Abstract
The antifungal activity of caspofungin acetate (CAS) alone and in combination with voriconazole (VRC) was evaluated in an immunosuppressed transiently neutropenic guinea pig model of invasive aspergillosis. Guinea pigs were immunosuppressed with triamcinolone at 20 mg/kg of body weight/day subcutaneously beginning 4 days prior to lethal intravenous challenge with Aspergillus fumigatus and were made temporarily neutropenic with cyclophosphamide administered at 150 mg/kg intraperitoneally (i.p.) 1 day prior to challenge. Therapy with i.p. CAS at 1 and 2.5 mg/kg/day (with and without oral VRC at 5 mg/kg/day), oral VRC at 5 mg/kg/day, or i.p. amphotericin B (AMB) at 1.25 mg/kg/day was begun 24 h after challenge and was continued for 5 days. Mortality occurred in 12 of 12 untreated controls, whereas mortality occurred in 4 of 12 and 6 of 12 guinea pigs treated with CAS at 1 and 2.5 mg/kg/day, respectively, and in 3 of 12 guinea pigs treated with AMB. No mortality occurred among animals treated with CAS at 1 mg/kg/day plus VRC at 5 mg/kg/day, CAS at 2.5 mg/kg/day plus VRC at 5 mg/kg/day, or VRC at 5 mg/kg/day alone. Both CAS regimens increased the survival times and reduced the colony counts in tissue compared with those for the controls. Treatment with VRC and AMB significantly reduced the colony counts in the tissues of selected animals compared with those in the tissues of the controls. Treatment with VRC and AMB also resulted in reductions in colony counts in tissues compared with those in the tissues of animals treated with CAS (the difference was not statistically significant) and improved the survival times but did not sterilize tissues. Combination therapies with CAS plus VRC at either dose reduced colony counts in tissues 1,000-fold over those for the controls and were the only regimens that significantly reduced the numbers of positive cultures. The combinations of CAS plus VRC were highly effective in this model and should be further evaluated for use against invasive aspergillosis.
Invasive aspergillosis remains a common and difficult clinical problem (8, 22). At present, amphotericin B deoxycholate is the standard therapy for invasive aspergillosis, but amphotericin B therapy is toxic and may be ineffective at well-tolerated doses (8, 15, 22). Recently, a variety of new antifungal agents, mostly azoles, have been tested against Aspergillus spp. Previously, the only azole with activity against Aspergillus has been itraconazole, which, in the standard formulation, may be erratically absorbed. Lipid formulations of amphotericin B and the intravenous form of itraconazole address and improve on some of the difficulties encountered with the older formulations (15, 18, 22). Recently, voriconazole, which is available both orally and intravenously, has been shown to have significant activity against invasive aspergillosis (14). With conventional therapy, outcomes of invasive aspergillosis remain poor, so that novel therapeutic agents used singly or in combination therapy, especially in high-risk patients, may increasingly be used (11).
Caspofungin acetate, a 1,3-β-d-glucan synthase inhibitor and a new antifungal agent in the echinocandin family, is effective in vitro against Aspergillus spp. and may prove beneficial in the treatment of invasive aspergillosis in that it has a target different from that of the azoles, which block the synthesis of ergosterol from lanosterol (13, 25). Inhibition of glucan synthesis is an attractive target for antifungal agents, since the absence of homologous enzymes in humans may afford a high degree of selectivity of the agent for certain pathogenic fungi (2, 6). Another approach to improving antifungal therapies has been in the use of combination therapy. Additive to synergistic effects may be seen with certain drug combinations, and these effects might have significant clinical relevance (4, 6). The potential beneficial effects from the use of combination antifungal therapy include the ability to use reduced doses of toxic drugs, which may lead to fewer side effects; increased spectra of activity or enhanced fungicidal effects; and the prevention of the emergence of drug resistance (6, 26, 27).
In this study, caspofungin was evaluated in an immunosuppressed transiently neutropenic guinea pig model of invasive aspergillosis. In this lethal model, guinea pigs were made leukopenic and additional immune system suppression was induced by the concomitant administration of steroids. Extensive infections developed throughout the liver, kidney, lung, and brain, analogous to the situation in animals with clinically disseminated invasive aspergillosis (14, 19-21). In these experiments, we used a guinea pig model of invasive aspergillosis to evaluate the antifungal activities of caspofungin alone and in combination with a reduced dose of voriconazole against this disseminated disease (10, 14).
MATERIALS AND METHODS
Guinea pig model.
Male Hartley guinea pigs (weight, 0.5 kg) were immunocompromised and challenged with Aspergillus fumigatus as described previously (14). Briefly, the groups of guinea pigs were immunosuppressed daily with subcutaneous triamcinolone acetonide (Steris Laboratories, Inc., Phoenix, Ariz.) at 20 mg/kg of body weight beginning 4 days prior to challenge and were made temporarily neutropenic with one intraperitoneal dose of cyclophosphamide (Pharmacia Inc., Kalamazoo, Mich.) at 300 mg/kg. By use of this regimen for temporary immunosuppression, the total white blood cell counts of the guinea pigs were reduced to <1,000 mm3, with immunosuppression lasting through day 7. One day following the initiation of neutropenia, groups of 8 to 10 guinea pigs were sedated with ketamine HCl (44 mg/kg; Fort Dodge Laboratories Inc., Fort Dodge, Iowa), atropine (0.04 mg/kg Elkins-Sinn, Inc., Cherry Hill, N.J.), and xylazine (5 mg/kg; Agriculture Division, Bayer Corporation, Shawnee Mission, Kans.). A lethal bolus of 106 A. fumigatus conidia was then inoculated intravenously through the saphenous vein. Each group contained at least one untreated control guinea pig, for which the lethal challenge was fatal within 6 days of challenge, with a mean survival time of 4.5 ± 0.3 days (range, 3 to 6 days) after challenge. Daily doses of ceftazidime (100 mg/kg; SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.) were administered intramuscularly beginning on the day of challenge to prevent simultaneous bacterial infections. All animal research procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Antifungal agents.
Antifungal therapy included amphotericin B (Fungizone; Bristol-Myers Squibb Co., Princeton, N.J.), caspofungin acetate (Merck Research Laboratories, Rahway, N.J.), or voriconazole (Pfizer, Inc., Groton, Conn.) and was initiated 24 h after challenge with A. fumigatus conidia and continued for 5 days. Amphotericin B was diluted with 5% dextrose in sterile water at a ratio of 1.25 mg/ml of diluent and was given intraperitoneally at a dose of 1.25 mg/kg/day. Caspofungin was dissolved in sterile water to 2.5 mg/ml and was given intraperitoneally at a dose of 1 or 2.5 mg/kg/day. Both amphotericin B and caspofungin solutions were sterile filtered before injection into the guinea pigs. Voriconazole was suspended in polyethylene glycol 200 (Sigma Chemical, St. Louis, Mo.) and was administered orally twice a day as a 10-mg/ml suspension at 5 mg/kg/day.
Organ cultures.
Organs were cultured postmortem (after the death of the animal during treatment [n = 25] or 96 h after the completion of therapy in the remaining treated guinea pigs [n = 59]). Guinea pigs were killed by terminal exsanguination after being anesthetized with 44 mg of ketamine HCl per kg and 10 mg of xylazine per kg. Organs (brain, lung, liver, and kidneys) were removed aseptically and were cultured to determine the degree of infection with A. fumigatus. Organs were considered positive when two or more colonies of A. fumigatus were present on 1 g of minced tissues placed directly on Sabouraud dextrose plates (Becton Dickinson and Company, Cockeysville, Md.) or when semiquantitative cultures of tissue homogenates contained more than 20 CFU/g of tissue (12). The burdens of Aspergillus in tissues were evaluated with semiquantitative cultures that could detect from 20 to 20,000 CFU/g of tissue (23). Samples of each organ were finely chopped (manually), weighed, diluted 1:10 (wt/vol) with sterile saline, and homogenized for 25 s with an electric tissue homogenizer (IKA-Works, Inc., Cincinnati, Ohio). Duplicate 0.1- and 1.0-ml samples of the organ homogenate were plated on Sabouraud dextrose and incubated at 37°C for 48 h, and the colonies were counted. In combination, these two methods detected A. fumigatus organisms present at 2 to 20,000 CFU/g of tissue.
Challenge organism.
A. fumigatus isolate P171, a clinical isolate which we have previously used in our animal studies, was grown on Sabouraud dextrose slants at 37°C for 24 h. For injection into the guinea pigs, conidia were harvested by washing the surface of the slant with sterile saline and dislodging the conidia by gentle rubbing with a sterile glass rod. The resultant conidial suspension was adjusted to the desired concentration of 106 conidia/ml by counting with a hemacytometer. The counts were verified by duplicate serial plating on Sabouraud dextrose plates for determination of colony counts. In vitro studies with this isolate showed that the MICs at 24 and 48 h were 0.5 and 0.5 μg/ml, respectively, for voriconazole; 32 and 32 μg/ml, respectively, for caspofungin; and 0.25 and 0.25 μg/ml, respectively, for the combination of caspofungin plus voriconazole. The combination of these two drugs in vitro has been shown to have a fractional inhibitory concentration index (FIC) of 0.51 and a weak synergistic effect against this isolate (S. Perea, G. Gonzalez, A. W. Fothergill, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. F-87, p. 372, 2001).
Statistical analysis.
The Fisher exact test and the Wilcoxon rank sum test were used where appropriate. Statistical significance was defined as a P value <0.05; however, adjustments were made for multiple dose comparisons for each organ evaluated so that the level of significance was a P value <0.0025.
RESULTS
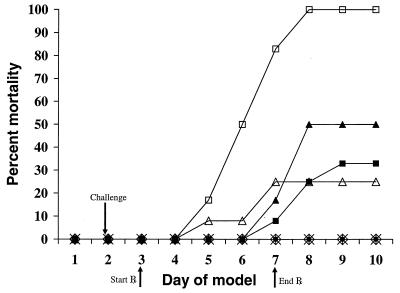
Experimental antifungal therapy with amphotericin B, caspofungin, voriconazole, or the combination of caspofungin and voriconazole initiated 24 h after lethal challenge with A. fumigatus conidia enhanced the period of survival compared with that for untreated infected controls, as shown in Fig. 1. By day 6 following challenge, the mean times (days) of survival for guinea pigs receiving amphotericin B at 1.25 mg/kg/day, voriconazole at 5 mg/kg/day, or caspofungin at 1 or 2.5 mg/kg/day alone or in combination with voriconazole at 5 mg/kg/day were significantly (P < 0.0025) improved compared with those for the controls. During the course of these experiments, all 12 control guinea pigs, 3 of 12 (25%) guinea pigs treated with amphotericin B, 4 of 12 (33%) guinea pigs treated with caspofungin at 1 mg/kg/day, and 6 of 12 (50%) guinea pigs receiving caspofungin at 2.5 mg/kg/day died, as shown in Table 1. There were no deaths in the voriconazole group or the combination therapy groups. Compared to the rate of mortality for the control animals, all treatment regimens significantly decreased the rate of mortality (P < 0.0025) by day 6 following challenge.
FIG. 1.
Cumulative mortality of 12 guinea pigs per treatment group treated with caspofungin (CAS), voriconazole (VRC), caspofungin plus voriconazole, or amphotericin B (AmB). Guinea pigs were challenged on the second day. Controls (□) received no antifungal therapy. Treatment of the guinea pigs with amphotericin B at 1.25 mg/kg/day (▵), caspofungin at 1 mg/kg/day (▪) or 2.5 mg/kg/day (▴), voriconazole at 5 mg/kg/day (⋄), caspofungin at 1 mg/kg/day plus voriconazole at 5 mg/kg/day (*), or caspofungin at 2.5 mg/kg/day plus voriconazole at 5 mg/kg/day (•) was initiated 24 h after challenge and was given daily for 5 days.
TABLE 1.
Mean times of survival in temporarily immunosuppressed guinea pigs
| Group (dose [mg/kg/day])a | Mortality (no. of animals that died/total no. of animals tested) [% of total]) | Mean survival time (days)b
|
|
|---|---|---|---|
| Mean ± SE | Range | ||
| Control | 12/12 (100) | 4.5 ± 0.29 | 3-6 |
| Caspofungin | |||
| 2.5 | 6/12 (50)c | 6.83 ± 0.37c | 5-8 |
| 1 | 4/12 (33)c | 7.33 ± 0.31c | 5-8 |
| Caspofungin (2.5) + voriconazole (5) | 0/12 (0)c | 8c | 8 |
| Caspofungin (1) + voriconazole (5) | 0/12 (0)c | 8c | 8 |
| Voriconazole (5) | 0/12 (0)c | 8c | 8 |
| Amphotericin B | 3/12 (25)c | 7.08 ± 0.50c | 3-8 |
There were 12 animals in each group.
Time of survival from day of challenge with A. fumigatus.
P < 0.0025 compared to controls.
The results of semiquantitative cultures of liver, lung, kidney, and brain tissue are presented in Table 2. Each of these tissues from the untreated control animals was extensively infected. Caspofungin at 1 mg/kg/day reduced the burden of Aspergillus in brain and liver tissue more than 10-fold compared with the burdens in the tissues of the control animals and also reduced the burdens in kidney tissue nearly 50-fold, while caspofungin at 2.5 mg/kg/day reduced the counts in kidney tissue more than 10-fold compared with the counts for the controls (P < 0.0025). Amphotericin B at 1.25 mg/kg/day, voriconazole at 5 mg/kg/day, and the combination of caspofungin and voriconazole also significantly reduced the colony counts in liver, kidney, and brain tissues compared to the counts in the tissues of the controls (P < 0.0025). Reductions in colony counts in the lung tissues of treated animals compared to those in the lung tissues of the controls were obtained with voriconazole at 5 mg/kg/day (P was not significant [NS]), amphotericin B at 1.25 mg/kg/day (P was NS), and the combinations of caspofungin at 1 or 2.5 mg/kg/day plus voriconazole at 5 mg/kg/day (P < 0.0025); however, treatment with either dose of caspofungin alone resulted in only slight improvements in the colony counts in lung tissue compared with those in the lung tissues of the controls.
TABLE 2.
Results of semiquantitative cultures of organs from guinea pigs treated with antifungal agents beginning 24 h after challenge and killed 96 h after completion of therapy
| Group (dose [mg/kg/day])a | Colony count (mean ± SE log10CFU/g of tissue)
|
|||
|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | |
| Control | 3.32 ± 0.20 | 0.90 ± 0.22 | 3.11 ± 0.19 | 3.03 ± 0.23 |
| Caspofungin | ||||
| 2.5 | 3.19 ± 0.45 | 0.42 ± 0.13 | 1.64 ± 0.31b | 2.97 ± 0.40 |
| 1 | 1.93 ± 0.48b | 0.56 ± 0.23 | 1.42 ± 0.39b | 1.47 ± 0.45 |
| Caspofungin (2.5) + voriconazole (5) | 0.32 ± 0.20b | 0.11 ± 0.08b | 0.34 ± 0.19b | 0.13 ± 0.11b |
| Caspofungin (1) + voriconazole (5) | 0.08 ± 0.04b | 0.05 ± 0.04b | 0.13 ± 0.11b | 0.19 ± 0.20b |
| Voriconazole (5) | 0.35 ± 0.19b | 0.30 ± 0.07 | 0.58 ± 0.19b | 0.13 ± 0.09b |
| Amphotericin B | 1.22 ± 0.37b | 0.38 ± 0.17 | 0.98 ± 0.32b | 0.87 ± 0.44b |
There were 12 animals in each group.
P < 0.0025 compared to controls.
Culture results for guinea pigs treated with caspofungin, amphotericin B, voriconazole, and caspofungin in combination with voriconazole are shown in Table 3. Positive culture results were obtained for all tissues examined from each of the untreated control animals. Neither amphotericin B at 1.25 mg/kg/day nor voriconazole at 5 mg/kg/day was effective at sterilizing liver, lung, or kidney tissue (P was NS compared to the results for the controls); however, amphotericin B at 1.25 mg/kg/day and voriconazole at 5 mg/kg/day were effective at sterilizing brain tissue (P < 0.0025). Caspofungin at either 1 or 2.5 mg/kg/day did not significantly sterilize tissues (P was NS compared to the results for the controls). All 12 animals treated with each agent alone had at least one positive culture of organ tissue. In contrast, treatments with the combinations of caspofungin and voriconazole were significantly more effective than any of the other therapeutic regimens in reducing the numbers of positive cultures of liver, lung, kidney, and brain tissues (P < 0.0025 compared to the results for the controls). Overall, only 3 of 12 (25%) animals receiving combination therapy with caspofungin at 1 mg/kg/day plus voriconazole at 5 mg/kg/day had any organs positive for Aspergillus by culture. With the latter regimen, only 8 of 48 (17%) of all tissues cultured were positive for Aspergillus, and of these 8 tissues positive by culture, the colony counts were at the lowest limit of detection of 0.3 log10 CFU/g of tissue in 6 of them; however, positive culture results were obtained for all 48 organs from infected controls. Similarly, 5 of 12 (42%) guinea pigs receiving combination therapy with caspofungin at 2.5 mg/kg/day plus voriconazole at 5 mg/kg/day had any organs positive for Aspergillus. Examination of all 48 cultures of organs from the group treated with the combination of caspofungin at 2.5 mg/kg/day and voriconazole at 5 mg/kg/day showed that 11 (23%) were positive, and 5 of these 11 cultures had colony counts at the limit of detection. In contrast, the 48 cultures of organs from the group treated with voriconazole at 5 mg/kg/day alone yielded 25 (52%) that were positive, although the colony counts were at the limit of detection in 15 of them. In addition, 36 of 48 (75%) and 40 of 48 (83%) organs from guinea pigs receiving caspofungin alone at 1 and 2.5 mg/kg/day were positive by culture, respectively.
TABLE 3.
Results of cultures of organs from temporarily immunosuppressed guinea pigs killed 96 h after completion of therapy
| Group (dose [mg/kg/day])a | No. of positive cultures/no. of guinea pig organs cultured
|
||||
|---|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | Any organ | |
| Control | 12/12 | 12/12 | 12/12 | 12/12 | 12/12 |
| Caspofungin | |||||
| 2.5 | 11/12 | 10/12 | 10/12 | 9/12 | 12/12 |
| 1 | 12/12 | 8/12 | 9/12 | 7/12 | 12/12 |
| Caspofungin (2.5) + voriconazole (5) | 3/12b | 2/12b | 4/12b | 2/12b | 5/12b |
| Caspofungin (1) + voriconazole (5) | 3/12b | 2/12b | 2/12b | 1/12b | 3/12b |
| Voriconazole 5 | 6/12 | 8/12 | 8/12 | 3/12b | 12/12 |
| Amphotericin B | 8/12 | 6/12 | 8/12 | 4/12b | 11/12 |
There were 12 animals in each group
P < 0.0025 compared to controls.
DISCUSSION
Caspofungin has both in vitro and in vivo activities against a variety of fungi, including Aspergillus spp., although in vitro variations in activities by strain and species have been reported (2, 3, 9). Assessment of the in vitro activity of caspofungin against Aspergillus remains difficult because of the profound morphological changes that the drug effects on the hyphae (1, 3, 7), which are partially inhibited and which appear short, stubby, and highly branched (3). Despite the difficulties involved with the in vitro assessment of antifungal activity, caspofungin remains an attractive compound for use against Aspergillus spp. due to its favorable toxicity profile and ability to be administered intravenously. The antifungal activity of caspofungin is similar to that of amphotericin B against Aspergillus in immunosuppressed mice (2), although its fungicidal effects occur at a slower rate than those of amphotericin B (6), and furthermore, it has a target that is not found in mammals, which itself introduces the possibility that this drug has reduced toxicity (6).
Voriconazole is a new triazole antifungal that has excellent oral bioavailability, a broad spectrum of antifungal activity, including potent activity against Aspergillus, and an extended half-life and may also be administered intravenously (5, 16, 17). In a previous study, we examined the efficacy of voriconazole at 5 and 10 mg/kg/day in this model of experimental invasive aspergillosis. We found that both doses equally prolonged survival and that both doses significantly reduced the colony counts in tissues compared to those in the tissues of untreated controls. However, in terms of reducing the total number of positive cultures, we also found that only the 10-mg/kg/day dosing regimen was effective in reducing the number of positive cultures, with 13 of 32 (41%) cultures of tissue obtained from animals treated with a dose of 10 mg/kg/day and 18 of 32 (56%) cultures of tissue obtained from animals treated with a dose of 5 mg/kg/day being positive (14).
In the present study, we examined the in vivo interaction between caspofungin alone and caspofungin in combination with the new triazole voriconazole against experimental invasive aspergillosis in a guinea pig model. Treatment with caspofungin, particularly at 1 mg/kg/day, was seen to reduce the rate of mortality (33%) and also to increase the mean time of survival (which, at 7.33 ± 0.31 days, was nearly equal to that achieved with amphotericin B) compared to the survival times of untreated control animals. Similar results of studies of the activity of caspofungin against experimental A. fumigatus infections in mice immunosuppressed with cyclophosphamide have been reported previously; the rates of survival in three trials with this dose ranged from 50 to 92% (2). Furthermore, in our experiments, as noted above, treatment with caspofungin reduced the colony counts in liver, kidney, and brain tissues by 10-fold to nearly 50-fold compared to the colony counts in those tissues of the controls. An exception to this trend was seen with caspofungin at 2.5 mg/kg/day; the colony counts in the brain and liver tissues of animals treated with caspofungin at this dose were indistinguishable from those in the tissues of the controls. A recent study of the activity of the echinocandin LY-303366 against A. fumigatus in lethally challenged rabbits obtained results similar to those reported here. In that study, the rate of mortality and the level of antigenemia were reduced compared to those in the controls, but the burden of Aspergillus in tissue was not reduced. The latter was hypothesized to have been related to the presence of damaged hyphal elements that retained a degree of viability (24). Additionally, since determination of tissue burden may have limited sensitivity and may not always correlate well with fungal growth, some researchers have suggested the use of a quantitative PCR-based assay to assess drug efficacy (6a).
Previous in vitro studies with the A. fumigatus isolate that we used in our animal model showed that the isolate was susceptible to voriconazole (MIC at 48 h, 0.5 μg/ml), caspofungin (MIC at 48 h, 32 μg/ml), and the combination of caspofungin and voriconazole (MIC at 48 h, 0.25 μg/ml). The combination of these two drugs in vitro, for which the FIC index was 0.51, showed a weak synergistic effect against this isolate. In the former in vitro study, interactions between caspofungin and voriconazole were examined by using 48 clinical Aspergillus isolates representing four species obtained from patients with invasive aspergillosis. MICs were determined by the NCCLS broth microdilution methodology 17a. An FIC index of <1 was detected in 87.5% of the interactions, with marked synergy (<0.5) seen in 14 of 48 (29%) of the interactions; an additive effect, defined as an FIC index of 1.0, was observed in 4.2% of the interactions; and a subadditive effect, defined as an FIC index of 1.0 to 2.0, was found in 8.3% of the interactions. Antagonism was not observed in that study of 48 Aspergillus isolates (Perea et al., Abstr. 101st Gen. Meet. Am. Soc. Microbiol.).
The encouraging results obtained with the combination of caspofungin and voriconazole were borne out by the in vivo studies described here. While combination therapies with caspofungin and voriconazole were as effective as voriconazole alone in reducing rates of mortality, with the combination therapies the colony counts were reduced compared to those obtained with either amphotericin B alone or voriconazole alone. Furthermore, only combination therapy with caspofungin and voriconazole resulted in cultures more sterile than those achieved with the other therapeutic regimens examined in these experiments, with the tissues from 9 of 12 (75%) of the animals receiving combination therapy with caspofungin at 1 mg/kg/day plus voriconazole at 5 mg/kg/day and 7 of 12 (58%) of the animals receiving combination therapy with caspofungin at 2.5 mg/kg/day plus voriconazole at 5 mg/kg/day showing no colony growth on culture. In comparison, in these experiments either caspofungin alone or voriconazole alone did not sterilize the tissues of any of the animals. One important aspect of the use of caspofungin is in the realm of combination therapy with other antifungal drugs. Our data support our own in vitro work and add support to the work of others (6) that combination therapy with caspofungin may have important clinical relevance.
Although it remains somewhat controversial whether there is a correlation between in vitro antifungal susceptibility and in vivo efficacy, therapeutic regimens with caspofungin and voriconazole appear to represent a viable alternative for the effective treatment of Aspergillus infections. Further clinical studies are needed to investigate the usefulness of combination therapy for invasive aspergillosis.
Acknowledgments
This work was supported by a grant from Merck & Company, Inc.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi, F., L. F. Di Francesco, P. Compagnucci, D. Arzeni, A. Giacometti, and G. Scalise. 1998. In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J. Antimicrob. Chemother. 41:59-65. [DOI] [PubMed] [Google Scholar]
- 5.Barry, A. L., and S. D. Brown. 1996. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob. Agents Chemother. 40:1948-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2000. Influence of human sera on the in vitro activity of the echinocandin caspofungin (MK-0991) against Aspergillus fumigatus. Antimicrob. Agents Chemother. 44:3302-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George, D., D. Kordick, P. Miniter, T. F. Patterson, and V. T. Andriole. 1993. Combination therapy in experimental invasive aspergillosis. J. Infect. Dis. 168:692-698. [DOI] [PubMed] [Google Scholar]
- 11.Graybill, J. R. 1992. Future directions of antifungal chemotherapy. Clin. Infect. Dis. 14(Suppl. 1):S170-S181. [DOI] [PubMed] [Google Scholar]
- 12.Graybill, J. R., and S. R. Kaster. 1984. Experimental murine aspergillosis. Comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am. Rev. Respir. Dis. 129:292-295. [PubMed] [Google Scholar]
- 13.Groll, A. H., B. M. Gullick, R. Petraitiene, V. Petraitis, M. Candelario, S. C. Piscitelli, and T. J. Walsh. 2001. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob. Agents Chemother. 45:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob. Agents Chemother. 44:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano-Chiu, M., S. Arikan, V. L. Paetznick, E. J. Anaissie, and J. H. Rex. 1999. Optimizing voriconazole susceptibility testing of Candida: effects of incubation time, endpoint rule, species of Candida, and level of fluconazole susceptibility. J. Clin. Microbiol. 37:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, M. V., J. Yates, and C. A. Hitchcock. 1997. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob. Agents Chemother. 41:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Odds, F. C., M. Oris, P. Van Dorsselaer, and F. Van Gerven. 2000. Activities of an intravenous formulation of itraconazole in experimental disseminated Aspergillus, Candida, and Cryptococcus infections. Antimicrob. Agents Chemother. 44:3180-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson, T. F., A. W. Fothergill, and M. G. Rinaldi. 1993. Efficacy of itraconazole solution in a rabbit model of invasive aspergillosis. Antimicrob. Agents Chemother. 37:2307-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson, T. F., D. George, R. Ingersoll, P. Miniter, and V. T. Andriole. 1991. Efficacy of SCH 39304 in treatment of experimental invasive aspergillosis. Antimicrob. Agents Chemother. 35:1985-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson, T. F., D. George, P. Miniter, and V. T. Andriole. 1991. The role of fluconazole in the early treatment and prophylaxis of experimental invasive aspergillosis. J. Infect. Dis. 164:575-580. [DOI] [PubMed] [Google Scholar]
- 22.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, J. R. Graybill, et al. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, T. F., P. Miniter, J. Dijkstra, F. C. Szoka, Jr., J. L. Ryan, and V. T. Andriole. 1989. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J. Infect. Dis. 159:717-724. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, J., K. Schock, S. Marino, and V. T. Andriole. 2000. Efficacies of two new antifungal agents, the triazole ravuconazole and the echinocandin LY-303366, in an experimental model of invasive aspergillosis. Antimicrob. Agents Chemother. 44:3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryder, N. S. 1999. Activity of terbinafine against serious fungal pathogens. Mycoses 42:115-119. [PubMed] [Google Scholar]
- 26.Ryder, N. S., and I. Leitner. 2001. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med. Mycol. 39:91-95. [DOI] [PubMed] [Google Scholar]
- 27.Sanati, H., C. F. Ramos, A. S. Bayer, and M. A. Ghannoum. 1997. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob. Agents Chemother. 41:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]