Abstract
AZD2563, a novel oxazolidinone, and a selection of comparator drugs that included linezolid, erythromycin, clindamycin, quinolones, and gentamicin were tested against 384 Staphylococcus aureus (176 oxacillin-resistant S. aureus [ORSA]) and 219 coagulase-negative staphylococci (CoNS; 162 oxacillin resistant) by reference microdilution (all strains) and agar dilution (30 strains) methods. The following results were noted for AZD2563. (Note that, for comparison only, a breakpoint of ≤4 μg/ml [the breakpoint of linezolid] was used for this study, although a susceptibility breakpoint for AZD2563 has not been determined.) For S. aureus, the MIC at which 50% of the isolates tested are inhibited (MIC50) was 1 μg/ml, the MIC90 was 2 μg/ml, and the percent susceptibility was 100%. For CoNS, the MIC50 was 0.5 μg/ml, the MIC90 was 1 μg/ml, and the percent susceptibility was 100%. ORSA and OR-CoNS strains were equally inhibited by AZD2563 and linezolid. AZD2563 demonstrated antistaphylococcal activity comparable to that of linezolid.
The resistance profiles of gram-positive pathogens have rapidly changed during the past 10 years. The threat of oxacillin-resistant or glycopeptide-intermediate staphylococci, penicillin- and macrolide-resistant pneumococci, and vancomycin-resistant enterococci has necessitated the development of new antimicrobial classes, such as the oxazolidinones (2, 10). These oxazolidinones inhibit protein synthesis by acting against the formation of the 70S initiation complex, and they are generally considered to be bacteriostatic (2, 7, 10).
The first oxazolidinone to be licensed for clinical use was linezolid (2, 10). Multicenter or focused in vitro studies of linezolid against gram-positive cocci have proven linezolid to be a valuable therapeutic option, even when multidrug-resistant isolates were tested (3-7). Oxazolidinones have also demonstrated activity against less common gram-positive organisms, such as Bacillus spp., Corynebacterium spp., and Listeria spp. (2).
This study examines the comparative potency of AZD2563 (Fig. 1) tested against staphylococcal isolates. AZD2563 is one of a new series of compounds that have a C-5 substitution with O- or N-linked, five- and six-member aromatic heterocycles (M. B. Gravestock, M. J. Betts, E. Chawner, L. Dawson, A. McGregor, S. D. Mills, R. G. Wilson, L. Woods and A. Wookey, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1203, 2001). The isolates studied include oxacillin-resistant and -susceptible strains of Staphylococcus aureus and coagulase-negative staphylococci (CoNS). Reference broth microdilution methods were used to compare AZD2563 MIC results against those for linezolid and other antimicrobial agents (8).
FIG. 1.
Chemical structure of AZD2563.
(This study was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 22 to 25 September, 2001 [abstr. no. F-1029].)
A total of 603 clinical strains were selected from the culture collection at JMI Laboratories (North Liberty, Iowa); all had been isolated in the preceding 12 months. There were 384 S. aureus strains and 219 CoNS strains (10 species). Of those isolates, 176 of the S. aureus isolates and 162 of the CoNS isolates were oxacillin resistant, and 10 S. aureus isolates were consistent with vancomycin-intermediate susceptibility (VISA) by the definitions of Walsh et al. (12). Identification of each isolate to species level was performed independently at two laboratories.
AZD2563 powder was obtained from AstraZeneca (Macclesfield, United Kingdom), linezolid was obtained from Pharmacia and Upjohn (Kalamazoo, Mich.), and all other antimicrobials (quinupristin-dalfopristin [Q/D], vancomycin, erythromycin, clindamycin, levofloxacin, and oxacillin) were provided by their respective manufacturers or purchased from Sigma Chemicals (St. Louis, Mo.). Frozen-form, reference broth microdilution panels were prepared by TREK Diagnostics (Westlake, Ohio) and were stored at −80°C until used.
All isolates were tested for antimicrobial susceptibility by reference broth microdilution methods (8). For comparative purposes, agar dilution MICs (8) were also determined for 30 staphylococcal isolates selected from various species to provide a representative range of oxazolidinone MICs. All AZD2563 and linezolid MICs were identical by each reference method (data not shown). Quality control was performed with Enterococcus faecalis ATCC 29212 and S. aureus ATCC 29213 (9). The interpretive breakpoint criteria of the National Committee for Clinical Laboratory Standards (NCCLS) (9) were used throughout, except for AZD2563, for which the susceptibility breakpoint has not yet been defined. A breakpoint was chosen at ≤4 μg/ml (equal to linezolid) for purposes of comparison only.
Table 1 lists the antimicrobial activities for AZD2563 compared to those of the other antimicrobials tested against the 603 recent staphylococcal isolates. When comparing the two oxazolidinones directly against S. aureus, AZD2563 (MIC at which 50% of the isolates tested are inhibited [MIC50], 1 μg/ml) proved to be equally active to or twofold more potent than linezolid (MIC50, 2 μg/ml). The MICs of both AZD2563 and linezolid were within a narrow range (0.25 to 4 μg/ml for AZD2563 and 0.5 to 4 μg/ml for linezolid) (Fig. 2). Also, oxacillin susceptibility or resistance did not influence the MIC results for the oxazolidinones. Among the other antimicrobial agents tested, only vancomycin (MIC90, 1 μg/ml) and Q/D (MIC90, 0.5 to 1 μg/ml) possess a spectrum of activity comparable to that exhibited by the oxazolidinones. However, it should be noted that 1% of the isolates tested are currently resistant to Q/D (MIC, ≥4 μg/ml). All of the other antimicrobial agents tested were less active against S. aureus isolates and were consistently less effective against the oxacillin-resistant strains (≤35.2% susceptible). The VISA isolates, for which vancomycin MICs were 8 to 12 μg/ml with elevated inocula, did not exhibit oxazolidinone MIC results that differed significantly from the total population of S. aureus MICs (12).
TABLE 1.
In vitro activity of AZD2563 compared to those of 10 other antimicrobial agents tested against 384 strains of S. aureus and 219 strains of CoNS
| Organism (no. of strains tested) and antimicrobial agent | MIC (μg/ml)a
|
||
|---|---|---|---|
| 50% | 90% | Range (% susceptible)b | |
| S. aureus | |||
| Oxacillin susceptible (208) | |||
| AZD2563 | 1 | 2 | 0.25-4 |
| Linezolid | 2 | 2 | 0.5-(100.0) |
| Q/D | 0.25 | 0.5 | 0.06-1(100.0) |
| Vancomycin | 1 | 1 | 0.25-1(100.0) |
| Azithromycin | ≤2 | >8 | ≤2->4(81.7) |
| Clarithromycin | ≤2 | >4 | ≤2->4(80.3) |
| Erythromycin | 0.25 | >16 | ≤0.12->16(81.3) |
| Clindamycin | ≤0.5 | ≤0.5 | ≤0.5->4(95.2) |
| Gentamicin | ≤0.25 | 1 | ≤0.25->8(94.7) |
| Levofloxacin | 0.12 | 0.5 | 0.03->8(94.7) |
| Oxacillin | 0.25 | 0.5 | 0.06-2(100.0) |
| Oxacillin resistant (176) | |||
| AZD2563 | 1 | 2 | 0.25-2 |
| Linezolid | 2 | 2 | 0.5-4(100.0) |
| Q/D | 0.5 | 1 | 0.25->8(97.7) |
| Vancomycin | 1 | 1 | 0.5-2(100.0) |
| Azithromycin | >4 | >4 | ≤2->4(8.5) |
| Clarithromycin | >4 | >4 | ≤2->4(10.8) |
| Erythromycin | >16 | >16 | ≤0.25->16(9.1) |
| Clindamycin | >4 | >4 | ≤0.5->4(25.6) |
| Gentamicin | >8 | >8 | ≤0.25->8(35.2) |
| Levofloxacin | 8 | >8 | 0.06->8(9.7) |
| Oxacillin | >16 | >16 | 4->16(0.0) |
| CoNS | |||
| Oxacillin susceptible (57) | |||
| AZD2563 | 0.5 | 1 | 0.25-2 |
| Linezolid | 1 | 1 | 0.5-2(100.0) |
| Q/D | 0.12 | 0.25 | 0.06-0.5(100.0) |
| Vancomycin | 1 | 2 | 0.5-2(100.0) |
| Azithromycin | ≤2 | >4 | ≤2->4(59.6) |
| Clarithromycin | ≤2 | >4 | ≤2->4(61.4) |
| Erythromycin | 0.25 | >16 | ≤0.12->16(61.4) |
| Clindamycin | ≤0.5 | >4 | ≤0.5->4(87.7) |
| Gentamicin | ≤0.25 | >8 | ≤0.25->8(89.5) |
| Levofloxacin | 0.12 | 8 | 0.06->8(84.2) |
| Oxacillin | 0.12 | 0.25 | 0.03-0.25(100.0) |
| Oxacillin resistant (162) | |||
| AZD2563 | 0.5 | 1 | 0.12-1 |
| Linezolid | 1 | 2 | 0.5-2(100.0) |
| Q/D | 0.25 | 0.5 | 0.06-1(100.0) |
| Vancomycin | 1 | 2 | 0.25-4(100.0) |
| Azithromycin | >4 | >4 | ≤2->4(21.6) |
| Clarithromycin | >4 | >4 | ≤2->4(21.6) |
| Erythromycin | >16 | >16 | ≤0.1->16(21.6) |
| Clindamycin | ≤0.5 | >4 | ≤0.5->4(64.8) |
| Gentamicin | 8 | >8 | ≤0.25->8(48.1) |
| Levofloxacin | 2 | >8 | 0.06->8(50.6) |
| Oxacillin | >16 | >16 | 0.5->16(0.0) |
MICs were determined by the NCCLS broth microdilution method (8); 50% and 90%, MIC50 and MIC90, respectively.
Percentages of strains susceptible by NCCLS criteria are listed in parentheses (9). No breakpoint for AZD2563 has been determined, but the proportion of strains inhibited at ≤4 μg/ml was used for comparisons to linezolid (100.0% susceptible overall).
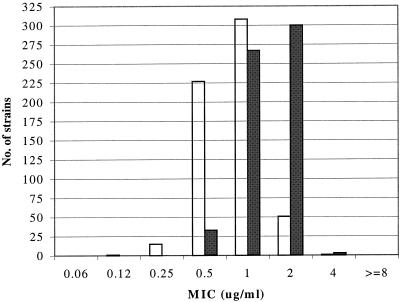
FIG. 2.
Distributions of MICs of AZD2563 (open bars) and linezolid (solid bars) tested against 603 recent isolates of staphylococci.
Table 1 also compares the activity of AZD2563 to those of the other antimicrobial agents tested against the 219 CoNS isolates. All CoNS isolates proved to be susceptible to AZD2563 (MIC90, 1 μg/ml), linezolid (MIC90, 1 to 2 μg/ml), Q/D (MIC90, 0.25 to 0.5 μg/ml), and vancomycin (MIC90, 2 μg/ml). When comparing the oxazolidinones alone, AZD2563 (MIC50, 0.5 μg/ml) was slightly more active against the CoNS isolates than linezolid (MIC50, 1 μg/ml). The only other antimicrobial agent that was more potent by weight than AZD2563 against the CoNS isolates was Q/D (MIC50, 0.25 μg/ml), yet the overall susceptibility rates of both agents appeared equivalent. The rates of susceptibility for oxacillin-resistant CoNS against the macrolides (three tested), clindamycin, gentamicin, levofloxacin, and oxacillin ranged from 21.6 to 64.8%. Oxacillin susceptibility or resistance among the CoNS strains did not affect the activity of either oxazolidinone.
For all 603 isolates of staphylococci tested, the distribution of MIC results for AZD2563 was 0.12 to 4 μg/ml (mode, 1 μg/ml), and that for linezolid was 0.5 to 4 μg/ml (mode, 2 μg/ml) (Fig. 2). A clear majority of AZD2563 MICs were 0.5 or 1 μg/ml, compared to MICs of 1 or 2 μg/ml for linezolid.
The oxazolidinone linezolid has become a useful alternative to glycopeptide therapy of serious gram-positive infections (2, 10). With very rare exceptions (4, 11), the linezolid MICs remain at ≤4 μg/ml, which is the value initially selected to represent oxazolidinone susceptibility (1). Improvements in the oxazolidinone class have recently focused on the reduction of adverse events, improvement of pharmacokinetic (PK) profiles, and increasing potency against gram-positive and -negative pathogens. AZD2563 appears to have achieved improved activity compared to linezolid when testing staphylococcal isolates, including VISA (reported here) and oxacillin-resistant or multidrug-resistant isolates (P. J. Turner, A. Wookey, J. M. Greenhalgh, M. Eastwood, and J. Clarke, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1024, 2001). Furthermore, preliminary pharmacodynamic (PD) results indicate that the 24-h area under the concentration-time curve (AUC)/MIC is the PK/PD parameter predicting in vivo AZD2563 success, and a prolonged postantibiotic effect was noted (not observed with linezolid). Additional physiologically based PK simulations have predicted AZD2563 will be a long-acting agent with once-daily dosing (W. A. Craig and D. R. Andes, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1037, 2001; P. A. Arundel, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1039, 2001).
Previous geographically diverse samples of gram-positive pathogens have demonstrated that linezolid possesses a complete spectrum of activity against initial clinical isolates of staphylococci (3, 5, 6). Oxazolidinone-resistant strains of enterococci and S. aureus have been observed very rarely—usually following prolonged courses of linezolid therapy (4, 11), a phenomenon also encountered with Q/D and glycopeptides. AZD2563 joins linezolid as an oxazolidinone having favorable class characteristics, but also demonstrating improved antistaphylococcal potency (MIC90, 1 to 2 μg/ml) and favorable PK/PD features (once-daily dosing). We eagerly await clinical trial results and expanded in vitro studies against other clinically important gram-positive pathogens.
Acknowledgments
The coauthors express their gratitude to K. Meyer, J. Jones, M. A. Pfaller, L. Deshpande, P. Rhomberg, and W. Howard for technical and manuscript preparation support.
This investigation was funded by AstraZeneca via an education or research grant.
REFERENCES
- 1.Biedenbach, D. J., and R. N. Jones. 1997. Disk diffusion test interpretive criteria and quality control recommendations for testing linezolid (U-100766) and eperezolid (U-100592) with commercially prepared reagents. J. Clin. Microbiol. 35:3198-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diekema, D. J., and R. N. Jones. 2000. Oxazolidinones: a review. Drug 59:7-16. [DOI] [PubMed] [Google Scholar]
- 3.Gemmell, C. G. 2001. Susceptibility of a variety of clinical isolates to linezolid: a European inter-country comparison. J. Antimicrob. Chemother. 48:47-52. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179.. [DOI] [PubMed] [Google Scholar]
- 5.Henwood, C. J., D. M. Livermore, A. P. Johnson, D. James, M. Warner, A. Gardiner, and The Linezolid Study Group. 2000. Susceptibility of Gram-positive cocci from 25 UK hospitals to antimicrobial agents including linezolid. J. Antimicrob. Chemother. 46:931-940. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. N., C. H. Ballow, D. J. Biedenbach, and The ZAPS Study Group Medical Centers. 2001. Multi-laboratory assessment of the linezolid spectrum of activity using the Kirby-Bauer disk diffusion method: report of the Zyvox antimicrobial potency study (ZAPS) in the United States. Diagn. Microbiol. Infect. Dis. 40:59-66. [DOI] [PubMed] [Google Scholar]
- 7.Jones, R. N., D. M. Johnson, and M. E. Erwin. 1996. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob. Agents Chemother. 40:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Norrby, R. 2001. Linezolid: a review of the first oxazolidinone. Expert Opin. Pharmacother. 2:293-302. [DOI] [PubMed] [Google Scholar]
- 11.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, Jr., and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 12.Walsh, T. R., A. Bolmstrom, A. Qwarnstrom, P. Ho, M. Wootton, R. A. Howe, A. P. MacGowan, and D. Dickema. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]