Abstract
Salicylate induces multiple antibiotic resistance in various bacterial species. Here we investigated the effect of salicylate on the susceptibility of Mycobacterium tuberculosis to a range of antituberculosis (anti-TB) drugs. In the presence of salicylate, the killing effects of isoniazid (INH), rifampin (RMP), ethambutol (EMB), streptomycin (STR), and p-aminosalicylate (PAS) were reduced, as shown with a tetrazolium redox dye viability assay and a bacterial survival assay. Salicylate-induced resistance was more pronounced for PAS, STR, and EMB but was not apparent for INH and RMP when salicylate and the anti-TB agents were incorporated into 7H11 plates. The significance of these findings for TB treatment needs to be further evaluated in vivo.
Salicylate is a ubiquitous molecule that is involved in systemic acquired disease resistance in plants (9). Salicylate is the active component of aspirin, which is one of the most popular medicines used in the prevention and treatment of a wide variety of disease conditions. During the analysis of the effect of organic acids on the oxidative metabolism of the tubercle bacillus in the 1940s, Bernheim found that salicylate increased the oxygen consumption of the bacilli, whereas structurally related benzoic acid had a much weaker effect (1). Based on this observation, Lehmann and colleagues identified the salicylate analogue p-aminosalicylate (PAS) as an effective antituberculosis (anti-TB) agent (now used as a second-line TB drug) (5), even though the mode of action of PAS is probably not directly related to the effect of salicylate on tubercle bacilli.
Salicylate was first described to induce a multiple antibiotic resistance (Mar) phenotype in Escherichia coli (10), and it is now known to induce antibiotic resistance in a variety of bacterial species (9). In the E. coli system, salicylate induces antibiotic resistance by binding to MarR and activating the transcription of marA and marB. MarA and MarB regulate a range of genes that confer a Mar phenotype, including down-regulation of OmpF porin expression via micF antisense RNA to limit the entry of antibiotics (2) and switching on of efflux pumps such as AcrAB to more effectively extrude antibiotics from the cells (7). However, salicylate also induces antibiotic resistance through a Mar-independent pathway, since a Mar deletion strain still showed resistance in the presence of salicylate (2). In gram-positive bacteria such as Staphylococcus aureus, salicylate induces resistance to antibiotics such as fluoroquinolone (4), although the mechanism involved is unknown. In this study, we investigated the effect of salicylate on the susceptibility of Mycobacterium tuberculosis to anti-TB drugs and found that salicylate induced resistance to several anti-TB agents.
We first determined the sensitivity of M. tuberculosis to salicylate in order to assess the effect of salicylate on susceptibility to anti-TB drugs. Three-week-old stationary-phase M. tuberculosis H37Ra or H37Rv cultures or a 5-day-old Mycobacterium smegmatis strain mc26 culture grown in 7H9 liquid medium with albumin-dextrose-catalase (ADC) enrichment (Difco) were tested for susceptibility to varying concentrations of salicylate (sodium salt) on 7H11 agar at pH 6.8 as described previously (11). The susceptibility of E. coli strain DH5α to salicylate was determined on Luria-Bertani agar plates. M. tuberculosis strain H37Ra or H37Rv was found to have a salicylate MIC of 250 μg/ml (1.5 mM) on 7H11 agar plates at pH 6.8. On the other hand, M. smegmatis and E. coli were more resistant to salicylate, with a MIC of at least 1,000 μg/ml (6.25 mM) at pH 6.8 for both organisms.
Two types of assays, the tetrazolium redox dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay and the CFU assay, were used to assess the viability of the bacterial cells when exposed to anti-TB drugs in the presence of salicylate. MTT is a yellow redox dye that is converted to purple formazan by live cells, and the degree of bacterial cell viability can be assessed by the MTT assay (11, 12). A similar assay, called the Alamar Blue assay, is also a tetrazolium redox dye assay and has been used in antimycobacterial drug screens (3). We first established the sensitivity of the MTT assay as a measure of cell viability and its relationship to CFU counts. An M. tuberculosis H37Ra culture was grown in 7H9-ADC medium at 37°C for 4 weeks. The CFU count of the culture was determined to be 5 × 10 7 colonies/ml. Meanwhile, the same culture was diluted in 7H9 medium at 1:2, 4, 8, 16, 32, 64, 128, and 256 (in a volume of 200 μl) in Eppendorf tubes, followed by addition of 50 μl of a 2-mg/ml MTT solution. After incubation at 37°C for 2 h, the reaction was stopped by adding a drop of 10% sodium dodecyl sulfate to dissolve the purple formazan. The colored mixture was then resuspended and subjected to optical density measurements at 590 nm (OD590). A linear relationship between the MTT OD values and CFU was found at cell concentrations ranging from 2.5 × 106 to 5 × 107 bacilli/ml (data not shown). The MTT assay can be used satisfactorily to quantify the viability of the bacilli within appropriate cell concentrations.
To investigate the effect of salicylate on drug susceptibility in M. tuberculosis, an H37Ra cell suspension (107 bacilli/ml) was incubated with or without 0.5 mM salicylate and the following various anti-TB drugs: isoniazid (INH; 0.2 μg/ml), rifampin (RMP; 4 μg/ml), ethambutol (EMB; 2 μg/ml), streptomycin (STR; 8 μg/ml) and PAS (0.5 μg/ml). After incubation at 37°C for 3 days, the bacilli were washed with 7H9 medium and subcultured 1:10 to fresh 7H9 medium in triplicate in a 96-well microtiter plate. The plate was further incubated without shaking at 37°C for 6 days, after which the bacterial viability was assessed by using the MTT assay (12). The susceptibility of M. tuberculosis H37Ra to INH, PAS, and EMB was reduced as shown by the increase in viable cells manifested by higher MTT OD readings in the presence of 0.5 mM salicylate than in its absence (Table 1). However, salicylate did not appear to induce any significant resistance to STR and RMP as judged by the MTT assay (Table 1).
TABLE 1.
Effect of salicylate on susceptibility of M. tuberculosis to the drugs by the MTT assay
| Drug | MTT OD readings
|
|
|---|---|---|
| No salicylate | 0.5 mM salicylate | |
| Drug-free control | 1.58 ± 0.35 | 1.47 ± 0.09 |
| INH | 0.38 ± 0.02 | 0.55 ± 0.01 |
| EMB | 0.85 ± 0.15 | 1.40 ± 0.19 |
| RMP | 0.12 ± 0.01 | 0.13 ± 0.01 |
| STR | 0.26 ± 0.23 | 0.27 ± 0.23 |
| PAS | 0.65 ± 0.14 | 0.81 ± 0.02 |
In separate experiments, the M. tuberculosis H37Ra culture was preincubated with salicylate for 3 days, followed by addition of anti-TB drugs and then incubation for 3 days. The viability of the culture was determined with the MTT assay. Similar results as those shown in Table 1 were obtained, indicating that salicylate added either before or simultaneously with the anti-TB drugs did not appear to make a significant difference in inducing the drug resistance (data not shown). In addition, unlike salicylate, structurally related compounds such as aspirin and benzoic acid (sodium salt) did not induce drug resistance in M. tuberculosis (data not shown).
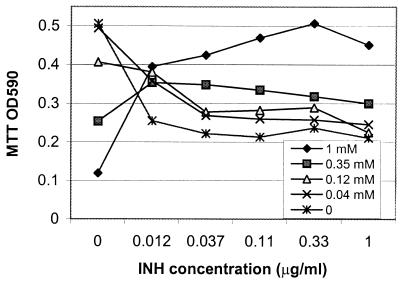
The effect of salicylate on INH susceptibility was analyzed in more detail. Various concentrations of salicylate and INH were added simultaneously to the H37Ra cell suspension (5 × 107 bacilli/ml). The mixtures were incubated at 37°C for 5 days and the viability of the bacilli was determined by the MTT assay. Salicylate induced significant resistance to INH at concentrations of 0.125 to 1 mM, especially at the higher salicylate concentration of 1 mM (Fig. 1). Surprisingly, INH also interfered with the inhibitory effect of salicylate at 0.35 and 1 mM, since salicylate alone at these two concentrations inhibited M. tuberculosis to a greater extent than in the presence of INH. The mechanism by which INH interferes with the activity of salicylate remains to be determined.
FIG. 1.
Effect of various concentrations of salicylate on induction of INH resistance. Various concentrations of salicylate (1, 0.35, 0.125, and 0.04 mM) and INH (1, 0.33, 0.11, 0.037, 0.012, and 0 μg/ml) were incubated with M. tuberculosis H37Ra bacilli (5 × 107/ml) in Sauton's medium in 96-well microtiter plates at 37°C without shaking for 5 days. The viability of the bacilli was assessed by the MTT assay and expressed as the OD590. The OD readings represent averages of duplicate samples.
To confirm the results of the MTT viability assay, we performed a CFU assay on an M. tuberculosis H37Ra culture treated with anti-TB drugs in the presence of salicylate. Salicylate at 0.125, 0.25, and 0.5 mM and INH, STR, EMB, and RMP at 0.2, 8, 2, and 4 μg/ml, respectively, were added simultaneously to H37Ra cell suspensions prepared from a 2-week-old culture in 7H9 medium. The mixtures were incubated at 37°C for 3 days, and the viability of bacilli was assessed by CFU counts on 7H11 plates after removal of the drugs and salicylate (Table 2 and Fig. 2). The presence of salicylate significantly reduced the killing effects of these drugs, with a 2.5-, 19-, 32-, and 40-fold higher CFU count for EMB, INH, STR, and RMP compared to values in the absence of salicylate. It is worth noting that while the CFU assay showed that salicylate significantly reduced the killing activity of STR and RMP (Table 2), the resistance induced by salicylate was not obvious in the MTT assay (Table 1). This is presumably due to differences in the nature of the two assays, as the MTT assay only measures the metabolic activity of the bacilli and may not correlate with CFU counts in bacterial populations that have a reduced metabolic activity but retain the ability to form CFU.
TABLE 2.
Effect of salicylate on susceptibility of M. tuberculosis to the drugs by the CFU assay
| Drug (concn) | CFU (103/ml)a
|
|
|---|---|---|
| No salicylate | Salicylate (0.125 mM) | |
| Drug-free control | 260 | 250 |
| INH (0.2 μg/ml) | 5.3 | 100 |
| SM (8 μg/ml) | 7.3 | 230 |
| RMP (2 μg/ml) | 4 | 160 |
| EMB (4 μg/ml) | 93 | 240 |
The CFU data in the table represent the average of triplicate samples.
FIG. 2.
Effect of salicylate exposure on inducing resistance to INH. Salicylate at 0 (A), 0.125 (B), 0.25 (C), or 0.5 (D) mM and INH at 0.2 μg/ml were added to an M. tuberculosis H37Ra cell suspension (5 × 107 bacilli/ml) derived from a 10-day-old culture with occasional agitation. The mixtures were incubated at 37°C for 3 days, and INH and salicylate were removed by washing. Triplicate dilutions (10−1, 10−2, 10−3, and 10−4, from left to right) of the washed bacterial cell suspension were spotted on 7H11 plates, which were then incubated for 4 weeks at 37°C. INH killed H37Ra more effectively in the absence of salicylate (A) than in its presence (B, C, and D).
The effect of salicylate was also investigated in a conventional drug susceptibility testing setting in which anti-TB agents and salicylate were incorporated into 7H11 agar medium. M. tuberculosis H37Ra cultures were grown in the presence or absence of salicylate (0.5 mM), followed by plating on 7H11 plates containing 0.2 mM salicylate or no salicylate and anti-TB agents. INH at 0.1 and 0.2 μg/ml, PAS at 0.5 and 1 μg/ml, EMB at 2 and 4 μg/ml, RMP at 2 and 4 μg/ml, and STR at 2 and 4 μg/ml were incorporated into the 7H11 agar medium. The plates were incubated at 37°C for 3 weeks, after which the effect of salicylate on drug susceptibility was examined. The salicylate effect was best seen with PAS and EMB (Fig. 3) and STR, but not for the other agents, INH and RMP (data not shown). Compared with the cultures grown in the absence of salicylate, the presence of salicylate (0.5 mM) in the liquid culture prior to exposure to drug did not make any significant difference in terms of inducing resistance to PAS, STR, or EMB. The presence of salicylate in the plate appeared to be more important in this test. The level of induced resistance in M. tuberculosis was less than two times the MIC and was best seen on plates containing various inocula sizes with the same drug concentration in the presence or absence of salicylate (Fig. 3). The level of salicylate-induced resistance was comparable to that of salicylate-induced fluoroquinolone resistance in S. aureus (4). To determine if the salicylate-induced drug resistance in the avirulent strain H37Ra is also true for the virulent strain, we tested M. tuberculosis H37Rv in a similar manner as described above for H37Ra by using the 7H11 plate assay with or without salicylate. Indeed, the same salicylate-induced resistance to PAS, STR, and EMB seen in avirulent strain H37Ra was also observed with virulent strain M. tuberculosis H37Rv.
FIG. 3.
Effect of salicylate on induction of resistance to PAS and EMB. The susceptibilities of M. tuberculosis H37Ra to PAS (1 μg/ml) and EMB (4 μg/ml) were determined on 7H11 plates. Triplicate dilutions (10−1, 10−2, and 10−3) of the H37Ra liquid culture with or without 0.5 mM salicylate were plated onto 7H11 agar plates with or without 0.2 mM salicylate, which were then incubated at 37°C for 3 weeks. The activities of PAS and EMB were reduced in the presence of salicylate.
In this study, by using different assays (MTT and CFU) we found that the presence of salicylate reduced the susceptibility of M. tuberculosis to several structurally unrelated antimycobacterial agents, including INH, EMB, PAS, STR, and RMP. Like E. coli (10), the level of salicylate-induced resistance in M. tuberculosis is generally low, no more than two times the MIC by the plate method. This low level of salicylate-induced resistance is also seen with other bacteria exposed to salicylate (4, 10). The decreased killing of bacteria by antibiotics, which causes an increased number of survivors in the presence of salicylate, has been shown to facilitate the emergence of genetically drug-resistant mutants (4). It remains to be determined if salicylate could facilitate emergence of drug-resistant M. tuberculosis mutants.
The mechanism of the salicylate-induced resistance in M. tuberculosis remains to be determined. One possible mechanism is that M. tuberculosis may have a Mar-like regulatory mechanism as in E. coli. Overexpression of the E. coli marA on a multicopy plasmid in the fast growing organism M. smegmatis mediates resistance to multiple antimycobacterial agents, indicating the presence of a mar-like regulatory system in this organism (6). However, MarA overexpression was not performed with M. tuberculosis in that study. A MarA homolog (Rv1931) is present in the M. tuberculosis genome; however, the degree of homology of Rv1931 to MarA is quite low. Preliminary studies showed that the Rv1931 mRNA was not induced by salicylate (data not shown), indicating that Rv1931 is unlikely to be responsible for the salicylate-induced drug resistance in M. tuberculosis. On the other hand, salicylate may reduce the permeability of the mycobacterial cell membrane as a mechanism for the salicylate induced drug resistance in M. tuberculosis. A preliminary study using C14-INH has suggested that the uptake of INH by M. tuberculosis H37Ra is reduced in the presence of salicylate (data not shown). The possibility of an involvement of efflux pumps in salicylate-induced resistance in M. tuberculosis remains to be tested. Further studies are needed to determine the precise mechanism of the salicylate-induced drug resistance in M. tuberculosis. Because salicylate is widely used in the prevention and treatment of diverse disease conditions at concentrations (up to 2 mM) (8) that are known to induce resistance to various drugs in M. tuberculosis in vitro, it will be of interest to assess the effect of salicylate on the treatment of TB in animal models and in humans.
Acknowledgments
This work was supported by NIH grants AI40584 and AI44063 and the Potts Memorial Foundation. A.S. was supported by a fellowship from the Swiss Science Foundation.
We thank Lee Rosner for critical reading of the manuscript and Hongling Guo for help with some experiments.
REFERENCES
- 1.Bernheim, F. 1940. The effect of salicylate on the oxygen uptake of the tubercle bacillus. Science 92:204.. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, S. P., S. B. Levy, J. Foulds, and J. L. Rosner. 1993. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 175:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, L., and S. G. Franzblau. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafson, J. E., P. V. Candelaria, S. A. Fisher, J. P. Goodridge, T. M. Lichocik, T. M. McWilliams, C. T. Price, F. G. O'Brien, and W. B. Grubb. 1999. Growth in the presence of salicylate increases fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 43:990-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann, J. 1946. Determination of pathogenicity of tubercle bacilli by their intermediate metabolism. Lancet 250:14-15. [DOI] [PubMed] [Google Scholar]
- 6.McDermott, P. F., D. G. White, I. Podglajen, M. N. Alekshun, and S. B. Levy. 1998. Multidrug resistance following expression of the Escherichia coli marA gene in Mycobacterium smegmatis. J. Bacteriol. 180:2995-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotz, P. H. 1985. In W. N. Kelley, E. D. Harris, S. Ruddy, and C. B. Sledge (ed.), Textbook of rheumatology, 2nd ed., vol. 2. Saunders, Philadelphia, Pa.
- 9.Price, C. T., I. R. Lee, and J. E. Gustafson. 2000. The effects of salicylate on bacteria. Int. J. Biochem. Cell. Biol. 32:1029-1043. [DOI] [PubMed] [Google Scholar]
- 10.Rosner, J. L. 1985. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 82:8771-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun, Z., and Y. Zhang. 1999. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuberc. Lung Dis. 79:319-320. [DOI] [PubMed] [Google Scholar]
- 12.Vandiviere, H. M., W. H. Gentry, and H. S. Willis. 1952. A rapid chemical test of total viability for suspensions of tubercle bacilli. Am. Rev. Tuberc. 66:95-98. [DOI] [PubMed] [Google Scholar]