Abstract
The incidence of cytomegalovirus (CMV) retinitis is declining in AIDS patients but remains a significant clinical problem in patients with organ transplants and bone marrow transplants. Prophylaxis with ganciclovir (GCV) or valganciclovir reduces the incidence of CMV disease but may lead to the emergence of drug-resistant virus with mutations in the UL97 or UL54 gene. It would be useful to have other types of oral therapy for CMV disease. We synthesized hexadecyloxypropyl and octadecyloxyethyl derivatives of cyclic cidofovir (cCDV) and cidofovir (CDV) and found that these novel analogs had 2.5- to 4-log increases in antiviral activity against CMV compared to the activities of unmodified CDV and cCDV. Multiple-log increases in activity were noted against laboratory CMV strains and various CMV clinical isolates including GCV-resistant strains with mutations in the UL97 and UL54 genes. Preliminary cell studies suggest that the increase in antiviral activity may be partially explained by a much greater cell penetration of the novel analogs. 1-O-Hexadecyloxypropyl-CDV, 1-O-octadecyloxyethyl-CDV, and their corresponding cCDV analogs are worthy of further preclinical evaluation for treatment and prevention of CMV and herpes simplex virus infections in humans.
Although the incidence and prevalence of cytomegalovirus (CMV) retinitis in AIDS patients are declining due to the use of highly active antiretroviral therapies (12), CMV continues to be a major cause of opportunistic infections in patients with allogeneic bone marrow transplants (BMTs) and solid-organ transplants (6). In transplant patients, the incidence of CMV infection increases with the duration and degree of immunosuppression, approximating 70% in allogeneic BMT patients who are CMV seropositive (2) and in patients receiving solid-organ transplants from CMV-seropositive donors (4, 18). CMV disease is associated with a high risk of morbidity and mortality in solid-organ transplant and allogeneic BMT patients (6). While prophylaxis with ganciclovir (GCV) significantly reduces the incidence of CMV disease in transplant recipients, drug resistance may emerge because of mutations in the UL97 gene, which catalyzes the initial phosphorylation of GCV, or in the UL54 polymerase gene of the virus (for a review, see reference 5). Current therapies for CMV disease in transplant patients are based primarily on intravenous therapy with GCV, cidofovir (CDV), or foscarnet (phosphonoformate) or, more recently, with oral valganciclovir.
It would be useful to identify more effective oral therapies for the treatment of CMV disease in allogeneic bone marrow, stem cell, or solid-organ transplant patients and in CMV retinitis patients with AIDS. We have developed a strategy to improve the antiviral activity and oral absorption of acyclovir (ACV) and GCV by covalently attaching alkoxyalkyl or alkoxyglyceryl residues to the phosphate of ACV monophosphate or GCV monophosphate (1, 8, 9). These ether lipid analogs generally show severalfold increases in activity over the activity of underivatized ACV or GCV and provide increased oral absorption in rodents (8). In woodchucks with hepatitis, 1-O-hexadecyloxypropyl-phospho-ACV reduced woodchuck hepatitis virus DNA levels in plasma by nearly 2 logs after 4 weeks of treatment with 10 mg/kg of body weight twice daily, but a five times greater oral dose of ACV (molar basis) had no effect (7).
To determine if more effective and less toxic forms of CDV or cyclic CDV (cCDV) can be designed, we synthesized several alkoxyalkyl analogs of these compounds and evaluated their antiviral activities against human CMV (HCMV) and herpes simplex virus (HSV) by DNA reduction and plaque reduction assays with cells infected with various wild-type and GCV-resistant strains of CMV and HSV type 1 (HSV-1). Surprisingly, we detected multiple-log enhancement of the in vitro antiviral activities of the alkoxyalkyl analogs compared with the activity of underivatized cCDV or CDV.
MATERIALS AND METHODS
Chemistry. (i) General.
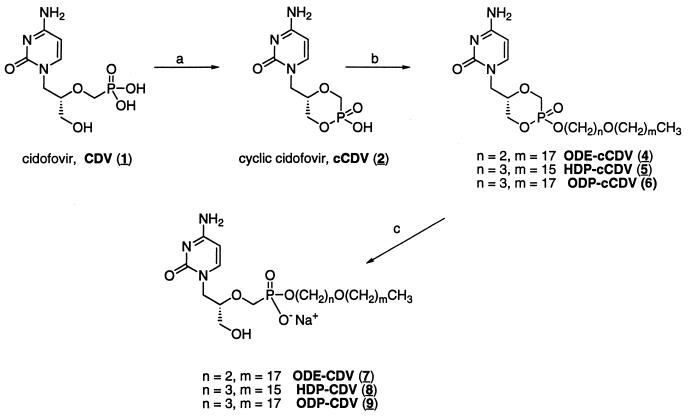
All products were homogeneous by thin-layer chromatography (TLC), performed on Analtech 250-μm Silica Gel GF Uniplates and visualized under UV light with phospray (Supelco, Bellafonte, Pa.) and by charring. Chromatographic purification was done by the flash method with Merck silica gel 60 (240 to 400 mesh). 1H and 31P nuclear magnetic resonance (NMR) spectra were recorded at 400 MHz on a Varian HG-400 spectrophotometer with tetramethylsilane (internal) and 85% D3PO4 in D2O (external) as references for 1H and 31P (0.00 ppm), respectively. Electrospray ionization mass spectroscopy (ESI) was performed by Mass Consortium (San Diego, Calif.). CDV (compound 1) was provided by Gilead Sciences, Inc. (Foster City, Calif.). The synthesis and characterization of compounds 2, 4, 5, 7, and 8 have been reported previously (10) (Fig. 1).
FIG. 1.
Synthesis of alkoxyalkyl analogs of CDV and cCDV. Reagents: a, N,N-dicyclohexyl-morpholinocarboxamidine, 1,3-dicyclohexylcarbodiimide, pyridine, 100°C; b, ODE-Br, ODP-Br, or HDP-Br, N,N-dimethylformamide, 80°C; c, 0.5 M NaOH. The underscored numbers in parentheses correspond to the compound numbers used throughout the report.
1-Bromo-3-octadecyloxypropane.
Triphenylphosphine (10.0 g, 38 mmol) was added to a cooled (0°C) solution of 3-octadecyloxy-1-propanol (5.0 g, 15 mmol) and carbon tetrabromide (10.6 g, 32 mmol) in dichloromethane (70 ml) in 2-g portions over 30 min. The reaction mixture was stirred for 45 min at 0°C and then for 1 h at room temperature. The reaction mixture was concentrated, and the residue was dissolved in ether. After the mixture was stirred for 1 h, it was filtered and the filtrate was concentrated. The residue was purified by flash chromatography. Elution with 90% hexane-10% ethyl acetate yielded 4.3 g (77%) as a colorless oil. 1H NMR δ 0.88 (t, 3-H), 1.25 (br s, 30-H), 1.56 (m, 2-H), 2.09 (p, 2-H), 3.42 (t, 2-H), 3.49 to 3.54 (two triplets, 4-H). Electrospray mass spectroscopy (MS), positive and negative, failed to give a molecular ion.
(ii) cCDV-hexadecyl ester (compound 3).
A mixture of compound 2 (188 mg, 0.34 mmol) and 1-bromohexadecane (520 mg, 1.8 mmol) in N,N-dimethylformamide (25 ml) was stirred and heated to 80°C for 6 h. The mixture was concentrated, and the residue was purified by flash chromatography. Elution with 10% methanol (MeOH)-90% CH2Cl2 yielded 58 mg of compound 3 (33%) as a white powder. 1H NMR δ (dimethyl sulfoxide [DMSO]-d6) 0.85 (t, 3-H), 1.23 (broad s, 26-H), 1.60 (m, 2-H), 3.55 to 4.20 (m, 9-H), 5.6 (dd, 1-H), 7.18 and 7.04 (broad d, 2-H), 7.57 and 7.45 (d, 1-H); 31P NMR δ +13.60 and +12.48 (mixture of axial and equatorial diastereomers) (13); MS (ESI) m/z 486 (M+ H)+, 484 (M− H)−. TLC Rf = 0.9 (CHCl3-MeOH-concentrated NH4OH-H2O; 80:20:1:1).
(iii) cCDV-octadecyloxypropyl ester (compound 6).
A mixture of compound 2 (1.02 g, 1.8 mmol) and 1-bromo-3-octadecyloxypropane (2.82 g, 7.5 mmol) in N,N-dimethylformamide (35 ml) was stirred and heated (80°C) for 6 h. The mixture was then concentrated in vacuo, and the residue was purified by flash chromatography. Elution with 10% MeOH-90% CH2Cl2 afforded 450 mg of a white powder (41% yield). High-pressure liquid chromatography, TLC, and spectroscopic analysis showed the presence of two diastereomeric (axial and equatorial) alkylation products. 1H NMR δ (DMSO-d6) 0.85 (t, 3-H), 1.23 (broad s, 30-H), 1.47 (m, 2-H), 1.84 (p, 2-H), 3.55 to 4.20 (m,13-H), 5.70 (dd, 1-H), 7.18 and 7.04 (broad d, 2-H), 7.55 and 7.45 (d, 1-H); 31P NMR δ +13.61 and +12.31; MS (ESI) m/z 572 (M+ H)+, 570 (M− H)−. TLC Rf = 0.9 (CHCl3-MeOH-concentrated NH4OH-H2O; 80:20:1:1).
(iv) CDV-octadecyloxypropyl ester (compound 9).
Compound 6 (230 mg, 0.38 mmol) was dissolved in 0.5 M NaOH (5 ml), and the mixture was stirred at room temperature for 1.5 h. The solution was neutralized with acetic acid, and the precipitate was isolated by filtration and then purified by flash column chromatography. The product (133 mg, 58%) was eluted with CH2Cl2-MeOH (70:30). 1H NMR δ (DMSO-d6) 0.86 (t, 3-H), 1.24 (broad s, 30-H), 1.47 (m, 2-H), 1.73 (p, 2-H), 3.20 to 3.89 (m, 13-H), 5.72 (m, 1-H), 7.21 (d, 2-H), 7.54 (d, 1-H); 31P NMR δ +13.98; MS (ESI) m/z 584 (M+ Na)+, 560 (M− H)−. TLC Rf = 0.27 (CHCl3-MeOH-concentrated NH4OH-H2O; 80:20:1:1).
Preparation of control and drug-containing liposomes for antiviral assays.
For the in vitro studies, 1-O-hexadecyloxypropyl-cCDV (HDP-cCDV), 1-O-hexadecyloxypropyl-CDV (HDP-CDV), 1-O-octadecyloxyethyl-cCDV (ODE-cCDV), 1-O-octadecyloxyethyl-CDV (ODE-CDV), 1-O-octadecyloxypropyl-cCDV (ODP-cCDV), 1-O-octadecyloxypropyl-CDV (ODP-CDV), and hexadecyl-cCDV (HD-cCDV) were incorporated into liposomes. Chloroform solutions of the phospholipids, cholesterol (CH), and drugs were mixed to provide dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylglycerol (DOPG), CH, and an alkoxyalkanol-CDV or alkoxyalkanol-cCDV analog at a molar ratio of 50/10/30/10. Control liposomes were prepared without drug and had a DOPC-DOPG-CH composition of 60/10/30. The chloroform was removed under a stream of nitrogen, and the thin lipid film was hydrated by the addition of 360 μl of 250 mM sorbitol-20 mM sodium acetate (pH 5.5). The small multiple-dose vial was sealed under nitrogen with a Teflon-lined cap and sonicated for 1 h at 42°C. The clear preparation of sonicated liposomes, representing a nominal drug concentration of 5 mM, was diluted sequentially with Dulbecco's modified Eagle's medium containing 4% fetal bovine serum to provide the indicated range of concentrations, and the medium was added to the virus-infected cells as indicated below.
Antiviral assays for activities against various strains of CMV and HSV-1 in vitro.
Antiviral activity against HCMV (AD169) or HSV-1 was determined by a DNA reduction assay with MRC-5 human lung fibroblast cells with DNA probes supplied by Diagnostic Hybrids, Athens, Ohio, as described previously (9) or by plaque reduction assay with human foreskin fibroblast cells infected with various strains of HCMV or HSV-1 (11). The results of antiviral assays with HDP-CDV presented to cells in dilute DMSO were similar to those obtained with the compound presented to cells in liposomes, and blank liposome controls had no effect on viral replication. The antiviral activities of the various alkoxyalkyl esters of CDV and cCDV were also determined in CMV-infected murine, rat, and guinea pig embryonic fibroblast cells by plaque reduction assays (11, 14). The cytotoxic concentration of drug which reduced the viable cell number by 50% (CC50) was determined. In the plaque reduction assays, cytotoxicity was determined by measurement of neutral red uptake (14).
RESULTS
MRC-5 human lung fibroblasts were infected with HCMV (AD169) or HSV-1, and the antiviral activities of CDV and cCDV were assessed by DNA reduction assay (Table 1). Against HCMV the 50% effective concentrations (EC50s) for CDV and cCDV were similar (0.46 to 0.47 μM). The alkoxyalkyl analogs ODE-CDV, ODP-CDV, and HDP-CDV were 4 to 5 logs more active against HCMV, with EC50s ranging from 2 × 10−6 to 3 × 10−5 μM. In cells infected with HSV-1, CDV and cCDV reduced viral replication by 50% at 3.3 and 2.3 μM, respectively. Again, the alkoxyalkyl analogs of CDV were most active, with EC50s of 0.0001 to 0.003 μM. HDP-CDV was the most active of these three compounds. The alkoxyalkyl analogs of cCDV were less active than the corresponding CDV compounds. We also synthesized the 16-carbon straight-chain alkyl ester of cCDV, HD-cCDV, which lacks the oxygen group two or three carbons from the ester functionality. Interestingly, this compound is 133 to 400 times less active than ODE- or HDP-cCDV, esters of octadecylethanediol and hexadecylpropanediol, respectively (Table 1). The cytotoxicities of the alkoxyalkyl esters of CDV and cCDV in MRC-5 cells were greater than those observed with CDV or cCDV, but the selectivities of the HDP, ODE, and ODP derivatives of cCDV and CDV against CMV or HSV-1 increased greatly because of the marked increases in antiviral activity. In contrast, the compound lacking the oxygen heteroatom in the alkyl chain, HD-cCDV, exhibited greater toxicity and less antiviral activity (Table 1).
TABLE 1.
Antiviral activities and selectivities of CDV compounds in HCMV or HSV-1-infected MRC-5 human lung fibroblast measured by DNA reduction assaya
| Compound | EC50 (μM)
|
Toxicity (CC50 [μM]) | Selectivity for:
|
||
|---|---|---|---|---|---|
| HCMV | HSV-1 | HCMV | HSV-1 | ||
| CDV | 0.46 ± 0.08 (4) | 3.3 ± 3.7 (3) | >1,000 | >303 | >2,174 |
| ODE-CDV | 2 × 10−5 ± 3 × 10−5 (6) | 0.001 ± 0.002 (3) | 210 | 2 × 105 | 10 × 106 |
| HDP-CDV | 2 × 10−6 ± 3 × 10−6 (4) | 0.0001 ± 0.0001 (4) | 10 | 1 × 105 | 5 × 106 |
| ODP-CDV | 3 × 10−5 ± 4 × 10−5 (4) | 0.003 ± 0.001 (3) | 32 | 1 × 104 | 1 × 106 |
| cCDV | 0.47 ± 0.13 (3) | 2.3 ± 1.5 (3) | >1,000 | >2,128 | >435 |
| HD-cCDV | 0.04 ± 0.01 (3) | 3.1 ± 2.4 (3) | 6.5 | 163 | 2.0 |
| ODE-cCDV | 1 × 10−4 ± 1 × 10−4 (4) | 0.005 ± 0.005 (3) | 320 | 3 × 106 | 6 × 104 |
| HDP-cCDV | 3 × 10−4 ± 3 × 10−4 (6) | 0.04 ± 0.03 (3) | 320 | 1 × 106 | 8 × 103 |
| ODP-cCDV | 3 × 10−4 ± 3 × 10−4 (3) | 0.35 ± 0.18 (3) | 320 | 1 × 106 | 900 |
The values are means ± standard deviations. Numbers in parentheses represent number of replicates. Selectivity is CC50/EC50.
We also evaluated the activities of the analogs of CDV and cCDV against HSV-1 and HSV-2 by the plaque reduction assay. CDV and cCDV appeared to be less active against HSV-1 by the plaque reduction assay (Table 2) than by the DNA reduction assay, with EC50s of 18.0 and 30.6 μM, respectively, compared with EC50s of 3.3 and 2.3 μM, respectively, by the DNA reduction assay (Table 1). The EC50s of the alkoxyalkyl analogs were also higher by the plaque reduction assay than by the DNA reduction assay. Nevertheless, increases in antiviral activity of 2.39 to 2.81 logs were noted with HDP-CDV and ODE-CDV, respectively, compared with the activity of unmodified CDV. Somewhat lesser increases in activity were noted with the analogs of cCDV versus those of unmodified cCDV when the activities were measured by the plaque reduction assay (Table 2).
TABLE 2.
Activities of alkoxyalkyl esters of CDV and cCDV against HSV-1 and HSV-2 by plaque reduction assay
| Compound | EC50 (μM)
|
|||
|---|---|---|---|---|
| HSV-1
|
HSV-2
|
|||
| Assay 1 | Assay 2 | Assay 1 | Assay 2 | |
| CDV | 22.5 | 7.9 | 13.2 | 7.9 |
| HDP-CDV | 0.08 | 0.04 | 0.13 | 0.03 |
| ODE-CDV | 0.03 | 0.012 | 0.03 | 0.03 |
| cCDV | 28.4 | 10.6 | 11.2 | 7.6 |
| HDP-cCDV | 0.9 | 0.25 | 0.35 | 0.11 |
| ODP-cCDV | 0.5 | 0.2 | 0.28 | 0.12 |
The antiviral activities of the HDP and ODE analogs of cCDV and CDV were also examined by the plaque reduction assay with human foreskin fibroblast cells infected with various laboratory strains and clinical isolates of HCMV, and the antiviral activities of these compounds were compared with those of GCV, cCDV, and CDV (Table 3). In general, when the antiviral activities of CDV and cCDV were compared to those of the respective HDP and ODE esters, multiple-log increases in antiviral activities were observed. For example, for strain AD169, the EC50 of CDV was 0.38 μM, whereas the EC50s of both HDP-CDV and ODE-CDV were 0.0009 μM, representing increases in activity of 2.6 logs for the new analogs. Similar results were obtained with the Towne, Davis, and C9208/5-4-2 strains of wild-type HCMV (Table 3). Although the Toledo strain was much less sensitive to CDV (EC50, 13.8 μM), the HDP-CDV and ODE-CDV analogs were both substantially more active, with EC50s of 0.025 μM, representing an increase in antiviral activity of 2.74 logs. Nearly 3-log increases in antiviral activities were noted with the alkoxyalkanol analogs of CDV against cells infected with the Coffman, C8708/17-1-1, and C9208/5-4-2 strains of CMV. Similar findings were obtained with HDP-cCDV and ODE-cCDV, except that these analogs were generally somewhat less active than the corresponding analogs of CDV (Table 3).
TABLE 3.
Activities of GCV, CDV, cCDV, and alkoxyalkyl esters of CDV and cCDV against HCMV replication measured by plaque reduction assay
| Isolatea | EC50 (μM)b
|
||||||
|---|---|---|---|---|---|---|---|
| GCV | CDV | HDP-CDV | ODE-CDV | cCDV | HDP-cCDV | ODE-cCDV | |
| AD169 | 2.75 ± 1.6 | 0.38 ± 0 | 0.0009 ± 0.0001 | 0.0009 ± 0.0001 | 0.31 ± 0.02 | 0.001 ± 0 | 0.0018 ± 0.001 |
| Towne | 4.3 ± 0 | 0.4 ± 0.11 | 0.0009 ± 0 | 0.0009 ± 0.0001 | 0.48 ± .02 | 0.001 ± 0 | 0.001 ± 0 |
| Davis | 5.1 ± 0 | 0.66 ± 0.3 | 0.00095 ± 0.00007 | 0.0009 ± 0.0001 | 0.45 ± 0.07 | 0.001 ± 0 | 0.001 ± 0 |
| Toledo | 19.6 ± 7.2 | 13.8 ± 7.3 | 0.025 ± 0.007 | 0.025 ± 0.02 | 17.1 ± 8 | 0.055 ± 0.03 | 0.055 ± 0.03 |
| Coffman | 4.7 ± 0 | 0.87 ± 0.15 | 0.001 ± 0 | 0.001 ± 0 | 1.2 ± 0.5 | 0.0015 ± 0.0007 | 0.002 ± 0.0007 |
| C8708/17-1-1 | 2.6 ± 0.9 | 1.1 ± 0.5 | 0.001 ± 0 | 0.001 ± 0 | 2.1 ± 0.4 | 0.0015 ± 0.0007 | 0.0025 ± 0.002 |
| C9208/3-3-1 | 4.25 ± 1.2 | 0.95 ± 0.6 | 0.001 ± 0 | 0.001 ± 0 | 1.3 ± 0.3 | 0.00095 ± 0.00007 | 0.001 ± 0 |
| C9208/5-4-2 | 1.6 ± 0.42 | 0.41 ± 0.09 | 0.00085 ± 0.0002 | 0.001 ± 0 | 1.1 ± 0.26 | 0.00095 ± 0.00007 | 0.001 ± 0 |
All isolates are wild type.
The values are the means ± standard deviations of two or more determinations.
The activities of GCV, CDV, cCDV, and the alkoxyalkyl esters of CDV and cCDV were also evaluated against a panel of drug-resistant HCMV mutants kindly provided to E. R. Kern by Karen Biron of GlaxoSmithKline, Research Triangle Park, N.C., and Donald Coen, Boston, Mass. (Table 4). The EC50s of GCV for GCV-resistant strains with mutations in the UL97 gene were 3.7 to 16.4 times greater than the average EC50 (3.61 μM) for the seven wild-type strains (Table 5). CDV and cCDV retained nearly full activity against the strains with mutations in the UL97 gene, and their alkoxyalkyl esters were 2.5 to 2.98 logs more active than the underivatized nucleotide phosphonates. A mutant of CMV with a mutation in the DNA polymerase gene (UL54), mutant GDGP53, exhibited 15 to 22 times greater resistance to cCDV and CDV and 15 times greater resistance to GCV than the wild type. Interestingly, the alkoxyalkyl esters of cCDV and CDV retained substantial activities against this mutant with a mutation in the DNA polymerase gene; HDP-CDV and ODE-CDV both had EC50s of 0.02 μM, a 3.4-log increase in activity compared with that of unmodified CDV. HDP-cCDV and ODE-cCDV were also active, showing 2.4- to 2.5-log increases in activity compared with that of cCDV against the mutant with the polymerase mutation. A double mutant, mutant 759D100, which has mutations in both the DNA polymerase (G987A in UL54) and in UL97 (deletion of 590 to 593 in UL97) was the mutant most resistant to GCV, but it was somewhat less cross resistant than the mutant with a mutation in the polymerase gene, mutant GDGP53. The antiviral activities of the alkoxyalkanol analogs of cCDV and CDV against mutant 759D100 were intermediate between those against the mutant with the UL97 gene mutation and the mutant with the polymerase gene mutation. Against mutant 759D100, the HDP and ODE esters of cCDV and CDV were 2.2 to 2.5 logs more active than the unmodified phosphonates (Table 5).
TABLE 4.
Drug-resistant HCMV isolates
| Isolate | Drug(s) to which the isolate is resistant | Affected gene | Mutation | Refer- ence |
|---|---|---|---|---|
| C9209/1-4-4a | GCV | UL97 | M460V | 3 |
| C8914-6a | GCV | UL97 | L595F | 3 |
| C8805/37-1-1a | GCV | UL97 | M460V | 3, 15 |
| GDGP53b | GCV, CDV | UL54 (Pol) | G987A | 16 |
| 759D100b | GCV | UL54/UL97 | G987A/Δ590-93 | 17 |
Provided by Karen Biron.
Provided by Donald Coen.
TABLE 5.
Effects of GCV, CDV, cCDV, and alkoxyalkyl esters against drug-resistant isolates of HCMV by plaque reduction assaya
| Isolate | EC50 (μM)
|
||||||
|---|---|---|---|---|---|---|---|
| GCV | CDV | HDP-CDV | ODE-CDV | cCDV | HDP-cCDV | ODE-cCDV | |
| Wild typea | 3.61 ± 1.3 | 0.68 ± 0.29 | 0.0009 ± 0.0006 | 0.00096 ± 0.00005 | 0.99 ± 0.63 | 0.0011 ± 0.0003 | 0.0015 ± 0.0006 |
| Drug-resistant mutants | |||||||
| C9209/1-4-4 | 59.3 ± 0.2 | 2.3 ± 0.28 | 0.003 ± 0 | 0.0040 ± 0.001 | 1.95 ± 0.9 | 0.005 ± 0 | 0.0045 ± 0.0007 |
| C8914-6 | 13.5 ± 2 | 0.99 ± 0.01 | 0.0025 ± 0.0007 | 0.0010 ± 0 | 1.45 ± 0.21 | 0.005 ± 0.001 | 0.0045 ± 0.0007 |
| C8805/37-1-1 | 47.4 ± 1.1 | 0.84 ± 0.2 | 0.00095 ± 0.00007 | 0.00095 ± 0.00007 | 0.96 ± 0.2 | 0.001 ± 0 | 0.0010 ± 0 |
| GDGP53 | 54.6 ± 23 | 15.7 ± 14.1 | 0.020 ± 0.009 | 0.020 ± 0.008 | 15.9 ± 12.2 | 0.06 ± 0.05 | 0.050 ± 0.03 |
| 759D100 | 177 ± 28.2 | 2.0 ± 0.56 | 0.0065 ± 0.0007 | 0.0055 ± 0.0007 | 2.5 ± 0.1 | 0.015 ± 0.007 | 0.0150 ± 0.004 |
The values are the means ± standard deviations for the seven wild-type strains for which the results are presented in Table 3 (the Toledo strain was omitted from the analysis).
HDP-CDV and ODE-CDV were also highly active against a panel of strains of CMV from animals, including CMV strains from the mouse, rat, and guinea pig. The most active compounds were HDP-CDV and ODE-CDV, with EC50s of 0.0009 to 0.005 μM, whereas the EC50 of unmodified CDV was 0.26 μM. Similar trends were noted with the cCDV series of compounds (Table 6).
TABLE 6.
Activities of alkoxyalkyl esters of CDV and cCDV against murine, rat, and guinea pig CMV strains
| Compound | EC50 (μM)a
|
||
|---|---|---|---|
| MCMV | RCMV | GpCMV | |
| GCV | 9.0 ± 4.9 | 48.2 ± 1.1 | 239.0 ± 10.6 |
| CDV | 0.26 ± 0.02 | 0.30 ± 0.03 | 0.31 ± 0.003 |
| HDP-CDV | 0.0009 ± 0 | 0.004 ± 0.001 | 0.0009 ± 0.00007 |
| ODE-CDV | 0.001 ± 0 | 0.005 ± 0 | 0.0009 ± 0.00007 |
| cCDV | 0.39 ± 0.05 | 0.46 ± 0.05 | 0.50 ± 0.26 |
| HDP-cCDV | 0.003 ± 0 | 0.005 ± 0 | 0.001 ± 0 |
| ODE-cCDV | 0.004 ± 0 | 0.005 ± 0 | 0.001 ± 0 |
The values are the means ± standard deviations of two assays. Abbreviations: MCMV, murine CMV; RCMV, rat CMV; GpCMV, guinea pig CMV.
DISCUSSION
The covalent addition of an alkoxyalkyl ester group to the phosphonate of CDV or cCDV resulted in remarkable increases in antiviral activities against CMV and HSV-1 in vitro. The cytotoxicities of the analogs were also increased, but selectivity (CC50/EC50) was increased substantially in most cases. When the activities were measured by plaque reduction assay, the increases in activities observed with the alkoxyalkyl analogs of CDV and cCDV were 1 to 2 logs less than those noted by DNA reduction assay. However, the activities of CDV and cCDV were similar by the two assays. Alkyl ether analogs of both ethanediol and propanediol were highly active. Although we have not done extensive structure-activity analyses, both 16- and 18-carbon alkyl ether chains were highly effective. By the DNA reduction assay, the analogs of cCDV were less active than the corresponding CDV compounds. Interestingly, when cCDV was coupled directly to hexadecanol, a long-chain alcohol 16 carbons in length, a 2-log drop in antiviral activity was noted, demonstrating the importance of the oxygen heteroatom. The oxygen heteroatom may make the analogs subject to rapid enzymatic conversion to cCDV or CDV, precursors of the active antiviral CDV diphosphate (CDV-PP). This must be confirmed by metabolic studies comparing the conversion to cCDV by using radiolabeled HD-cCDV and HDP-cCDV incubated with cell homogenates or subcellular membrane fractions. Preliminary studies indicate that HDP-CDV is metabolized by an intracellular enzyme of the phospholipase C type (unpublished observation).
The HDP and ODE derivatives of CDV exhibited 2.5- to 4-log increases in antiviral activity depending on the antiviral assay used (Tables 1 and 2). The mechanism of the increased activity remains to be determined. However, CDV enters cells by pinocytosis, which may greatly restrict passage of unmodified drug into cells. Preliminary studies in our laboratory with 14C-labeled CDV and HDP-CDV indicate that the amount of HDP-CDV that enters the cell is increased by several logs; intracellular CDV-PP can easily be detected when 10 μM HDP-CDV is used, but when 10 μM CDV is used, the intracellular levels of CDV-PP are substantially lower (unpublished data). Full assessment of the mechanisms of the increased activities of HDP-CDV and ODE-CDV awaits determination of comparative levels of intracellular CDV monophosphate and CDV-PP in cells incubated with equimolar concentrations of 14C-labeled CDV and the respective analogs. However, our preliminary studies suggest that enhanced cell uptake is a major factor in the 2.5- to 4-log increases in antiviral activity which have been documented.
The alkoxyalkyl analogs of CDV and cCDV also showed multiple-log increases in antiviral activities against multiple clinical isolates of HCMV (Table 3). One of these isolates, Towne, exhibited reduced susceptibility to GCV, cCDV, and CDV, with EC50s 3 to 10 times greater than those for laboratory HCMV and clinical isolates. Although the Towne strain was also relatively resistant to the alkoxyalkyl analogs of CDV, the EC50s were still low (0.025 to 0.055 μM) and were more than 2.5 logs lower than those of underivatized CDV or cCDV. In addition, all of the alkoxyalkyl analogs tested had multiple-log increases in activity compared with that of CDV against GCV-resistant HCMV mutants (Table 5). Most of these strains have mutations in the UL97 gene, which controls the phosphorylation of GCV. Interestingly, a DNA polymerase mutant, mutant GDGP53, which exhibits about 10-fold greater resistance to CDV and cCDV and 50-fold greater resistance to GCV than the wild type, remains sensitive to HDP-CDV and ODE-CDV, with EC50s of 0.02 μM (Table 5). Although this represents a 20-fold decrease in antiviral activity compared with that of wild-type strains of HCMV, HDP-CDV may still be useful against mutants of this type in vivo. Similar results were observed with a double mutant with mutations in both the UL97 and the UL54 genes.
HDP-CDV and ODE-CDV are also highly active against orthopoxviruses such as vaccinia virus and cowpox virus (10) and monkeypox virus and smallpox virus (John Huggins, personal communication). The EC50s of HDP-CDV and ODE-CDV for the various poxviruses are in the range of 0.01 to 0.8 μM, making these agents of interest as potential treatments for smallpox, should the disease reappear.
In conclusion, long-chain alkyl ethers of propanediol or ethanediol covalently linked to cCDV or CDV provide multiple-log increases in antiviral activity against laboratory wild-type strains, various clinical isolates, and GCV-resistant strains of HCMV in vitro. The most active compounds were HDP-CDV and ODE-CDV, with EC50s of 2 × 10−6 and 2 × 10−5 μM, respectively. Based on our previous research, compounds of this type may be orally bioavailable (6-8). Further evaluation of this approach is warranted to assess the suitability of HDP-CDV and ODE-CDV for further development for the treatment or prevention of human infections with the herpesvirus group of viruses and poxviruses.
Acknowledgments
This work was supported in part by NIH grants EY11834, DAMD 17-01-2-0071, AI41928, and AI-29164; by the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Medical Center; and by Public Health Service contracts NO1-AI-85347 and NO1-AI-15439 from the National Institutes of Allergy and Infectious Diseases, NIH, Bethesda, Md.
REFERENCES
- 1.Beadle, J. R., G. D. Kini, K. A. Aldern, M. F. Gardner, K. N. Wright, R. J. Ryback, E. R. Kern, and K. Y. Hostetler. 2000. Synthesis and antiviral evaluation of 1-O-hexadecylpropane-diol-3-P-acyclovir: efficacy against HSV-1 infection in mice. Nucleosides Nucleotides 19:471-480. [DOI] [PubMed] [Google Scholar]
- 2.Boeckh, M., T. A. Gooley, D. Myerson, T. Cunningham, G. Schoch, and R. A. Bowden. 1996. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogenic marrow transplantation: a randomized double-blind study. Blood 88:4063-4071. [PubMed] [Google Scholar]
- 3.Chou, S., A. Erice, M. C. Jordan, G. M. Vercellotti, K. R. Michels, C. L. Talarico, S. C. Stanat, and K. K. Biron. 1995. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J. Infect. Dis. 171:576-583. [DOI] [PubMed] [Google Scholar]
- 4.Dunn, D. L., K. J. Gillingham, M. A. Kramer, W. J. Schmidt, A. Enrice, H. H. Balfour, Jr., P. F. Gores, R. W. Gruessner, A. J. Matas, W. D. Payne, D. E. R. Sutherland, and J. S. Najarian. 1994. A prospective randomized study of acyclovir versus ganciclovir plus human immune globulin prophylaxis of cytomegalovirus infection after solid organ transplant. Transplantation 57:876-884. [DOI] [PubMed] [Google Scholar]
- 5.Erice, A. 1999. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 12:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebart, H., L. Kanz, G. Jahn, and H. Einsele. 1998. Management of CMV infection after solid organ or stem-cell transplantation: current guidelines and future prospects. Drugs 55:59-72. [DOI] [PubMed] [Google Scholar]
- 7.Hostetler, K. Y., J. R. Beadle, W. E. Hornbuckle, C. A. Bellezza, I. A. Tochkov, P. J. Cote, J. L. Gerin, B. E. Korba, and B. C. Tennant. 2000. Antiviral activities of oral 1-O-hexadecylpropanediol-3-phosphoacyclovir and acyclovir in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 44:1964-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hostetler, K. Y., J. R. Beadle, G. D. Kini, M. F. Gardner, K. N. Wright, T.-H. Wu, and B. A. Korba. 1997. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir in hepatitis B virus infection in vitro. Biochem. Pharmacol. 53:1815-1822. [DOI] [PubMed] [Google Scholar]
- 9.Hostetler, K. Y., R. J. Rybak, J. R. Beadle, C. B. Hartline, M. F. Gardner, K. A. Aldern, K. N. Wright, and E. R. Kern. 2001. In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3-phospho-penciclovir in cytomegalovirus and herpes simplex virus infections. Antivir. Chem. Chemother. 11:373-381. [DOI] [PubMed] [Google Scholar]
- 10.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxylalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern, E. R., J. C. Overall, Jr., and L. A. Glasgow. 1973. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine, J. Infect. Dis. 128:290-299. [DOI] [PubMed] [Google Scholar]
- 12.Murphy, E. L., A. C. Collier, L. A. Kalish, S. F. Assmann, M. F. Para, T. P. Flanigan, P. N. Kumar, L. Mintz, F. R. Wallach, and G. J. Nemo. 2001. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann. Intern. Med. 135:17-26. [DOI] [PubMed] [Google Scholar]
- 13.Oliyai, R., J.-P. Shaw, C. M. Sueoke-Lennen, K. C. Cundy, M. N. Arimilli, R. J. Jones, and W. A. Lee. 1999. Aryl ester prodrugs of cyclic HPMPC I: physicochemical characterization and in vitro biological stability. Pharm. Res. 16:1687-1693. [DOI] [PubMed] [Google Scholar]
- 14.Qiu, Y.-L., M. B. Ksebati, R. G. Ptak, B. Y. Fan, J. M. Breitenback, J.-S. Lin, Y.-C. Cheng, E. R. Kern, J. C. Drach, and J. Zemlicka. 1998. (Z)- and (E)-2-((Hydroxymethyl)cyclopropylidene)methyladenine and -guanosine. New nucleoside analogs with a broad-spectrum antiviral activity. J. Med. Chem. 41:10-23. [DOI] [PubMed] [Google Scholar]
- 15.Stanat, S. C., J. E. Reardon, A. Erice, M. C. Jordan, W. L. Drew, and K. K. Biron. 1991. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob. Agents Chemother. 35:2191-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan, V., K. K. Biron, C. Talarico, S. C. Stanat, M. Davis, L. M. Pozzi, and D. M. Coen. 1993. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Chemother. 37:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 18.Winston, D. J., D. Wirin, A. Shaked, and R. W. Busuttil. 1995. Randomized comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet 346:69-74. [DOI] [PubMed] [Google Scholar]