Emax, EP50, and gamma values are given as means and SEMs.
The equation for the inhibitory effect sigmoidal pharmacodynamic model is Emax{1 − [Cgamma/(Cgamma + EP50gamma)]}, where C is the pharmacodynaic parameter.
The equation for the inhibitory effect pharmacodynamic model is Emax{1 − [C/(C + EP50)]}, where C is the pharmacodynamic parameter.
FIG. 4.
Plots of the relationships between pharmacodynamic parameters and the residual OPC score after 15 days of therapy with CD-ITRA oral solution at 2.5 mg/kg QD or 2.5 mg/kg BID. The triangles represent the relationship for each individual patient, while the line describes the relationship predicted by the pharmacodynamic model on the basis of the sum of the observed values.
DISCUSSION
The results of our study demonstrate that oral CD-ITRA is safe, well tolerated, and effective in the treatment of recurrent OPC in pediatric patients with advanced HIV disease featuring low CD4+-cell counts and a high prevalence of fluconazole-resistant Candida isolates. To our knowledge, this is the first therapeutic study of oral CD-ITRA in pediatric patients. As assessed by a scoring system that quantifies the extent of mucosal candidiasis in the oropharynx, both investigated dosage regimens showed highly significant therapeutic activity. Only two patients (7.6%) discontinued treatment prematurely due to study drug-related adverse events. No patients had to prematurely discontinue treatment for laboratory abnormalities. A comparison of the plasma pharmacokinetics of the two dosage regimens revealed a trend toward nonlinear drug disposition and increased systemic drug exposure in patients receiving the BID regimen, which was associated with a higher percentage of patients achieving a complete clinical response. Although pharmacodynamic modeling revealed significant relationships between pharmacodynamic parameters and the residual OPC score at the end of treatment, there was no meaningful correlation between the in vitro susceptibility of baseline isolates to ITRA and the response to therapy. All patients with fluconazole-resistant isolates responded to therapy with CD-ITRA.
In adults, oral ITRA in capsule form is usually well tolerated at dosages of up to 400 mg/day. Adverse effects tend to be transient and may include gastrointestinal disturbances (<10%), hypertriglyceridemia (9%), hypokalemia and elevated levels of hepatic transaminases (5% each), and rash or pruritus, headaches or dizziness, and pedal edema (<2% each) (31). The favorable safety profile of oral CD-ITRA observed in pediatric patients enrolled in our study is consistent with that reported for immunocompromised pediatric patients with cancer or following liver transplantation and receiving the compound at 5 mg/kg QD (7) or 2.5 mg/kg BID (29) for approximately 14 days. These favorable safety data, however, stand somewhat in contrast to those of a third study that evaluated the safety and tolerance of CD-ITRA administered at dosages of 5 mg/kg QD and 2.5 mg/kg BID for a median duration of 37 days for antifungal prophylaxis in children undergoing hematopoietic stem cell transplantation (9). In that study, only 45% of enrolled patients completed prophylaxis in accordance with the protocol, and 18% withdrew because of adverse events that included vomiting (12%), abnormal liver function tests (5%), and abdominal pain (3%). Of note, most adverse events in our patients were gastrointestinal in nature. Indeed, gastrointestinal intolerance related to the osmotic properties of the cyclodextrin carrier (30, 36) appears to be the dose-limiting toxicity of oral CD-ITRA. In a comparative study of adult patients with acute leukemia, 46% of patients receiving a daily dosage of 800 mg stopped treatment early because of severe nausea and vomiting (11). The identical dosage in capsule form was well tolerated by all patients. Patients receiving 400 mg of the oral cyclodextrin solution per day had no gastrointestinal adverse effects.
With the exception of pharmacokinetics in infants and young children <5 years old, the pharmacokinetics of CD-ITRA in pediatric patients do not appear to be fundamentally different from those in adults. These data may reflect the disposition of a highly lipophilic compound (26) and stand in contrast to data for the highly water-soluble triazole fluconazole, whose disposition in the body is determined by the relative water content and glomerular filtration (17). In 26 infants and children 6 months to 12 years old, with cancer (n = 20) or undergoing liver transplantation, and receiving CD-ITRA at 5 mg/kg QD (7), plasma drug concentrations were substantially lower than those reported in adult cancer patients (24, 25), particularly for infants <2 years old. However, in a second pharmacokinetic study of children 1.7 to 14.3 years old, with cancer, and receiving CD-ITRA in the form of a split dosing regimen of 2.5 mg/kg BID, peak and trough drug concentrations were similar to or slightly higher than those in adults (29). Nevertheless, there was a similar trend toward less exposure in the group of children ≤5 years old. Peak and trough drug levels, as well as the AUC as a measure of exposure during the dosing interval for the cohort receiving 2.5 mg/kg BID in our study, were very similar to the values observed for the corresponding age segment in the latter study. In addition, both dosage regimens tested in this study yielded peak and trough drug levels similar to or slightly higher than those reported for immunocompromised adults receiving similar dosage regimens (2, 24). In several studies, in comparison to the results for QD dosing, trough levels of ITRA and OH-ITRA were consistently higher after BID dosing with the same total daily dosage (7, 24, 29). Given the concentration- and time-dependent pharmacodynamics of the antifungal azoles (15), BID dosing thus appears more appropriate, despite the prolonged half-life that by itself would suggest QD dosing.
An important finding of our study was the impact of concomitant therapy with antiretroviral protease inhibitors on the disposition of ITRA. Antiretroviral protease inhibitors are both substrates and inhibitors of CYP3A4 and glycoprotein P, which also play a major role in the intestinal and hepatic metabolism of ITRA (23, 35). Patients receiving concomitant protease inhibitors exhibited higher mean peak levels in plasma, higher AUC values, and a slower clearance of both ITRA and its hydroxylated metabolite following a single CD-ITRA dose of 2.5 mg/kg. These data would be consistent with decreased presystemic elimination of ITRA by enterocytes and/or hepatocytes following concurrent oral administration of both drugs and enhanced oral bioavailability. While we would not advocate routine therapeutic monitoring of ITRA concentrations in the treatment of OPC and esophageal candidiasis, such monitoring may be indicated when clinically relevant drug-drug interactions are suspected or when therapy with ITRA fails to be effective. In the latter circumstance, our pharmacodynamic modeling of residual OPC score and Cmin (Fig. 4) would suggest that one achieve and maintain an ITRA trough level of 0.25 to 0.5 μg/ml.
At both investigated dosage regimens, CD-ITRA was highly effective for the treatment of OPC in patients with advanced HIV disease featuring low CD4+-cell counts, poor nutritional status, prior opportunistic infections, and concomitant broad-spectrum antibiotic therapy. A significantly higher percentage of patients in the cohort receiving 2.5 mg/kg BID achieved a complete clinical response at the end of therapy, indicating dose-dependent antifungal efficacy in this situation. However, it is also conceivable that the better clinical response and the apparently nonlinear disposition were caused by the higher percentage of patients receiving concomitant protease inhibitors.
Similar to findings for adults (3, 8, 22, 28), patients with fluconazole-resistant isolates in this study responded to therapy with CD-ITRA. Pharmacodynamic modeling revealed equally good fits for AUC0-12, Cmax, and Cmin with antifungal efficacy, as assessed by the residual OPC score, whereas MIC-related pharmacodynamic parameters had less good fits. These findings are consistent with the exposure-dependent pharmacodynamics of the antifungal azoles (15) and the poor correlation of the MIC with the antifungal efficacy of CD-ITRA in the treatment of OPC. Eighty-five percent of patients with isolates considered resistant in vitro according to current breakpoints (19, 27) showed clearance or improvement of oropharyngeal lesions. These findings are in line with those of Phillips and coworkers, who noted a correlation between clinical response and in vitro susceptibility for fluconazole but not for CD-ITRA (22). These data indicate that current NCCLS breakpoints for susceptibility to ITRA may not be applicable for the treatment of OPC with the cyclodextrin formulation. One explanation for these data would be the topical effect of the cyclodextrin solution with exposure of the mucosa to excessive concentrations of the compound. Another explanation may be provided by the enhanced systemic delivery of this lipophilic triazole to the oroesophageal epithelium, as indicated by a significant accumulation in esophageal tissue relative to plasma (5) and significant pharmacodynamic correlations of parameters of drug exposure with antifungal efficacy, as demonstrated in our study.
In summary, CD-ITRA was well tolerated and efficacious in the treatment of OPC in HIV-infected children ≥5 years old and adolescents, including patients with fluconazole-resistant isolates. Pharmacodynamic modeling revealed significant concentration-response relationships; in contrast, no relationship was found between the MIC for the infecting organism and antifungal efficacy. Pharmacokinetic parameters appeared similar to those reported for adult patients. Based on safety, plasma pharmacokinetics, and antifungal efficacy, a dosage of 2.5 mg/kg BID is recommended for the treatment of OPC in HIV-infected pediatric patients ≥5 years old.
REFERENCES
- 1.Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Enhanced bioavailability of itraconazole in hydroxypropyl-beta-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob. Agents Chemother. 42:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartledge, J. D., J. Midgely, B. G. Gazzard. 1997. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J. Clin. Pathol. 50:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartledge, J. D., J. Midgley, M. Youle, and B. G. Gazzard. 1994. Itraconazole cyclodextrin solution—effective treatment for HIV-related candidosis unresponsive to other azole therapy. J. Antimicrob. Chemother. 33:1071-1073. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1994. Revised classification system for human immunodeficiency virus infection in children less then 13 years of age. Morb. Mortal. Wkly. Rep. 43:1-10. [Google Scholar]
- 5.Darouiche, R. O., A. Setoodeh, and E. J. Anaissie. 1995. Potential use of a simplified method for determination of itraconazole levels in plasma and esophageal tissue by using high-performance liquid chromatography. Antimicrob. Agents Chemother. 39:757-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Beule, K., and J. Van Gestel. 2001. Pharmacology of itraconazole. Drugs 61(Suppl. 1):27-37. [DOI] [PubMed] [Google Scholar]
- 7.de Repentigny, L., J. Ratelle, J. M. Leclerc, G. Cornu, E. M. Sokal, P. Jacqmin, and K. De Beule. 1998. Repeated-dose pharmacokinetics of an oral solution of itraconazole in infants and children. Antimicrob. Agents Chemother. 42:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichel, M., G. Just-Nubling, E. B. Helm, and W. Stille. 1996. Itraconazole suspension in the treatment of HIV-infected patients with fluconazole-resistant oropharyngeal candidiasis and esophagitis. Mycoses 39(Suppl. 1):102-106. [DOI] [PubMed] [Google Scholar]
- 9.Foot, A. B., P. A. Veys, and B. E. Gibson. 1999. Itraconazole oral solution as antifungal prophylaxis in children undergoing stem cell transplantation or intensive chemotherapy for haematological disorders. Bone Marrow Transplant. 24:1089-1093. [DOI] [PubMed] [Google Scholar]
- 10.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd. ed., p. 455-459.
- 11.Glasmacher, A., C. Hahn, E. Molitor, G. Marklein, T. Sauerbruch, and I. G. Schmidt-Wolf. 1999. Itraconazole through concentrations in antifungal prophylaxis with six different dosing regimens using hydroxypropyl-beta-cyclodextrin oral solution or coated-pellet capsules. Mycoses 42:591-600. [DOI] [PubMed] [Google Scholar]
- 12.Graybill, J. R., J. Vazquez, R. O. Darouiche, R. Morhart, D. Greenspan, C. Tuazon, L. J. Wheat, J. Carey, I. Leviton, R. G. Hewitt, R. R. MacGregor, W. Valenti, M. Restrepo, and B. L. Moskovitz. 1998. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am. J. Med. 104:33-39. [DOI] [PubMed] [Google Scholar]
- 13.Groll, A. H. 2001. Drug therapy in pediatric patients. Lancet 357:719.. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 15.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 2001. Antifungal pharmacodynamics: concentration-effect relationships in vitro and in vivo. Pharmacotherapy 21:133S-148S. [DOI] [PubMed] [Google Scholar]
- 16.Gubbins, P. O., B. J. Gurley, and J. Bowman. 1998. Rapid and sensitive high performance liquid chromatographic method for the determination of itraconazole and its hydroxy-metabolite in human serum. J. Pharm. Biomed. Anal. 16:1005-1012. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J. W., N. L. Seibel, M. Amantea, P. Whitcomb, P. A. Pizzo, and T. J. Walsh. 1992. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J. Pediatr. 120:987-993. [DOI] [PubMed] [Google Scholar]
- 18.Muller, F. M., A. H. Groll, and T. J. Walsh. 1999. Current approaches to diagnosis and treatment of fungal infections in children infected with human immunodeficiency virus. Eur. J. Pediatr. 158:187-199. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard. NCCLS document M27-T. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Penzak, S. R., and P. O. Gubbins. 1998. Preventing and treating azole-resistant oropharyngeal candidiasis in HIV-infected patients. Am. J. Health Syst. Pharm. 55:279-283. [DOI] [PubMed] [Google Scholar]
- 21.Phillips, P., K. De Beule, G. Frechette, S. Tchamouroff, B. Vandercam, L. Weitner, A. Hoepelman, G. Stingl, and B. Clotet. 1998. A double-blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clin. Infect. Dis. 26:1368-1373. [DOI] [PubMed] [Google Scholar]
- 22.Phillips, P., J. Zemcov, M. Mahmood, J. S. Montaner, K. Craib, and A. M. Clarke. 1996. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS 10:1369-1376. [DOI] [PubMed] [Google Scholar]
- 23.Piscitelli, S. C., C. Flexner, J. R. Minor, M. A. Polis, and H. Masur. 1996. Drug interactions in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 23:685-693. [DOI] [PubMed] [Google Scholar]
- 24.Prentice, A. G., D. W. Warnock, S. A. Johnson, P. C. Taylor, and D. A. Oliver. 1995. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J. Antimicrob. Chemother. 36:657-663. [DOI] [PubMed] [Google Scholar]
- 25.Prentice, A. G., D. W. Warnock, S. A. Johnson, M. J. Phillips, and D. A. Oliver. 1994. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J. Antimicrob. Chemother. 34:247-252. [DOI] [PubMed] [Google Scholar]
- 26.Reed, M. D., and J. B. Besunder. 1989. Developmental ontogenic basis of drug disposition. Pediatr. Clin. North Am. 36:1053-1074. [DOI] [PubMed] [Google Scholar]
- 27.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, A. L. Barry, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 28.Saag, M. S., W. J. Fessel, C. A. Kaufman, K. W. Merrill, D. J. Ward, B. L. Moskovitz, C. Thomas, N. Oleka, J. A. Guarnieri, J. Lee, L. Brenner-Gati, and M. Klausner. 1999. Treatment of fluconazole-refractory oropharyngeal candidiasis with itraconazole oral solution in HIV-positive patients. AIDS Res. Hum. Retrovir. 15:1413-1417. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt, C., Y. Perel, J. L. Harousseau, S. Lemerle, E. Chwetzoff, J. P. le Moing, and J. C. Levron. 2001. Pharmacokinetics of itraconazole oral solution in neutropenic children during long-term prophylaxis. Antimicrob. Agents Chemother. 45:1561-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, D. A. 1999. Itraconazole in cyclodextrin solution. Pharmacotherapy 19:603-611. [DOI] [PubMed] [Google Scholar]
- 31.Tucker, R. M., Y. Haq, D. W. Denning, and D. A. Stevens. 1990. Adverse events associated with itraconazole in 189 patients on chronic therapy. J. Antimicrob. Chemother. 26:561-566. [DOI] [PubMed] [Google Scholar]
- 32.Vanden Bossche, H., F. Dromer, I. Improvisi, M. Lozano-Chiu, J. H. Rex, and D. Sanglard. 1998. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 36(Suppl. 1):119-128. [PubMed] [Google Scholar]
- 33.Van de Velde, V. J., A. P. Van Peer, J. L. Heykants, R. J. Woestenborghs, P. Van Rooy, K. L. De Beule, and G. F. Cauwenbergh. 1996. Effect of food on the pharmacokinetics of a new hydroxypropyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy 16:424-428. [PubMed] [Google Scholar]
- 34.Vazquez, J. A. 1999. Options for the management of mucosal candidiasis in patients with AIDS and HIV infection. Pharmacotherapy 19:76-87. [DOI] [PubMed] [Google Scholar]
- 35.Venkatakrishnan, K., L. L. von Moltke, and D. J. Greenblatt. 2000. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin. Pharmacokinet. 38:111-180. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335-347. [PubMed] [Google Scholar]