Abstract
Members of a family of N-arylsulfonyl hydrazones have been identified as novel inhibitors of IMP-1, a metallo-β-lactamase of increasing prevalence. Structure-activity relationship studies have indicated a requirement for bulky aromatic substituents on each side of the sulfonyl hydrazone backbone for these compounds to serve as efficient inhibitors of IMP-1. Molecular modeling has provided insight into the structural basis for the anti-metallo-β-lactamase activity exhibited by this class of compounds.
The ever-increasing incidence of microbial resistance to β-lactam antibiotics has been traced, in a large number of instances, to the ability of the organisms to produce β-lactamases, enzymes capable of deactivating these antibiotics by hydrolysis (16, 22, 28). Consequently, there is a considerable interest in the development of β-lactamase inhibitors that would allow for the continued use of β-lactam antibiotics in clinical therapy. To date, four distinct classes, designated A, B, C, and D, of β-lactamases have been identified (1, 22, 28). The members of the A, C, and D classes have been shown to use a serine residue as a nucleophile in their catalytic mechanism. In sharp contrast, class B β-lactamases are metalloenzymes, with zinc ions fulfilling their metal requirement. Studies have been directed for the most part toward screening and/or development of potential inhibitors of class A β-lactamases. As a consequence of these studies, clavulanate and penicillanic acid sulfones (e.g., sulbactam and tazobactam), which function as potent mechanism-based inhibitors of these enzymes (6, 16), have been successfully exploited in clinical medicine for the continued use of penicillins in combating bacterial infections (22, 30).
During the past few years, class B β-lactamases have attracted considerable attention in view of their ability to function as carbapenemases (9, 35) as well as their ability to utilize inhibitors of class A enzymes as substrates (33). All metallo-β-lactamases isolated to date are chromosome encoded, with the exception of the IMP and VIM enzymes found in certain strains of Pseudomonas aeruginosa, Serratia marcescens, and other gram-negative bacteria, which are of plasmid origin (21). Based on the differences in their primary structures, these enzymes have been classified into three subfamilies (11). The B1 subfamily comprises some of the most extensively investigated metallo-β-lactamases (the commonly used abbreviated names are given in parentheses), such as those derived from Bacteroides fragilis (CcrA), Bacillus cereus (BcII), and P. aeruginosa (IMP-1). The metallo-β-lactamases from Aeromonas species (CphA and ImiS) belong to the B2 subfamily, while the Stenotrophomonas maltophilia enzyme (L1) serves as a prototype of the B3 subfamily. The three-dimensional structures of several metallo-β-lactamases, including IMP-1, L1, CcrA, and BcII, have been elucidated by X-ray crystallographic analysis, revealing the overall protein folds to be similar (7, 43).
Some of the reported inhibitors of these enzymes include trifluoromethyl alcohols and ketones (41), amino acid-derived hydroxamates (42), thiols (3, 4, 12, 13), thioester derivatives (13, 14, 31, 32), cysteinyl peptides (5), biphenyl tetrazoles (38, 39), mercaptocarboxylates (24, 30), 1β-methylcarbapenem derivatives (25, 26), a synthetic cephamycin (34), and 2,3-disubstituted succinic acid derivatives (40).
With a view to developing potent inhibitors of metallo-β-lactamases, we initiated the design, synthesis, and systematic screening of N-arylsulfonyl hydrazones (hereafter referred to as sulfonyl hydrazones) for their ability to inhibit IMP-1. The choice of this particular enzyme as the target was motivated by its impending emergence in other pathogenic organisms via the facile interspecies transfer of the plasmid-borne blaIMP gene (19, 44). This report concerns a detailed analysis of the structure-activity relationship of sulfonyl hydrazones as inhibitors of IMP-1. Molecular modeling studies, based on the recently published crystal structure of an IMP-1 enzyme-mercaptocarboxylate inhibitor complex (7), were used to correlate the observed structure-activity relationship with interactions between these compounds and the enzyme.
MATERIALS AND METHODS
Materials.
Nitrocefin was obtained from Oxoid (Basingstoke, United Kingdom). Glutathione S-transferase gene fusion vector pGEX-4T-3, glutathione-Sepharose 4B, and MonoQ ion-exchange resin were purchased from Amersham Pharmacia (Uppsala, Sweden). pCIP4 was kindly provided by M. Galleni (Laboratoire d'Enzymologie and Centre d'Ingénierie des Protéines, Institut de Chimie, Université de Liège, Liège, Belgium).
β-Lactamase preparations.
PCR was used to retrieve the gene encoding the B. cereus 5/B/6 metallo-β-lactamase (BcII) (20), which is homologous (only 17 amino acid residues being different out of a total of 227 such residues) to the processed form of the protein from strain 569/H/9 (2, 15). An EcoRI-XhoI fragment of this PCR product was inserted into pGEX-4T-3 for expression of the enzyme as a glutathione S-transferase fusion protein (37). The expression of the fusion protein was induced in Escherichia coli DH5α, and the protein was recovered from the cell extract by adsorption to the affinity matrix, glutathione-Sepharose 4B. Following the recovery of metallo-β-lactamase by treatment with thrombin (37), the protein was further purified by chromatography on an anionexchange matrix, MonoQ.
IMP-1 metallo-β-lactamase was expressed in E. coli BL21(DE3) carrying pCIP4 and purified as reported by Laraki et al. (18).
The homogeneity of metallo-β-lactamases was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to the method of Laemmli (17). Electrospray ionization (ESI) mass spectrometry (MS) was used to verify the masses of the purified proteins: (i) IMP-1, 25,112 ± 3 Da, in agreement with that expected from the amino acid sequence (19); and (ii) BcII, 25,478 ± 2 Da, in agreement with that expected for BcII β-lactamase (20) with a five-amino-acid-residue segment (Gly-Ser-Pro-Asn-Ser) from vector pGEX-4T-3 at the N terminus of the protein. The kinetic parameters for nitrocefin hydrolysis (kcat, 41 s−1; Km, 49 μM) obtained with the BcII enzyme are similar to those reported for the wild-type enzyme preparation (29).
Enzyme concentrations were determined from the absorbance at 280 nm (ɛ280 values of 44,380 M−1 cm−1 and 30,440 M−1 cm−1 for IMP-1 and BcII, respectively).
Synthesis of inhibitors. (i) General procedure for the preparation of arylsulfonyl hydrazides.
To a stirred solution of anhydrous hydrazine (12.5 mmol) in CH2Cl2 (5 ml) was added a solution of the appropriate sulfonyl chloride (2.5 mmol) over 2 min. The reaction mixture was stirred for 15 min, and the pH was subsequently adjusted to approximately 11 with 10% Na2CO3. The layers were separated, and the aqueous phase was extracted with CH2Cl2 (three times with 25 ml each time). The combined organic layers were dried over MgSO4 and filtered, and the solvent was removed under reduced pressure. No further purification of the product was required.
(ii) General procedure for the preparation of arylsulfonyl hydrazones.
A mixture of the required sulfonyl hydrazide (0.5 mmol) and the appropriate aldehyde (0.5 mmol) was dissolved in hot ethyl alcohol (2 ml). After the mixture was cooled to room temperature, the product was precipitated by the dropwise addition of water. Following filtration, the precipitate was washed with cold H2O (2 ml) and hexane (2 ml) and dried in vacuo.
All synthetic compounds were fully characterized by 1H nuclear magnetic resonance (NMR) spectrometry (300 or 500 MHz), 13C NMR spectrometry (75 MHz), and MS. Mass spectra were recorded at the McMaster University Mass Spectrometry Laboratory, Hamilton, Ontario, Canada.
Metallo-β-lactamase assays.
A spectrophotometric method based on the use of a chromophoric substrate, nitrocefin (27), was used for the measurement of β-lactamase activity with the aid of a Cary5 spectrophotometer (Varian, Mississauga, Ontario, Canada). All assays were performed with HEPES buffer (50 mM, pH 7.3) at 30°C. The procedure involved the preparation of the following stock solutions: (i) nitrocefin (2 mM) in HEPES buffer (50 mM, pH 7.3) containing dimethyl sulfoxide (DMSO) (5% [vol/vol]) and (ii) metallo-β-lactamase (400 nM) in HEPES buffer containing ZnCl2 (100 μM) and bovine serum albumin (BSA) (100 μg/ml). The inclusion of BSA in the enzyme stock solution results in the protection of β-lactamase from denaturation at low protein concentrations and thus allows for a reliable and reproducible assessment of the initial rate of enzyme-catalyzed hydrolysis of its substrate, observations in agreement with those reported by Laraki et al. (18). At the low concentration (1 μg/ml) used in the assay, BSA showed no detectable interaction with the product of nitrocefin hydrolysis. Furthermore, the degree of inhibition was not significantly influenced by the presence of BSA up to a concentration, in the assay, of at least 20 μg/ml.
In a typical assay (final volume, 1 ml), metallo-β-lactamase (4 nM) in HEPES buffer was allowed to equilibrate at 30°C for 1 min. The reaction was initiated by the introduction of the substrate (100 μM for IMP-1 and 200 μM for BcII), and the increase in the absorbance at 482 nm was recorded.
For determination of the concentration required to effect 50% inhibition of enzyme activity (IC50), the enzyme was preincubated with the desired compound for 1 min at 30°C prior to the initiation of the assay by the addition of the substrate. The compounds to be tested were dissolved in DMSO prior to incubation with the enzyme, with the final concentration of DMSO in the assay not exceeding 1% (vol/vol). IC50s were deduced from a plot of percent loss of activity versus inhibitor concentration. The precision of these determinations was within the range of ±10%.
Determination of steady-state kinetic constants.
Estimates of steady-state kinetic parameters for the hydrolysis of nitrocefin (Km and kcat) were achieved by fitting the initial velocity data to the Michaelis-Menten equation with the software package Grafit 4.0 (Erithacus Software Ltd., Staines, United Kingdom). Inhibitor constants (Ki) were assessed according to the procedure of Dixon (10). The resulting data were used to generate a Cornish-Bowden plot (8) to establish the mode of inhibition exhibited by the compounds. A Δɛ482 value of 15,930 M−1 cm−1 for nitrocefin hydrolysis was used in the estimation of kcat values.
ESI MS.
ESI MS studies were performed with a QuattroII mass spectrometer (Micromass, Manchester, United Kingdom) equipped with an ESI source.
Molecular modeling.
The IMP-1-inhibitor (sulfonyl hydrazone) structural model was based on the recently reported X-ray crystal structure of the IMP-1 enzyme from P. aeruginosa in complex with a mercaptocarboxylate inhibitor (7). To construct a model of the inhibitor, a single X-ray crystal diffraction study of sulfonyl hydrazone 1 (Table 1) was performed. The obtained coordinates were used to generate a model of the hypothetical bound state by manually docking the hydrazone structure into the protein crystal structure containing the bound mercaptocarboxylate inhibitor (entry code, 1DD6) such that one of the sulfonyl group oxygen atoms (Pro-R) was positioned to establish contacts with both zinc ions (O—Zn1, 2.278 Å; O—Zn2, 2.260 Å), similar to those formed by the thiol group of the mercaptocarboxylate inhibitor (7). The mercaptocarboxylate structure was subsequently extracted, hydrogen atoms were added to all appropriate sites, and minimization of the resulting hydrazone-protein complex was carried out by using the MMFF94 molecular mechanics force field and the Anneal function within Sybyl 6.7 (Tripos, Inc., St. Louis, Mo.). Additional models of bound sulfonyl hydrazones were constructed by modifying the inhibitor structure within the protein-inhibitor complex, followed by minimization, as described above.
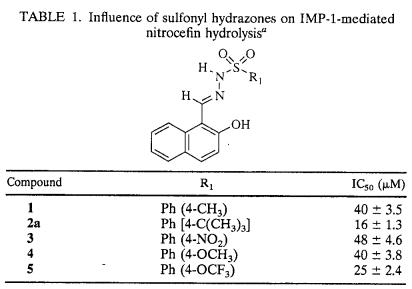
TABLE 1.
Influence of sulfonyl hydrazones on IMP-1-mediated nitrocefin hydrolysisa
Amino acid residues are labeled according to the recently proposed class B β-lactamase numbering scheme (11). For convenience, the formerly used numbering of IMP-1 amino acid residues is given in parentheses.
RESULTS AND DISCUSSION
Optimization of assay conditions.
The effect of any putative inhibitor on a target enzyme is generally assessed in reference to control experiments performed in its absence. Since the determination of the inhibitory potency of a given compound involves its preincubation with the target enzyme, the effect of such preexposure on the catalytic efficiency of IMP-1 appeared essential, especially in view of its lability at very low concentrations.
Such studies have revealed that the kinetic parameters (Km and kcat) depend on whether the assay is initiated by the addition of the enzyme to the substrate (no preincubation) or started by the addition of the substrate (prewarmed to 30°C) to the enzyme maintained at 30°C for 1 min (preincubation). In the former situation, Km and kcat values were 21 ± 2 μM and 320 ± 30 s−1, respectively, while under the latter conditions, these values were found to be 12 ± 1 μM and 260 ± 22 s−1, respectively. The latter values are in excellent agreement with those recorded in a recent report (23). Extension of the period of preincubation of IMP-1 beyond 1 min did not lead to further changes in the kinetic parameters of the enzyme. Hence, all experiments with IMP-1 were routinely performed subsequent to its preincubation for 1 min at 30°C.
In experiments which involved the testing of sulfonyl hydrazones for their inhibitory activity, the enzyme was preincubated with the desired compound for 1 min at 30°C (conditions similar to those noted above) prior to initiation of the assay by the addition of the substrate. Extending the period of incubation of the enzyme with the inhibitor beyond 1 min did not lead to further changes in enzymatic activity, indicating that sulfonyl hydrazones are relatively fast in exerting their inhibitory effect.
Structure-inhibitory activity relationship.
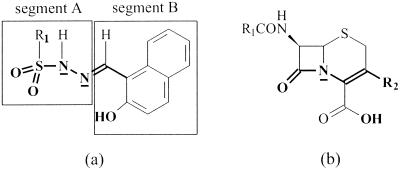
Two aspects of sulfonyl hydrazones, (i) the resemblance of their backbone structural elements to those in the scissile segment of cephalosporins, the natural substrates of these enzymes (Fig. 1), and (ii) their potential to function as moderate binding agents for divalent metal ions, provided an impetus for screening these compounds for their ability to function as novel inhibitors of metallo-β-lactamases, with the IMP-1 enzyme serving as the target protein.
FIG. 1.
Structural representation of sulfonyl hydrazone derivatives (a) and cephalosporins (b). Bonds and atoms in bold depict structural similarities between the classes of compounds.
For N-arylsulfonyl amino acids, metal complexation at high pH has been shown to involve the deprotonated sulfonamide nitrogen and the carboxylate function of the amino acid moiety (reference 36 and references therein). With regard to the selectivity in their action, arylsulfonyl compounds normally exhibit a weak affinity for zinc ions (36), a feature that would prevent them from interfering in the functioning of other Zn-containing proteins. However, their propensity to acquire an enhanced affinity for metal ions when present in a proper orientation could render them target specific in their function.
The sulfonyl hydrazones initially chosen for study of their potential anti-metallo-β-lactamase activity are listed in Table 1. For clarity in presentation, the sulfonyl hydrazine moiety of these compounds is designated segment A, while the component which contributes the carbonyl function for condensation with the hydrazide is designated segment B (Fig. 1a). The ligands for metal binding may be provided by either one or both of these segments. In segment A, the deprotonated sulfonamide and/or the sulfonyl oxygens might fulfill this function. The hydroxyl group in segment B might serve either as a metal binding ligand or as a crude mimic of the carboxylate group of cephalosporins (Fig. 1).
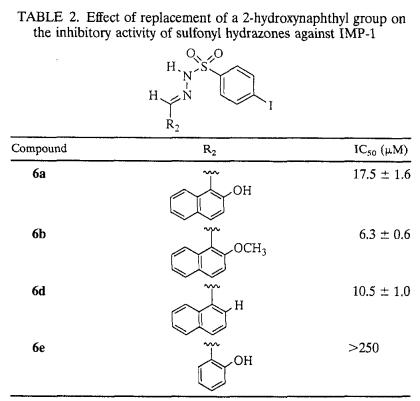
The details concerning the structure-activity relationship of the initially investigated sulfonyl hydrazones with substitutions on the benzene ring with IMP-1 are provided in Table 1. As indicated by the IC50s, the potencies of sulfonyl hydrazones were moderately affected by the nature of the substituent located on the aryl moiety of segment A. Compounds bearing larger substituents, such as a t-butyl group (compound 2a) or an OCF3 group (compound 5), tended to be more potent than other inhibitors listed in Table 1. It is interesting that the propensity of the para substituent to donate or withdraw electrons appears to play a less important role in tuning the inhibitory potencies of the compounds tested. On the other hand, replacement of the 2-hydroxynaphthyl substituent in segment B (as in compound 6a) with a 2-hydroxybenzene ring (as in compound 6e) resulted in more than a 15-fold increase in the IC50 (Table 2), suggesting the need for a bulky substituent at this location for the compound to be an effective inhibitor.
TABLE 2.
Effect of replacement of a 2-hydroxynaphthyl group on the inhibitory activity of sulfonyl hydrazones against IMP-1
In order to further assess the contribution of the 2-hydroxy group in segment B to the anti-metallo-β-lactamase activity, studies were extended to two analogs of compound 6a, one in which the hydroxyl substituent was deleted (compound 6d) and the other in which it was masked by methylation (compound 6b). Interestingly, both compounds, 6d and 6b, were slightly more effective as inhibitors of IMP-1 than the one with the hydroxyl group, 6a (Table 2). These findings tend to negate the earlier noted premise of the hydroxyl group at this location serving as an equivalent for the carboxyl function of cephalosporin substrates. In addition, its involvement as a metal binding ligand appears remote, especially since the enzymatic activity (with or without an inhibitor) of the protein is not significantly affected by the inclusion of 0.1 mM extraneous Zn2+ (data not shown). Furthermore, screening of some of the sulfonyl hydrazones examined in this study for their ability to inhibit the mammalian Zn2+-dependent enzyme carboxypeptidase A revealed these compounds to possess such low inhibitory activity that IC50s could not be obtained in most instances due to precipitation of the inhibitors at high concentrations. When IC50s could be estimated (e.g., with compound 11c), they were >10-fold those noted with IMP-1. Thus, these observations are consistent with the assertion that the inhibition of IMP-1 by sulfonyl hydrazones is not the result of nonspecific metal ion binding.
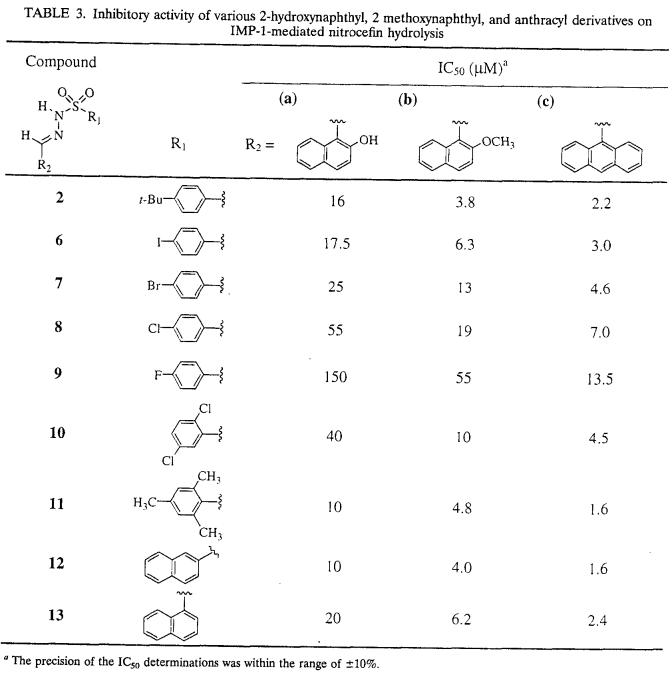
In an effort to further elucidate the structural features necessary for improved inhibition and thus to develop more potent metallo-β-lactamase inhibitors, a series of each of the 2-hydroxynaphthyl, 2-methoxynaphthyl, and anthracyl sulfonyl hydrazone derivatives with a variety of substitutions in the aromatic moiety in segment A were prepared, and their potential to inhibit IMP-1 was determined (Table 3). The rationale for the replacement of the naphthyl substituent with an anthracyl substituent was to elicit the influence of the hydrophobicity and/or the size of the aromatic ring system on the inhibitory potency of the compounds. As shown in Table 3, all anthracyl derivatives exhibited higher inhibitory activity (the IC50s decreased approximately two- to fourfold) than the corresponding 2-methoxynaphthyl derivatives (b versus c). Replacement of the para-iodo substituent with the lighter halogen atoms resulted in a substantial decrease in inhibitory efficiency, in the following order: I ≥ Br > Cl > F (compounds 6 to 9). This observation reinforces the view that the nature of the para substituents plays an important role in the binding of the sulfonyl hydrazones, with the anti-metallo-β-lactamase activity being directly proportional to the size of the substituent and inversely proportional to its electron-withdrawing property. Furthermore, the 2,5-dichloro derivatives 10, which tended to be slightly more potent than the para-chloro derivatives 8, exhibited potencies similar to those observed for the 4-bromo derivatives 7.
TABLE 3.
Inhibitory activity of various 2-hydroxynaphthyl, 2 methoxynaphthyl, and anthracyl derivatives on IMP-1-mediated nitrocefin hydrolysis
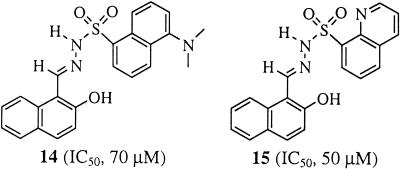
The most potent inhibitors within the series of compounds listed in Table 3 were 11c and 12c, each with an IC50 of 1.6 μM. These results are consistent with the observation that large substituents with less electron-withdrawing capacity improve the inhibition of the IMP-1 metallo-β-lactamase by sulfonyl hydrazone compounds. Sulfonyl hydrazones with a 2-substituted naphthyl moiety in segment A appear to be more effective as inhibitors than their 1-substituted counterparts (compare compounds 12 and 13). In addition, the introduction of a bulky dimethylamino substituent at position 5 of the 1-substituted naphthyl ring system, as in compound 14 (Fig. 2), led to a significant diminution of inhibitory potency. Such an adverse effect was also noted for the electron-withdrawing 8-quinolyl derivative (compound 15) (Fig. 2). In summary, these findings suggest that the inhibitory potency of sulfonyl hydrazones is sensitive to alterations in the orientation, size, and electronic properties of the aryl moiety in segment A.
FIG. 2.
Structural representation of compounds 14 and 15 and their IC50s.
Mode of inhibition of the IMP-1 metallo-β-lactamase by sulfonyl hydrazone compounds.
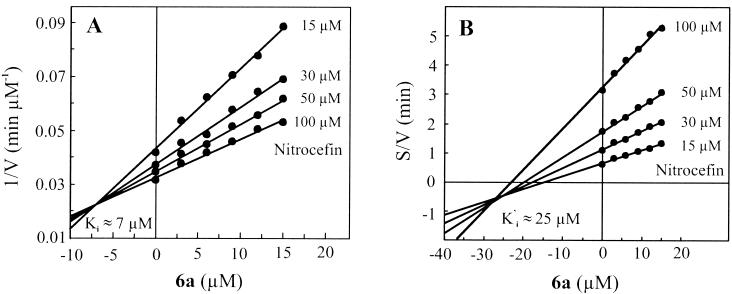
Compounds 6a and 11c were selected to identify the mode of their interactions with IMP-1. Analysis of the data with Dixon plots (Fig. 3A, compound 6a) indicated that both compounds function as reversible enzyme inhibitors, with calculated Ki values of 6.6 ± 1.1 and 0.7 ± 0.1 μM for compounds 6a and 11c, respectively. The mode of inhibition was determined with the aid of Cornish-Bowden plots (Fig. 3B, compound 6a), which showed that compounds 6a and 11c serve as mixed-type inhibitors of IMP-1, with Ki′ (dissociation constant for the enzyme-substrate-inhibitor complex) values of 25.3 ± 2.0 and 2.2 ± 0.2 μM, respectively. Since the magnitude of Ki is considerably smaller than that of Ki′, it would appear that the observed inhibition of IMP-1 by these compounds is mainly due to their specific interactions with the active site of the enzyme.
FIG. 3.
Dixon (A) and Cornish-Bowden (B) plots illustrating mixed-type inhibition of IMP-1-mediated nitrocefin hydrolysis by compound 6a. The inhibitor constant (Ki) was estimated by graphic analysis of the Dixon plot. Since Dixon plots are not suited for distinguishing between competitive and mixed-type inhibition kinetics, Cornish-Bowden plots were used to determine the mode of inhibition. V, initial rate (velocity) of the reaction; S, substrate concentration.
Studies with B. cereus 5/B/6 metallo-β-lactamase.
The ability of sulfonyl hydrazones to inhibit the metallo-β-lactamase from B. cereus (5/B/6), BcII, was also investigated. These studies revealed that these compounds had little or no adverse effect on the activity of BcII. Thus, compound 11c, which was most effective in inhibiting IMP-1 (Ki ≈ 0.7 μM), was found to be a poor inhibitor of BcII, causing approximately 30% inhibition at a concentration of 25 μM; other sulfonyl hydrazones (e.g., compounds 5, 6a or 6d, 9a, 11a, and 12a) were marginal in their action, with IC50s of ≥100 μM. In this connection, it is interesting that BcII (from strain 5/B/6), despite the presence of two Zn(II) binding sites, has been shown to be catalytically fully functional with just one metal ion bound to the active site (29). Although the catalytic competence of a mono-Zn form of IMP-1 remains to be established, the kinetic parameters of the binuclear enzyme have been documented (18). At the present time, it is not apparent whether the observed difference in the inhibitory potencies of sulfonyl hydrazones toward IMP-1 and BcII is a reflection of variations in the geometry of the active site(s) or is related to a change in the metal requirement for catalytic function.
Molecular modeling.
The recent release of the coordinates of IMP-1 in complex with a mercaptocarboxylate inhibitor (7) prompted us to construct a model for a possible mode of binding of sulfonyl hydrazones to the enzyme. Initially, a hypothetical model of compound 1 bound to the active site of IMP-1 was constructed as described in Materials and Methods.
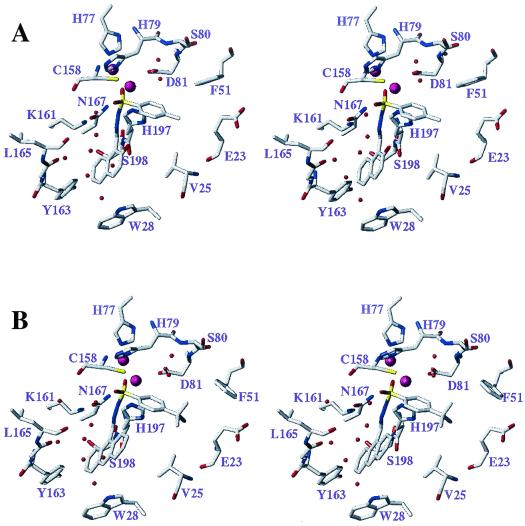
As shown in Fig. 4A, the Pro-S sulfonyl oxygen of compound 1 forms an H bond to the amide group of the side chain of Asn233 (167), consistent with the assumption that the sulfonamide group in this inhibitor may serve as a mimic of a tetrahedral intermediate involved in the hydrolysis of substrates (28, 43). Additional significant electrostatic stabilization of the negative charge on the nitrogen atom of the deprotonated sulfonamide group may arise by interaction with Zn1 and the protonated ɛ-NH2 group of Lys224 (161).
FIG. 4.
Stereoviews depicting the active sites of the modeled complexes of sulfonyl hydrazones 1 (A) and 2c (B) with the IMP-1 metallo-β-lactamase. The models are based on the published coordinates of an enzyme-mercaptocarboxylate inhibitor complex (7). Inhibitors and selected amino acid residues lining the binding site and discussed in the text are shown in capped-stick representation (carbon, gray; nitrogen, blue; oxygen, red; sulfur, yellow). The numbers shown denote the positions of amino acid residues in the primary structure of the enzyme. The zinc ions are depicted as large magenta spheres. Water molecules, which are proposed to contribute to a network of favorable interactions between the inhibitor and the protein (see the text), are shown as small red spheres.
Examination of the naphthyl ring of the inhibitor in this complex reveals a van der Waals (VdW) contact between C4-H of the naphthyl ring and the indole ring nitrogen of Trp64 (28), a residue which is part of a connecting turn (flap) linking two antiparallel β strands involved in inhibitor-substrate binding. A very similar interaction can be expected to occur in the complex of the salicylaldehyde-derived analog, compound 6e. However, since compound 6e is a poor inhibitor, such an interaction does not appear to be the sole contributor to the favorable interaction of the naphthyl ring with the active site of the enzyme. Indeed, a closer examination of the model depicted in Fig. 4A reveals that the second benzenoid ring establishes favorable VdW contacts with both the protein [C-6 of the naphthalene ring and Gly228 (164)] and two water molecules with C-7 of the naphthalene ring. The latter two molecules are part of a remarkable cluster of five water molecules filling the space between two loop regions that incorporate amino acid residues [Cys221 (158), Lys224 (161), His263 (197)] important for zinc ion and substrate binding. This water cluster involves H bonds among the water molecules as well as to the protein and may play an important role in stabilizing the active-site region.
In this model, the hydroxyl group of the 2-hydroxynaphthyl system is not near Lys224 (161), a feature that minimizes the importance of this functional group for the interaction of sulfonyl hydrazones with the enzyme. This finding is in full accordance with the structure-activity profile observed for this class of compounds.
It is interesting that a substantial skewing of the plane of the hydroxynaphthyl ring relative to the plane of the C=N group of the hydrazone linkage is observed in the model (Fig. 4A; naphthyl-C=N torsion, 52°), whereas a nearly coplanar arrangement of these groups, stabilized by an intramolecular H bond between the OH group and the C=N group, is found in the crystal structure of the free inhibitor. Thus, the complementary interaction of the inhibitor with the enzyme requires the concomitant destabilization of the above-mentioned intramolecular H bond. This feature may offer an explanation as to why the potency of the inhibitor is increased by replacement of the hydroxynaphthyl ring with a methoxynaphthyl or 9-anthracene ring system (Fig. 4B). In the latter two cases, the planes of the aryl group and the C=N group of the free inhibitors are intrinsically skewed, eliminating the need to deviate significantly from their minimum-energy conformations in order to bind to the active site of the enzyme.
The para substituent on the aromatic ring in this model is oriented toward a hydrophobic pocket lined by Phe87 (51), the β- and γ-CH2 groups of Glu59 (23), and one of the β-methyl groups of Val61 (25). The higher potencies of inhibitors with larger para substituents, such as the t-butyl group in compound 2c (Fig. 4B), are explicable largely on the basis of more extensive VdW contacts with this hydrophobic pocket.
In addition to the interactions noted above, the aromatic ring attached to the sulfonyl group (segment A) establishes a favorable contact with the carboxylate carbon atom of the zinc binding side chain of Asp120 (81). Furthermore, an interesting interaction is present between this ring and a water molecule which acts as an H-bond donor to the carboxylate group of Asp120 (81). This water molecule is oriented so as to serve as an H-bond donor to the face of the aromatic ring of the aryl sulfonamide portion of the inhibitor. This specific interaction may also partly explain the diminution in inhibitory potency observed with compounds of the para-halogen series in the order I ≥ Br > Cl > F, since the electron density in the ring system and, hence, the H-bond acceptor strength would be increasingly diminished in inhibitors of the same series.
In summary, the models presented in this study, although hypothetical, do provide some insights into possible specific interactions between sulfonyl hydrazones and the enzyme which help rationalize the observed structure-activity relationship at a molecular level. These insights may provide the basis for the development of more potent inhibitors of IMP-1.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
We are indebted to Gilles Lajoie, Dyanne Brewer, and Amanda Doherty-Kirby of the Department of Chemistry, University of Waterloo (currently at the University of Western Ontario, London, Ontario, Canada), for ESI MS measurements and Souzan Armstrong for preliminary studies concerning the stability of metallo-β-lactamases under assay conditions. We also thank Moreno Galleni for providing the plasmid used in this study as well as Matthew D. R. Brown and Nicholas J. Taylor for crystallographic data on tosylhydrazone.
REFERENCES
- 1.Ambler, R. P. 1980. The structure of β-lactamases. Philos. Trans. R. Soc. London B Biol. Sci. 289:321-331. [DOI] [PubMed] [Google Scholar]
- 2.Ambler, R. P., M. Daniel, J. Fleming, J.-M. Hermoso, C. Pang, and S. G. Waley. 1985. The amino acid sequence of the zinc-requiring β-lactamase II from the bacterium Bacillus cereus 569. FEBS Lett. 189:207-211. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bounaga, S., A. P. Laws, M. Galleni, and M. I. Page. 1998. The mechanism of catalysis and the inhibition of the Bacillus cereus zinc-dependent β-lactamase. Biochem. J. 331:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bounaga, S., M. Galleni, A. P. Laws, and M. I. Page. 2001. Cysteinyl peptide inhibitors of Bacillus cereus zinc β-lactamase. Bioorg. Med. Chem. 9:503-510. [DOI] [PubMed] [Google Scholar]
- 6.Bush, K., C. Macalintal, B. A. Rasmussen, V. J. Lee, and Y. Yang. 1993. Kinetic interaction of tazobactam with β-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concha, N. O., C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J. M. Frère, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. Crystal structure of the IMP-1 metallo β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4298. [DOI] [PubMed] [Google Scholar]
- 8.Cornish-Bowden, A. 1974. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 137:143-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cricco, J. A., and A. J. Vila. 1999. Class B β-lactamases: the importance of being metallic. Curr. Pharm. Des. 5:915-927. [PubMed] [Google Scholar]
- 10.Dixon, M. 1953. The determination of enzyme inhibitor constants. Biochem. J. 55:170-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, J. M. Frère, et al. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto, M., T. Takahashi, F. Yamashita, A. Koreeda, H. Mori, M. Ohta, and Y. Arakawa. 1997. Inhibition of the metallo-β-lactamase produced from Serratia marcescens by thiol compounds. Biol. Pharm. Bull. 20:1136-1140. [DOI] [PubMed] [Google Scholar]
- 13.Greenlee, M. L., J. B. Laub, J. M. Balkovec, M. L. Hammond, G. G. Hammond, D. L. Pompliano, and J. H. Epstein-Toney. 1999. Synthesis and SAR of thioester and thiol inhibitors of IMP-1 metallo-β-lactamase. Bioorg. Med. Chem. Lett. 9:2549-2554. [DOI] [PubMed] [Google Scholar]
- 14.Hammond, G. G., J. L. Huber, M. L. Greenlee, J. B. Laub, K. Young, L. L. Silver, J. M. Balkovec, K. D. Pryor, J. K. Wu, B. Leiting, D. L. Pompliano, and J. H. Toney. 1999. Inhibition of IMP-1 metallo-β-lactamase and sensitization of IMP-1-producing bacteria by thioester derivatives. FEMS Microbiol. Lett. 179:289-296. [DOI] [PubMed] [Google Scholar]
- 15.Hussain, M., A. Carlino, M. J. Madonna, and J. O. Lampen. 1985. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J. Bacteriol. 164:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles, J. R. 1985. Penicillin resistance: the chemistry of β-lactamase inhibition. Acc. Chem. Res. 18:97-104. [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frère, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laraki, N., M. Galleni, I. Thamm, M. L. Riccio, G. Amicosante, J. M. Frère, and G. M. Rossolini. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 43:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim, H. M., J. J. Pene, and R. W. Shaw. 1988. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase II structural gene. J. Bacteriol. 170:2873-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore, D. M., and N. Woodford. 2000. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 3:489-495. [DOI] [PubMed] [Google Scholar]
- 22.Matagne, A., A. Dubus, M. Galleni, and J.-M. Frère. 1999. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat. Prod. Rep. 16:1-19. [DOI] [PubMed] [Google Scholar]
- 23.Materon, I. C., and T. Palzkill. 2001. Identification of residues critical for metallo-β-lactamase function by codon randomization and selection. Protein Sci. 10:2556-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollard, C., C. Moali, C. Papamicael, C. Damblon, S. Vessilier, G. Amicosante, C. J. Schofield, M. Galleni, J. M. Frère, and G. C. Roberts. 2001. Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases: kinetic and spectroscopic studies. J. Biol. Chem. 276:45015-45023. [DOI] [PubMed] [Google Scholar]
- 25.Nagano, R., Y. Adachi, H. Imamura, K. Yamada, T. Hashizume, and H. Morishima. 1999. Carbapenem derivatives as potential inhibitors of various β-lactamases, including class B metallo-β-lactamases. Antimicrob. Agents Chemother. 43:2497-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagano, R., Y. Adachi, T. Hashizume, and H. Morishima. 2000. In vitro antibacterial activity and mechanism of action of J-111,225, a novel 1β-methylcarbapenem, against transferable IMP-1 metallo-β-lactamase producers. J. Antimicrob. Chemother. 45:271-276. [DOI] [PubMed] [Google Scholar]
- 27.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page, M. I., and A. P. Laws. 1998. The mechanism of catalysis and the inhibition of β-lactamases. Chem. Commun. 1609-1617.
- 29.Paul-Soto, R., R. Bauer, J. M. Frère, M. Galleni, W. Meyer-Klaucke, H. Nolting, G. M. Rossolini, D. de Seny, M. Hernandez-Valladares, M. Zeppezauer, and H.-W. Adolph. 1999. Mono- and binuclear Zn2+-β-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 274:13242-13249. [DOI] [PubMed] [Google Scholar]
- 30.Payne, D. J., W. Du, and J. H. Bateson. 2000. β-Lactamase epidemiology and the utility of established and novel β-lactamase inhibitors. Exp. Opin. Investig. Drugs 9:247-261. [DOI] [PubMed] [Google Scholar]
- 31.Payne, D. J., J. H. Bateson, B. C. Gasson, T. Khushi, D. Proctor, S. C. Pearson, and R. Reid. 1997. Inhibition of metallo-β-lactamase by a series of thiol ester derivatives of mercaptophenylacetic acid. FEMS Microbiol. Lett. 157:171-175. [DOI] [PubMed] [Google Scholar]
- 32.Payne, D. J., J. H. Bateson, B. C. Gasson, D. Proctor, T. Khushi, T. H. Farmer, D. A. Tolson, D. Bell, P. W. Skett, A. C. Marshall, R. Reid, L. Ghosez, Y. Combret, and J. Marchand-Brynaert. 1997. Inhibition of metallo-β-lactamase by a series of mercaptoacetic acid thiol ester derivatives. Antimicrob. Agents Chemother. 41:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prosperi-Meys, C., G. Llabres, D. de Seny, R. Paul-Soto, M. Hernandez-Valladares, N. Laraki, J. M. Frère, and M. Galleni. 1999. Interaction between class B β-lactamases and suicide substrates of active-site serine β-lactamases. FEBS Lett. 443:109-111. [DOI] [PubMed] [Google Scholar]
- 34.Quiroga, M. I., N. Franceschini, G. M. Rossolini, G. Gutkind, G. Bonfiglio, L. Franchino, and G. Amicosante. 2000. Interactions of cefotetan and the metallo-β-lactamases produced in Aeromonas spp. and in vitro activity. Chemotherapy 46:177-183. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saladini, M., D. Iacopino, and L. Menabue. 2000. Metal(II) binding ability of a novel N-protected amino acid. A solution-state investigation on binary and ternary complexes with 2,2′-bipyridine. J. Inorg. Biochem. 78:355-361. [DOI] [PubMed] [Google Scholar]
- 37.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 38.Toney, J. H., P. M. Fitzgerald, N. Grover-Sharma, S. H. Olson, W. J. May, J. G. Sundelof, D. E. Vanderwall, K. A. Cleary, S. K. Grant, J. K. Wu, J. W. Kozarich, D. L. Pompliano, and G. G. Hammond. 1998. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem. Biol. 5:185-196. [DOI] [PubMed] [Google Scholar]
- 39.Toney, J. H., K. A. Cleary, G. G. Hammond, X. Yuan, W. J. May, S. M. Hutchins, W. T. Ashton, and D. E. Vanderwall. 1999. Structure-activity relationships of biphenyl tetrazoles as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 9:2741-2746. [DOI] [PubMed] [Google Scholar]
- 40.Toney, J. H., G. G. Hammond, P. M. D. Fitzgerald, N. Sharma, J. M. Balkovec, G. P. Rouen, S. H. Olson, M. L. Hammond, M. L. Greenlee, and Y.-D. Gao. 2001. Succinic acids as potent inhibitors of plasmid-borne IMP-1 metallo-β-lactamase. J. Biol. Chem. 276:31913-31918. [DOI] [PubMed] [Google Scholar]
- 41.Walter, M. W., A. Felici, M. Galleni, R. Paul-Soto, R. M. Adlington, J. E. Baldwin, J. M. Frère, M. Gololobov, and C. J. Schofield. 1996. Trifluoromethyl alcohol and ketone inhibitors of metallo-β-lactamases. Bioorg. Med. Chem. Lett. 6:2455-2458. [Google Scholar]
- 42.Walter, M. W., M. Hernandez-Valladares, R. M. Adlington, G. Amicosante, J. E. Baldwin, J. M. Frère, M. Galleni, G. M. Rossolini, and C. J. Schofield. 1999. Hydroxamate inhibitors of Aeromonas hydrophila AE036 metallo-β-lactamase. Bioorg. Chem. 27:35-40. [Google Scholar]
- 43.Wang, Z., W. Fast, A. M. Valentine, and S. J. Benkovic. 1999. Metallo-β-lactamase: structure and mechanism. Curr. Opin. Chem. Biol. 3:614-622. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, M., S. Iyobe, M. Inoue, and S. Mitsuhashi. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 35:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]