Abstract
Cochleates containing amphotericin B (CAMB) were administered orally at doses ranging from 0 to 40 mg/kg of body weight/day for 14 days in a murine model of systemic aspergillosis. The administration of oral doses of CAMB (20 and 40 mg/kg/day) resulted in a survival rate of 70% and a reduction in colony counts of more than 2 logs in lungs, livers, and kidneys. Orally administered CAMB shows promise for the treatment of aspergillosis.
Aspergillus fumigatus causes a variety of diseases, including allergic bronchopulmonary aspergillosis in asthma patients and invasive pulmonary aspergillosis in immunocompromised patients (1, 7). Invasive pulmonary aspergillosis can be treated with broad-spectrum triazoles (2, 14) and echinocandins (14), yet amphotericin B (AmB) therapy remains the preferred treatment for severe Aspergillus infections (12). Unlike other classes of antifungals, AmB is highly toxic and shows poor oral bioavailability. In order to increase the therapeutic index of AmB, new lipid-based formulations have been developed (4, 5, 8). Despite improvement in the therapeutic index for liposomal AmB formulations, the overall prognosis for patients with invasive disease remains poor. Recently, cochleate delivery vehicles for amphotericin B have been introduced as a new platform for overcoming the poor oral bioavailability of AmB (9, 16, 17). It has been shown that orally administered cochleates containing amphotericin B (CAMB) were as effective as intraperitoneally (i.p.) administered deoxycholate AmB (DAMB) in protecting against mortality and reducing the fungal burden of tissues in a murine model of candidiasis (9). In this paper, we describe the efficacy in vivo of CAMB delivered orally in a mouse model of systemic aspergillosis.
CAMB were prepared by use of an aqueous/aqueous hydrogel binary system (9) and produced an MIC of <1 μg/ml for A. fumigatus challenge strain MSKCC R21 when tested in RPMI 1640 medium according to NCCLS protocol M38-P. CAMB were evaluated in a systemic aspergillosis model adapted from that described by Verweij and colleagues (13). Female BALB/c mice (20 to 22 g) were rendered susceptible to A. fumigatus infection by treatment with cyclophosphamide at 150 to 200 mg/kg of body weight via intravenous injection through the lateral tail vein 3 days prior to infection with 0.1 ml of saline containing 106 spores of A. fumigatus (MSKCC R21). Treatment was initiated immediately after infection by oral administration of CAMB for 14 days at 0 to 20 mg/kg/day for the group treated with 150 mg of cyclophosphamide/kg and at 0 to 40 mg/kg/day for the group treated with 200 mg of cyclophosphamide/kg. DAMB administered i.p. at 4 mg/kg/day was used as a positive control. Survival rates and tissue burden (CFU per gram) in kidneys, livers, and lungs were determined for each treatment.
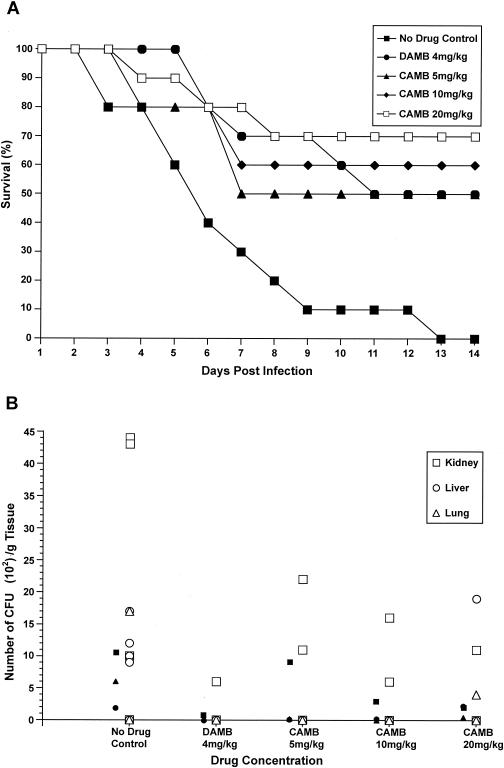
The efficacy in vivo of orally administered CAMB was evaluated in two separate trials of the disseminated aspergillosis model in which mice (10 per group) were treated daily for 14 days. In trial 1 (Fig. 1A), immunosuppression was induced with cyclophosphamide at 150 mg/kg and CAMB was administered orally (p.o.) at doses of 5, 10, and 20 mg/kg. Untreated control animals showed 100% mortality after 13 days. DAMB administered i.p. at 4 mg/kg/day resulted in 50% survival after 14 days. Orally administered CAMB produced a dose-dependent reduction of mortality, with CAMB at 10 mg/kg/day showing protection comparable to that of i.p.-delivered DAMB at 4 mg/kg/day at day 10 (P < 0.007). A dose-dependent reduction in fungal tissue burden, a semiquantitative parameter for Aspergillus, was observed for all target organs (kidney, liver, and lungs), with CAMB at 5 mg/kg/day being equivalent to DAMB in its ability to reduce organ load in the liver and lungs (P < 0.05) while CAMB at 20 mg/kg was required for maximum reduction in the kidneys.
FIG. 1.
(A) Survival of mice pretreated with cyclophosphamide at 150 mg/kg and then infected with A. fumigatus and given CAMB (p.o.) daily. Treatment of animals with CAMB (p.o.) at a dose of 5, 10, or 20 mg/kg/day or DAMB (i.p.) at a dose of 4 mg/kg/day resulted in a significant increase in survival relative to that of untreated (sham-treated) control animals, with conditional probability of survival values (Kaplan-Meier analysis; 95% confidence level) of P < 0.035, 0.008, 0.003, and 0.017, respectively. (B) Scatter plot indicating fungal tissue burden of the kidney (□), liver (○), and lungs (▵) of each animal treated as described above. Solid symbols represent the numeric averages of the fungal burden in a particular organ for all mice in a treatment group. A pair-wise comparison (Excel t test) of average CFU obtained from all organs at each drug concentration tested relative to the CFU from sham-treated control organs yielded significant differences between the results for treated and untreated animals (P < 0.05 for all drug concentrations tested).
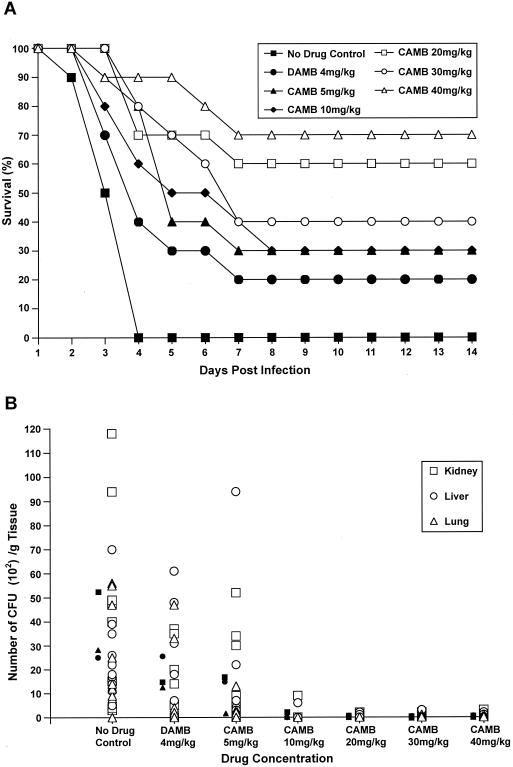
In trial 2, enhanced immunosuppression was achieved with cyclophosphamide at 200 mg/kg. As expected, the animals were more susceptible to infection, with 100% mortality observed after 4 days (Fig. 2A). CAMB increased survival in a dose-dependent manner, with CAMB at a dose of 40 mg/kg/day producing 90% survival after 4 days and 70% survival on day 14. In contrast, DAMB at 4 mg/kg showed only moderate protection. CAMB at 20 mg/kg produced 70% survival after 4 days and 60% survival on day 14. Lower doses also showed protection but typically produced less than 50% survival by day 5. The reduction in the tissue fungal burden of target organs largely paralleled the effects of CAMB on survival (Fig. 2B). A 2 to 3 log reduction in CFU/g of tissue was observed for CAMB (p.o.) at doses of ≥10 mg/kg/day, and Aspergillus was nearly eradicated at doses above 20 mg/kg/day.
FIG. 2.
(A) Survival of mice pretreated with cyclophosphamide at 200 mg/kg and then infected with A. fumigatus and given CAMB (p.o.) daily. Treatment of animals with CAMB (p.o.) at a dose of 5, 10, 20, 30, or 40 mg/kg/day or DAMB (i.p.) at a dose of 4 mg/kg/day resulted in a significant increase in survival relative to that of untreated (sham-treated) control animals, with conditional probability of survival values (Kaplan-Meier analysis; 95% confidence level) of P < 0.0010, 0.0070, 0.0004, 0.00017, 00016, and 0.0500, respectively. (B) Scatter plot indicating fungal tissue burden of the kidney (□), liver (○), and lungs (▵) of each animal treated as described above. A pair-wise comparison (Excel t test) of average CFU (solid symbols; see the legend to Fig. 1) from all organs at each drug concentration relative to the CFU from sham-treated control organs yielded significant differences between the results for treated and untreated animals, with P < 0.044 for CAMB at 5 mg/kg/day and P < 0.028 for CAMB at 10, 20, 30, and 40 mg/kg/day.
Amphotericin B remains the drug of choice for life-threatening invasive fungal infections. However, its narrow therapeutic index and adverse side effects, especially nephrotoxicity, limit its use (3, 10). Lipid-based delivery systems for AmB limit nephrotoxicity (6, 15) but are frequently utilized as second-line therapies because they are less stable and more expensive than DAMB. Recently, encapsulation of AmB in lipid-based cochleates resulted in particles that were highly efficacious when delivered orally in a murine model of candidiasis (9). In this study, the CAMB technology was extended to evaluate its efficacy for oral delivery in a murine aspergillosis model. It was found to be highly effective in reducing mortality and decreasing fungal tissue burden (Fig. 1 and 2). These results confirm that cochleates allow the systemic delivery of AmB after multiple oral dose administrations. A recent pharmacokinetic study confirmed that following multiple oral administrations of CAMB, AmB is distributed into the target tissues, with the highest concentration of AmB being in the kidneys, followed by the lungs, liver, spleen, and brain (11). In addition, an AmB concentration of 0.051 μg/ml was achieved in the blood following 10 consecutive-day doses at 10 mg/kg. The tissue-to-blood partition coefficient pattern was similar to the organ distribution pattern, indicating that there was predictable penetration of the drug in target tissues (11). The ability of cochleate vehicles to deliver systemic AmB after administration of multiple oral doses unambiguously points out the potential of CAMB formulations to treat and prevent invasive fungal infections. These preliminary experiments illustrate that CAMB is effective in reducing mortality from disseminated aspergillosis and support the notion that cochleates are promising vehicles for the oral delivery of AmB.
Acknowledgments
This research was supported by NIH contract no. AI-025141, SBIR Phase I no. 1 R43 AI46040-01 (L.Z.), SBIR Phase II no. 2 R44 AI46040-02 (L.Z.), and BioDelivery Sciences International, Inc.
REFERENCES
- 1.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 2.Denning, D. W., R. M. Tucker, L. H. Hanson, and D. A. Stevens. 1989. Treatment of invasive aspergillosis with itraconazole. Am. J. Med. 86:791-800. [DOI] [PubMed] [Google Scholar]
- 3.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 4.Gulati, M., S. Bajad, S. Singh, A. J. Ferdous, and M. Singh. 1998. Development of liposomal amphotericin B formulation. J. Microencapsul. 15:137-151. [DOI] [PubMed] [Google Scholar]
- 5.Hann, I. M., and H. G. Prentice. 2001. Lipid-based amphotericin B: a review of the last 10 years of use. Int. J. Antimicrob. Agents 17:161-169. [DOI] [PubMed] [Google Scholar]
- 6.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl.):S133-S144. [DOI] [PubMed] [Google Scholar]
- 7.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson, R. F., and M. C. Nahata. 1999. A comparative review of conventional and lipid formulations of amphotericin B. J. Clin. Pharm. Ther. 24:249-257. [DOI] [PubMed] [Google Scholar]
- 9.Santangelo, R., P. Paderu, G. Delmas, Z. W. Chen, R. Mannino, L. Zarif, and D. S. Perlin. 2000. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 44:2356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaya, B. P., J. P. Briggs, and J. Schnermann. 1995. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J. Am. Soc. Nephrol. 6:154-164. [DOI] [PubMed] [Google Scholar]
- 11.Segarra, I., D. A. Movshin, and L. Zarif. Tissue distribution of amphotericin B after multiple oral dosing of amphotericin B cochleate (CAMB) in a murine model. Pharm. Res., in press.
- 12.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 13.Verweij, P. E., K. L. Oakley, J. Morrissey, G. Morrissey, and D. W. Denning. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38:335-347. [PubMed] [Google Scholar]
- 15.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]
- 16.Zarif, L., J. R. Graybill, D. Perlin, L. Najvar, R. Bocanegra, and R. J. Mannino. 2000. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob. Agents Chemother. 44:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarif, L., J. R. Graybill, D. S. Perlin, and R. J. Mannino. 2000. Cochleates: new lipid-based drug delivery system. J. Liposome Res. 10:523-538. [Google Scholar]