Abstract
The high intrinsic penem resistance of Pseudomonas aeruginosa is due to the interplay among the outer membrane barrier, the active efflux system MexAB-OprM, and AmpC β-lactamase. We studied the roles of two other efflux systems, MexCD-OprJ and MexXY-OprM, in penem resistance by overexpressing each system in an AmpC- and MexAB-OprM-deficient background and found that MexAB-OprM is the most important among the three efflux systems for extrusion of penems from the cell interior.
Pseudomonas aeruginosa is a clinically significant pathogen exhibiting intrinsic and acquired resistance to various antimicrobial agents. This resistance is attributable to the limited permeability of the outer membrane and the extrusion of a wide variety of antibiotics from the cell interior by a tripartite multidrug efflux system, which is composed of membrane fusion protein-type periplasmic, resistance-nodulation-cell division-type inner-membrane, and outer-membrane efflux proteins (10). Among these efflux systems, MexAB-OprM (5, 9, 19) and MexXY-OprM (1, 11, 14) contribute to both intrinsic resistance and acquired resistance, while MexCD-OprJ (20) and MexEF-OprN (8) contribute only to acquired resistance in P. aeruginosa. Recently, Masuda et al. (12) reported the substrate specificities of MexAB-OprM, MexXY-OprM, and MexCD-OprJ. Although most antimicrobial agents are substrates of all three efflux systems, these systems have slight but significant differences in substrate specificities to β-lactams. MexAB-OprM extrudes the broadest variety of β-lactams, including penicillins, cephems, and meropenem-type carbapenems. MexXY-OprM and MexCD-OprJ extrude most penicillins but not carbenicillin, sulbenicillin, various cephems, and many carbapenems. Penem antibiotics display potent activities against a variety of gram-positive and gram-negative bacteria but not against P. aeruginosa (2, 3, 4, 13, 15, 16, 17, 21, 24). Studies with mutants that overproduce or lack MexAB-OprM demonstrated that this efflux system extrudes penem antibiotics (18). However, it is unclear whether MexXY-OprM and MexCD-OprJ extrude penem antibiotics.
We compared the susceptibilities of a series of previously described isogenic AmpC-lacking mutants, each of which constitutively overexpressed an individual efflux pump (12, 18) (Table 1). We used AmpC-lacking P. aeruginosa mutants because the presence of chromosomal AmpC β-lactamase makes it difficult to interpret data on the MICs of β-lactams, including penems, due to the interplay between β-lactamase and the efflux system(s). The MICs of various penems, norfloxacin, and tetracycline for the mutants were determined by the twofold agar dilution method (23) with L agar with an inoculum of 104 cells. These results are shown in Table 2. Although the susceptibilities of the mutant KG5002, which lacked MexAB-OprM, MexCD-OprJ, and MexXY-OprM, to all penems tested were reduced by the overexpression of MexAB-OprM, MexCD-OprJ, or MexXY-OprM, as were those to norfloxacin and tetracycline, the degree of reduction resulting from the overexpression of MexAB-OprM (128- to 4,096-fold reduction compared to the results for KG5002) was remarkably higher than that resulting from the overexpression of MexCD-OprJ (2- to 64-fold reduction compared to the results for KG5002) or MexXY-OprM (4- to 16-fold reduction compared to the results for KG5002). These results suggest that all of the efflux systems tested extrude penems, that MexAB-OprM pumps out penems more effectively than it pumps out norfloxacin and tetracycline, and that the extrusion potency of MexAB-OprM for penems is higher than those of MexCD-OprJ and MexXY-OprM.
TABLE 1.
Relevant bacterial strains and plasmids used in this study
| P. aeruginosa strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| KG2259 | nfxB ΔmexRAB-oprM | 7 |
| KG5002 | ΔmexXY ampC::ΩSm ΔmexRAB-oprM | 12 |
| KG5004 | ampC::ΩSm ΔmexXY, formerly N116 | 12 |
| KG5008 | ampC::ΩSm ΔmexXY, formerly N119 | 12 |
| KG5011 | nalB ΔmexAB, formerly N126 | 12 |
| KG5006 | ampC::ΩSm MexXY overexpressing, formerly N133 | 12 |
| KG2504 | ampC::ΩSm | 18 |
| KG2504F1 | nalB ampC::ΩSm | This study |
| KG2505 | ampC::ΩSm mexA::ΩSm | 18 |
| KG2505F1 | nfxB ampC::ΩSm mexA::ΩSm | This study |
| KG2505F1ΔD | nfxB ampC::ΩSm mexA::ΩSm mexD::ΩSm | This study |
| Plasmids | ||
| pKMJ075 | pMT5059 with mexD::ΩSm and Mob cassette; Cbr, Cmr, Smr | 6 |
| pKMM128 | pKMM002 derivative with oprM | 6 |
Abbreviations: Cbr, carbenicillin resistant; Cmr, chloramphenicol resistant; Smr, streptomycin resistant.
TABLE 2.
Susceptibilities of isogenic mutants of P. aeruginosa PAO to penems and other antimicrobial agents
| Strain | Phenotypea
|
MIC (μg/ml) ofb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB | XY | CD | M | J | Faropenem | Ritipenem | AMA3176 | Sulopenem | Sch29482 | Sch34343 | Norfloxacin | Tetracycline | |
| PAO1 | + | − | − | + | − | 512 | 128 | 128 | 32 | 256 | 128 | 0.5 | 8 |
| KG5002 | − | − | − | − | − | 1 | 2 | 1 | 0.125 | 0.5 | 1 | 0.063 | 1 |
| KG5004 | ++ | − | − | ++ | − | 4,096 | 256 | 1,024 | 64 | 2,048 | 1,024 | 2 | 32 |
| KG5006 | − | ++ | − | ++ | − | 4 | 8 | 8 | 0.5 | 8 | 8 | 2 | 16 |
| KG5008 | − | − | ++ | − | ++ | 16 | 4 | 64 | 2 | 32 | 32 | 16 | 32 |
| KG2504 | + | − | − | + | − | 256 | 32 | ND | 8 | 256 | ND | 0.5 | 8 |
| KG2504F1 | ++ | − | − | ++ | − | 2,048 | 128 | ND | 64 | 1,024 | ND | 2 | 32 |
| KG2505 | − | − | − | − | − | 1 | 2 | 1 | 0.125 | 1 | 1 | 0.25 | 2 |
| KG2505F1 | − | − | ++ | S | ++ | 16 | 4 | ND | 2 | 16 | ND | 8 | 16 |
| KG2505F1ΔD | − | − | − | S | − | 1 | 2 | ND | 0.125 | 1 | ND | 0.25 | 2 |
AB, MexAB; XY, MexXY; CD, MexCD; M, OprM; J, OprJ; +, wild-type-level expression; ++, overexpression; −, undetectable expression; S, lower, although significant, level of expression than the wild-type level.
Expression of the efflux systems was determined by immunoblot analysis using an antibody specific to a component of each efflux system as described in the text. ND, not done.
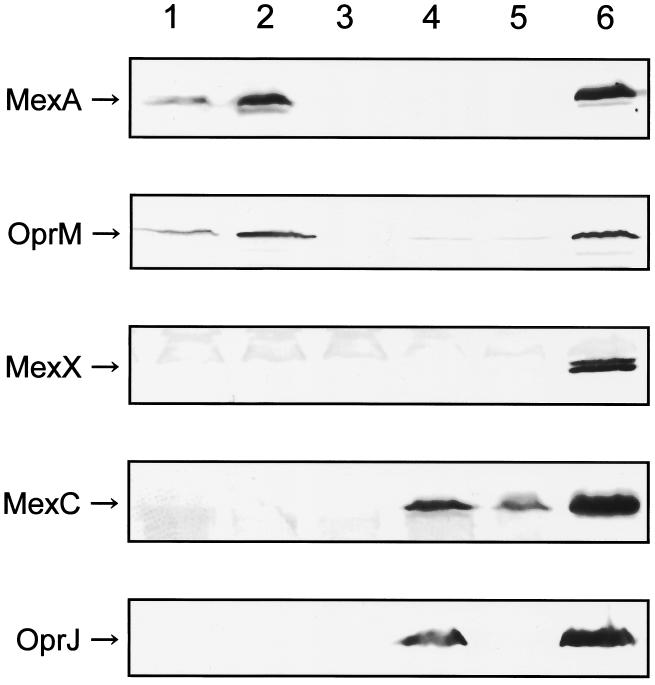
Four faropenem- and ritipenem-resistant mutants were spontaneously isolated from KG2504 (18), which has all three efflux system operons, on L agar (1.0% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl, and 1.5% [wt/vol] agar) plates containing twice the MIC of faropenem or ritipenem, respectively, per milliliter for these strains, and they were examined for their susceptibilities to penems, norfloxacin, and tetracycline and for the expression of efflux systems by immunoblot assay with antibodies (7) against a component of MexAB-OprM, antibodies (6) against a component of MexCD-OprJ, the antibody (11) against the periplasmic component, MexX, of MexXY-OprM, and the antibody (8) against the outer membrane component, OprN, of MexEF-OprN. All of the tested mutants showed identical resistance profiles and expression of efflux systems, and the resistance profiles and expression in KG2504F1, one of the isolated mutants, are shown in Table 2 and Fig. 1, respectively. KG2504F1 exhibited a resistance profile nearly identical to that of the strain overexpressing MexAB-OprM, KG5004 (Table 2). Immunoblot analysis of KG2504F1 revealed overexpression of MexA, MexB (data not shown), and OprM and undetectable expression of MexC, MexD (data not shown), OprJ, MexX, and OprN (data not shown) (Fig. 1, lane 2), indicating that it overexpressed only MexAB-OprM among the tested efflux systems. Similarly, four faropenem- and ritipenem-resistant mutants isolated in the same manner from the MexAB-deficient mutant, KG2505, showed identical phenotypes. The resistance profiles and expression in KG2505F1, one of the isolated mutants, are shown in Table 2 and Fig. 1, respectively. KG2505F1 showed a resistance profile nearly identical to that of the MexCD-OprJ hyperexpression strain, KG5008 (Table 2). Immunoblot analysis of KG2505F1 revealed overexpression of MexC, MexD (data not shown), and OprJ, very slight expression of OprM, and undetectable expression of MexA, MexB (data not shown), MexX, and OprN (data not shown) (Fig. 1, lane 4), indicating that it overexpressed only MexCD-OprJ among the tested efflux systems. These results show that MexAB-OprM is more effective in extruding penems than are the other tested efflux systems.
FIG. 1.
Detection of MexA, OprM, MexX, MexC, and OprJ by immunoblot analysis with the appropriate antibodies. Lane 1, KG2504; lane 2, KG2504F1; lane 3, KG2505; lane 4, KG2505F1; lane 5, KG2505F1ΔD; lane 6, positive controls (KG5004 for MexA and OprM, KG5006 for MexX, and KG5008 for MexC and OprJ).
MexXY expression, which is suppressed in wild-type strains such as PAO1 grown in ordinary nutrient media, is transiently derepressed from its suppression by the addition of antimicrobial agents such as tetracycline, erythromycin, and gentamicin (1, 11; T. Murata and N. Gotoh, unpublished data). To investigate whether penems induce MexXY expression, cells of mutants KG2504, KG2504F1, KG2505, and KG2505F1 were incubated and analyzed by immunoblot analysis as described previously (11). MexXY expression was not detected in cells that had been incubated with various concentrations (one-fourth the MIC to the MIC) of faropenem and ritipenem (data not shown). Thus, it was confirmed that MexXY has a trivial contribution to penem resistance, which is in accordance with the low resistance in cells overexpressing MexXY-OprM (Table 2).
Sequence data from the P. aeruginosa genome project have led researchers to predict the existence of at least six unidentified species of MexAB-OprM homologous resistance-nodulation-cell division (RND) family systems, in addition to the four efflux systems of P. aeruginosa PAO1 previously identified (22). Outer-membrane efflux proteins such as OprM and OprJ cooperatively function not only with native inner-membrane complexes such as MexAB and MexCD, respectively, but also with non-native inner-membrane complexes such as MexAB (for OprJ) (25), MexCD (for OprM) (6), and MexXY (for OprM) (11) as chimeric systems, indicating that the penem resistance observed in this study may be affected by the functional association of OprM and OprJ expressed in the tested strains with unknown efflux proteins. In order to investigate this possibility, we constructed a mexD::ΩSm mutant, KG2505F1ΔD, from KG2505F1 by homologous recombination using the pKMJ075 plasmid carrying mexD::ΩSm as described previously (6) and transformed KG5002 with an OprM expression plasmid, pKMM128 (6). Undetectable expression of MexD and overexpression of OprM, respectively, were confirmed in the two mutants by immunoblot analysis as described above (data not shown). The susceptibility of KG5002 to penems, norfloxacin, and tetracycline was not affected by the overexpression of OprM (data not shown). However, upon deletion of MexD, the susceptibilities of KG2505F1 to all penems tested increased to the same levels as those of KG5002, which lacks MexAB-OprM, MexCD-OprJ, and MexXY-OprM (Table 2), in spite of the low level of production of OprM in KG2505F1ΔD (Fig. 1, lane 5). This suggests that although we do not know whether an efflux system other than MexAB-OprM, MexCD-OprJ, and MexXY-OprM that is encoded on the P. aeruginosa chromosome contributes to penem resistance, no efflux system that functions cooperatively with OprM and OprJ is expressed. In contrast, loss of MexD did not increase the susceptibilities of KG2505F1 to tetracycline and norfloxacin to the levels of those of KG5002, although both of these agents are substrates for MexCD-OprJ (Table 2). This was probably due not to expression of an unknown efflux system but rather to the induced expression of MexXY in KG2505F1ΔD by tetracycline or norfloxacin, as reported previously (11).
Thus, we conclude that among the tested efflux systems, MexAB-OprM functions primarily and effectively in the extrusion of penem antibiotics in P. aeruginosa and that MexCD-OprJ is the compensatory system for penem efflux. Moreover, the potency of MexXY-OprM in the extrusion of penems is trivial. Penem antibiotics may not be able to be used for the treatment of P. aeruginosa infections because of the highly intrinsic resistance of this bacterium to these agents (18). However, overexpression of MexAB-OprM in strain KG5002, which lacks MexAB-OprM, MexCD-OprJ, and MexXY-OprM, causes increases of more than 1,000-fold in the MICs of faropenem, AMA3176, Sh29482, and Sch34343, whereas overexpression of MexCD-OprJ or MexXY-OprM did not cause comparable increases. This shows that these agents are useful tools for investigating the molecular mechanisms of extrusion by the efflux system, such as the substrate recognition mechanism. In fact, by using faropenem, we recently succeeded in isolating and characterizing genes encoding a substrate specificity-altered MexD mutant (N. Gotoh and T. Satou, unpublished data).
Acknowledgments
This research was supported by grants for scientific research to N.G. from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and from the Ministry of Health, Labour and Welfare of Japan.
REFERENCES
- 1.Aires, J. R., T. Köhler, H. Nikaido, and P. Plésiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., K. E. Aldridge, S. D. Allen, P. C. Fuchs, E. H. Gerlach, R. N. Jones, and M. A. Pfaller. 1988. In vitro activity of FCE 22101, imipenem, and ceftazidime against over 6,000 bacterial isolates and MIC quality control limits of FCE 22101. Eur. J. Clin. Microbiol. Infect. Dis. 7:794-798. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs, P. C., A. L. Barry, and D. L. Sewell. 1995. Antibacterial activity of WY-49605 compared with those of six other oral agents and selection of disk content for disk diffusion susceptibility testing. Antimicrob. Agents Chemother. 39:1472-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gootz, T., J. Retsema, A. Girard, E. Hamanaka, M. Anderson, and S. Sokolowski. 1989. In vitro activity of CP-65,207, a new penem antimicrobial agent, in comparison with those of other agents. Antimicrob. Agents Chemother. 33:1160-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotoh, N., N. Itoh, H. Tsujimoto, J. Yamagishi, Y. Oyamada, and T. Nishino. 1994. Isolation of OprM-deficient mutants of Pseudomonas aeruginosa by transposon insertion mutagenesis: evidence of involvement in multiple antibiotic resistance. FEMS Microbiol. Lett. 122:267-274. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh, N., H. Tsujimoto, A. Nomura, K. Okamoto, M. Tsuda, and T. Nishino. 1998. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 165:21-27. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh, N., H. Tsujimoto, M. Tsuda, K. Okamoto, A. Nomura, T. Wada, M. Nakahashi, and T. Nishino. 1998. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köhler, T., M. Michéa-Hamzehpour, U. Henze, N. Gotoh, L. K. Curty, and J. C. Pechere. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 9.Li, X.-Z., H. Nikaido, and K. Poole. 1995. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1948-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, X.-Z., L. Zhang, and K. Poole. 2000. Interplay between the MexA-MexB-OprM multidrug efflux system and the outer membrane barrier in the multiple antibiotic resistance of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:433-436. [DOI] [PubMed] [Google Scholar]
- 11.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda, K., K. Sasaki, K. Inoue, H. Kondo, M. Inoue, and S. Mitsuhashi. 1985. In vitro antibacterial activity of Sch 34343 and its stability to β-lactamases and renal dehydropeptidase 1. Antimicrob. Agents Chemother. 28:684-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neu, H. C., and P. Labthavikul. 1982. Antibacterial activity of an oral penem, Sch 29482. J. Antimicrob. Chemother. 9(Suppl.):49-57. [DOI] [PubMed] [Google Scholar]
- 16.Neu, H. C., N. X. Chin, and P. Labthavikul. 1985. The in-vitro activity of a novel penem FCE 22101 compared to other β-lactam antibiotics. J. Antimicrob. Chemother. 16:305-313. [DOI] [PubMed] [Google Scholar]
- 17.Nishino, T., Y. Maeda, E. Ohtsu, S. Koizuka, T. Nishihara, H. Adachi, K. Okamoto, and M. Ishiguro. 1989. Studies on penem antibiotics. II. In vitro activity of SUN5555, a new oral penem. J. Antibiot. 42:977-988. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto, K., N. Gotoh, and T. Nishino. 2001. Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay. Antimicrob. Agents Chemother. 45:1964-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X.-Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 21.Reeves, D. S., H. A. Holt, and M. J. Bywater. 1985. Comparative in-vitro activity of Sch 34343, a new penem antibiotic. J. Antimicrob. Chemother. 15(Suppl.):57-66. [DOI] [PubMed] [Google Scholar]
- 22.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 23.Washington, J. A., and A. L. Barry. 1974. Dilution test procedures, p. 410-417. In E. H. Lennette, E. H. Spaulding, and J. P. Traunt (ed.), Manual of clinical microbiology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 24.Wise, R., J. M. Andrews, and G. Danks. 1983. Comparison of in vitro activity of FCE 22101, a new penem, with those of other β-lactam antibiotics. Antimicrob. Agents Chemother. 24:909-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoneyama, H., A. Ocaktan, N. Gotoh, T. Nishino, and T. Nakae. 1998. Subunit swapping in the Mex-extrusion pumps in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 244:898-902. [DOI] [PubMed] [Google Scholar]