Abstract
The pathogenesis of infection with Yersinia pestis, the causative agent of plague, was examined following subcutaneous infection of BALB/c mice with a fully virulent strain expressing green fluorescent protein. Plate culturing, flow cytometry, and laser confocal microscopy of spleen homogenates throughout infection revealed three discernible stages of infection. The early phase was characterized by the presence of a small number of intracellular bacteria mostly within CD11b+ macrophages and Ly-6G+ neutrophils. These bacteria were not viable, as determined by plate culturing of spleen homogenates, until day 2 postinfection. Between days 2 and 4 postinfection, a plateau phase was observed, with bacterial burdens of 103 to 104 CFU per spleen. Flow cytometric analysis revealed that there was even distribution of Y. pestis within both CD11b+ macrophage and Ly-6G+ neutrophil populations on day 2 postinfection. However, from day 3 postinfection onward, intracellular bacteria were observed exclusively within splenic CD11b+ macrophages. The late phase of infection, between days 4 and 5 postinfection, was characterized by a rapid increase in bacterial numbers, as well as escape of bacteria into the extracellular compartment. Annexin V staining of spleens indicated that a large proportion of splenic neutrophils underwent rapid apoptosis on days 1 and 2 postinfection. Fewer macrophages underwent apoptosis during the same period. Our data suggest that during the early stages of Y. pestis infection, splenic neutrophils are responsible for limiting the growth of Y. pestis and that splenic macrophages provide safe intracellular shelters within which Y. pestis is able to grow and escape during the later stages of infection. This macrophage compliance can be overcome in vitro by stimulation with a combination of gamma interferon and tumor necrosis factor alpha.
The gram-negative bacterium Yersinia pestis is the etiological agent of plague. Plague is primarily a disease of rodents; however, humans are at risk of infection if they come into contact with an infected animal. Transmission is usually through the bite of an infected flea vector (21). This type of infection usually leads to the manifestation of bubonic plague with trafficking of bacteria to the local draining lymph node, which becomes swollen and tender following the formation of the characteristic bubo. Bacteria are subsequently seeded throughout the body and colonize major organs, including the liver and spleen, with high fever and prostration. During the later stages of the disease, the patient develops a bacteremia with blood culture counts in the range from 10 to 4 × 107 CFU/ml (20). Sometimes a bacteremia may occur without the formation of buboes. This septicemic form of plague is often hard to detect and results in a higher rate of mortality than the bubonic form due to delayed treatment (20). If during bubonic or septicemic plague colonization of the alveolar spaces occurs, then a secondary pneumonic plague develops, resulting in a highly transmissible disease with low infectious doses and mortality rates approaching 100% (20). The precise terminal stages of plague infection have not been identified but are similar to those of other systemic illnesses. Because an overwhelming septicemia is a strong feature during the later stages of Y. pestis infection, it seems likely that endotoxin is responsible for the systemic inflammatory response syndrome and its sequalae, such as disseminated intravascular coagulation (31).
Following infection, Y. pestis is phagocytosed at the site of infection by polymorphonuclear leukocytes (predominantly neutrophils) and macrophages. Histological evidence indicates that bacteria within neutrophils are killed, while bacteria within macrophages survive and go on to express various virulence determinants, which allows growth and eventual release from the macrophages (1, 5). For example, F1 antigen (7) and type III secretion systems/effectors (6) are expressed only at 37°C and have been shown to modulate the host response so that Y. pestis becomes resistant to subsequent phagocytosis. The use of these antiphagocytic mechanisms has led researchers to suggest that Y. pestis is predominantly an extracellular pathogen in the mammalian host (4, 6). This suggestion is supported by the results of recent vaccination studies with mice and recombinant F1 and V protein subunits, in which the major correlate of protection was the production of immunoglobulin G1 antibodies (35). In addition, passive transfer of F1 and V antibodies is sufficient to induce protection in SCID/Beige mice (12). However, a strong cell-mediated immune response to Y. pestis infection is seen in immunized mice, suggesting that immune cells are also needed to clear either extracellular Y. pestis that has been opsonized or intracellular bacteria within host cells. A T-cell component of protection against Y. pestis, in the absence of antibody, has long been established (32, 36). Furthermore, interleukin-4 knockout mice, which produce low titers of immunoglobulin G1 antibodies in response to vaccination with F1 and V, are still protected against challenge with fully virulent Y. pestis (8). In unvaccinated individuals, low doses of Y. pestis can be resolved following combined treatment with the Th1-associated cytokines gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (17). These studies suggest that cell-mediated immune responses are important in protection against Y. pestis.
The studies described above show that, although an antibody response is sufficient to protect against plague, the full response to vaccination is both humoral and cell mediated. In unvaccinated individuals, the interaction of the organism with the innate immune system, in particular the cellular response, is important in determining the outcome of the infection. To gain a better understanding of the cellular pathogenesis of plague, we studied the progression of infection in unvaccinated mice using a fully virulent strain of Y. pestis expressing green fluorescent protein (GFP). We demonstrated that in the spleens of BALB/c mice, Y. pestis is predominantly an intracellular pathogen, residing inside CD11b+ macrophages and Ly-6G+ neutrophils until the latest stages of infection (day 5 or 6), when extracellular bacteria can be detected by flow cytometry. Bacterial culture and flow cytometric analysis suggested that up to day 2 postinfection host neutrophils were able to control Y. pestis growth, probably through apoptosis, while host macrophages were compliant throughout the course of infection. We found that this Y. pestis-induced suppression of antibacterial macrophage activity could be overcome in vitro by addition of IFN-γ and TNF-α.
MATERIALS AND METHODS
Animals.
Six to 8-week-old female BALB/c mice were obtained from Charles River Laboratories (Margate, United Kingdom) and used for all experiments. Mice were culled as soon as they displayed terminal symptoms of Y. pestis infection. For this model of virulent Y. pestis infection by subcutaneous injection, the humane endpoint for BALB/c mice was usually between days 4 and 5 postinfection.
Bacterial strains, plasmids, growth conditions, and chemicals.
Y. pestis GB was grown aerobically at 28°C in blood agar base broth or on blood agar base or Congo red agar as described previously (19). Escherichia coli JM109 was cultured and stored as described by Sambrook et al. (27a). Ampicillin and kanamycin (Sigma, Poole, United Kingdom) were used at final concentrations of 55 and 50 μg ml−1. Isopropyl-d-thiogalactopyranoside (IPTG) was included when it was required at a final concentration of 1 mM. Restriction enzyme digestion, dephosphorylation with shrimp alkaline phosphatase, and DNA ligation were performed by standard procedures using enzymes provided by Roche (Lewes, United Kingdom). Plasmid DNA was isolated using kits provided by QIAGEN (Crawley, United Kingdom) according to the manufacturer's instructions.
Construction of Y. pestis expressing GFP.
The gfp gene encoding the “cycle 3” variant of GFP was isolated from pGFPuv (Clontech Laboratories Inc., United States) by digestion with SmaI and StuI and ligated into SamI-digested, dephosphorylated pTrc99A (Pharmacia) to generate pTrcGFP. The ligation was transformed into E. coli JM109 and plated on LB agar supplemented with ampicillin and IPTG. Colonies which fluoresced during exposure to UV light contained plasmids with inserts in the correct orientation for GFP expression driven by the Ptrc promoter of pTrc99A. The promoter and gfp gene were amplified from pTrcGFP by PCR using oligonucleotide primers GFPst1 (GTCGAGCTGCAGCGACTGCACGGTGCACCAA) and GFPst2 (CTATGTCTGCAGACACCAGACAAGTTGGTAATGG). The primers contained PstI sites (underlined), which allowed the PCR product to be digested with PstI and ligated into similarly digested and dephosphorylated pACYC177. The PCR was performed using 30 cycles of amplification (95°C for 15 s, 50°C for 15 s, 72°C for 30 s) with a Perkin-Elmer 9600 GeneAmp PCR system. The ligation was transformed into E. coli JM109. A kanamycin-resistant clone which was brightly fluorescent during exposure to UV light was chosen, and the plasmid was designated pACGFP. Plasmid pACGFP was transformed into Y. pestis GB by electroporation (0.2-cm cuvettes; 25 μF; 200 Ω; 1.6 V) using a Bio-Rad Electropulser. A kanamycin-resistant clone which was brightly fluorescent during exposure to UV light was designated Y. pestis(pACGFP).
Determination of the virulence of Y. pestis(pACGFP) in BALB/c mice.
The median lethal dose (MLD) is the median dose of bacteria required to induce morbidity or death. The MLD of the Y. pestis(pACGFP) recombinant was assessed by subcutaneous injection of groups of five female 8-week-old BALB/c mice (Charles River, United Kingdom) with serial dilutions of exponential-phase broth cultures grown at 28°C (27). Humane endpoints were strictly observed, and animals that were deemed incapable of survival were humanely culled by cervical dislocation. The times to humane death (typically 3 to 5 days) were recorded, and the MLD of the recombinant strain was determined by the method of Reed and Muench (22).
Colonization of spleens by Y. pestis(pACGFP).
Groups of five BALB/c mice were inoculated subcutaneously with approximately 1 × 105 CFU of Y. pestis and humanely culled at 6, 12, 24, 48, 72, 96, and 120 h postinfection. Numbers of bacteria were calculated by plating serial dilutions onto Congo red agar and incubating the plates at 28°C for 2 to 3 days. The results were expressed as geometric means plus 95% confidence limits.
Separation of target cell populations using magnetic beads.
Neutrophils and macrophages from Y. pestis(pACGFP)-infected mice were separated from a spleen cell suspension using the Minimacs system for cell separation according to the manufacturer's instructions (Miltenyi Biotech, United Kingdom). Briefly, individual suspensions were incubated with phycoerythrin (PE)-conjugated GR-1 antibody (antineutrophil; BD Pharmingen, United States) for 20 min on ice, washed with phosphate-buffered saline, and then incubated with anti-PE microbeads (Miltenyi Biotech, United Kingdom) for 30 min on ice. After washing, cells were passed through a column attached to a magnet so that GR-1+ cells remained in the column while unlabeled cells passed through as effluent. This procedure was repeated to improve the cell yield. The negative fraction was then labeled with anti-CD11b-coated microbeads (Miltenyi Biotech, United Kingdom) for 30 min on ice, and macrophages were separated from the effluent. The GR-1+, CD11b+, and GR-1− CD11b− fractions were stained with Cy-chrome-conjugated anti-CD11b (Serotec, United Kingdom), fixed in 4% paraformaldehyde for a minimum of 24 h, and analyzed by flow cytometry and laser confocal microscopy.
Flow cytometry and laser confocal microscopy.
Both intra- and extracellular fluorescent Y. pestis cells were detected using flow cytometry and laser confocal microscopy. Fixed samples were analyzed using a FACScan (BD Biosciences, United States), and fluorescence in the FL1 (p3) channel indicated that Y. pestis(pACGFP) was present. Intra- and extracellular bacteria were differentiated by cell size in the forward scatter channel. The data presented below are representative of entire spleen subset populations, since all target cells from individual spleens were acquired. Confirmation of the type of intracellular host of fluorescent bacteria was done using PE-conjugated anti-Ly-6G (BD Pharmingen, United States) for neutrophils and Cy-chrome-conjugated anti-CD11b (Serotec, United Kingdom) for macrophages. Appropriate isotype controls were used to define the location of analysis quadrants for PE and Cy-chrome staining. Spleen cells from age-matched, uninfected BALB/c mice were used to control for fluorescence in the FL1 channel. Apoptotic cells were detected using an Annexin V kit (BD Pharmingen, United States), in which cells were stained for the presence of the surface-expressed phospholipid phosphatidylserine, as well as the vital dyes propidium iodide and 7-amino-actinomycin D.
Cells were also examined using an Olympus IX70 inverted laser confocal scanning microscope with a ×60 water immersion lens. Images were excited at 488 nm and detected using 530-nm band-pass filters for GFP and a 565-nm long-pass filter for PE and Cy-chrome. Images were recorded and processed by using the Fluoview image system (Olympus, Germany).
Survival in cytokine-treated J774 macrophage cell cultures.
Y. pestis GB bacteria from frozen bead stocks were grown at 28°C as described previously (27). The precise number of viable bacteria was determined after dilutions of the broth were cultured on Congo red agar. Intracellular survival of Y. pestis was measured as described previously by Oyston et al. (18). J774 macrophages were seeded at a density of 5 × 105 cells ml−1 in L-15 medium supplemented with 2% fetal calf serum and 2 mM l-glutamine (Sigma, United Kingdom) in 24-well tissue culture dishes and cultured until they were confluent. The tissue culture medium was removed, 200 μl (107 CFU) of the bacterial suspension (resuspended in tissue culture medium) was added, and the cells were incubated at 37°C for 30 min. The suspension above the monolayer was removed, and the cells were washed three times with tissue culture medium. One milliliter of culture medium containing 10 μg ml−1 gentamicin (Sigma) was added, and the cells were incubated for 45 min at 37°C. The cells were washed twice with culture medium, and 1 ml of culture medium containing 2 μg ml−1 gentamicin was added to the cells. The cells were then incubated at 37°C. At selected times, the culture medium was removed, the cells were washed twice with fresh medium, and 1 ml of ice-cold 0.1% sodium deoxycholate (Sigma, United Kingdom) was added to the cells. Macrophages were lysed by vigorous aspiration. The lysate was diluted in phosphate-buffered saline, and the number of bacteria was determined after growth at 28°C for 48 h on Congo red agar. Duplicate samples were taken at all times, and the assay was repeated three times. At 12 to 16 h before infection with Y. pestis, macrophages were pretreated with either culture medium alone, 50 ng IFN-γ alone (4.21 × 105 U), 40 ng TNF-α alone (1.08 × 107 U), or both 50 μg IFN-γ and 40 μg TNF-α (all obtained from R & D Systems, United Kingdom). Once infected, macrophages were retreated with the combinations and concentrations of cytokines described above until the end of the experiment.
Statistical analyses.
The Student t test was used to determine whether there was a significant difference between arithmetic and geometric means for cell type and treatment group data, respectively.
RESULTS
Virulence of Y. pestis(pACGFP) in mice.
The Y. pestis recombinant expressing GFP was less virulent in mice than the wild-type GB strain. The previously reported MLD for intraperitoneal inoculation with the GB wild-type strain is 1 CFU (27). The recombinant carrying pACGFP was determined to have an MLD of 124 CFU, probably reflecting the biochemical burden of carrying the plasmid and constitutively expressing GFP.
Recovery of Y. pestis(pACGFP) from BALB/c mice.
Groups of five BALB/c mice were humanely culled on consecutive days after infection with 100 MLD (approximately 104 CFU) of Y. pestis(pACGFP), and their spleens were removed for assessment of the bacterial load (Fig. 1). Dilutions were plated on Congo red agar with and without 50 μg ml−1 kanamycin to evaluate the stability of pACGFP in vivo. The fact that there was no difference between the number of bacteria recovered in the presence of kanamycin and the number of bacteria recovered in the absence of kanamycin (P < 0.05) indicated that the plasmid was stable. Culturable bacteria were detected from day 2 postinfection onward. The number of bacteria remained relatively constant (between 103 and 104 CFU per spleen) between days 2 and 4 postinfection. After that, the number of bacteria increased rapidly to approximately 107 CFU per spleen.
FIG. 1.
Growth of Y. pestis(pACGFP) in the spleens of BALB/c mice following subcutaneous infection with approximately 100 MLD. Mice were infected subcutaneously in the hind thigh. Spleens were removed from groups of five mice at 6, 12, 24, 48, 72, 96, and 120 h postinfection. Log dilutions of each sample were plated in duplicate on Congo red agar plates containing kanamycin (kanamycin+) and not containing kanamycin (kanamycin−) and then incubated at 28°C for 2 days. The data are geometric means and standard deviations.
Intracellular and extracellular Y. pestis(pACGFP) pathogenesis over the course of infection.
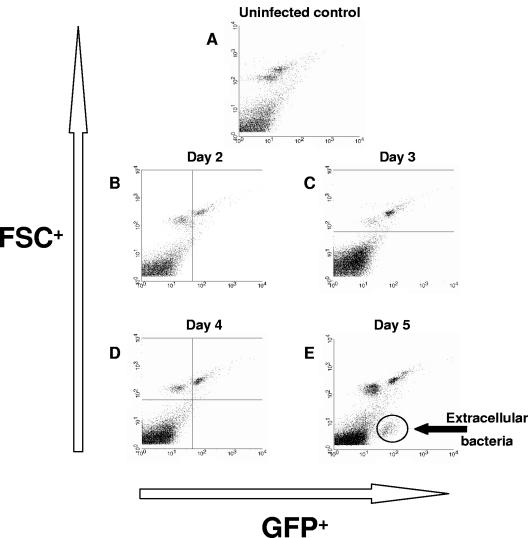
In order to determine the relative proportions of intra- and extracellular Y. pestis(pACGFP) present in the spleen over the course of infection, homogenates were analyzed by flow cytometry. Since the bacterial load data (Fig. 1) suggested that bacteria first get to the spleen on day 2 postinfection, samples were taken from day 2 postinfection onward (Fig. 2B to E), as well as from uninfected control mice (Fig. 2A). Cell size, determined by forward scatter, was plotted against fluorescence caused by GFP (Fig. 2). Both the upper left and upper right quadrants displayed eukaryotic cells, while the lower left and lower right quadrants displayed events that are approximately 1 log smaller (e.g., 1 μm), including bacteria. Interestingly, on days 2 to 4 postinfection (Fig. 2B to D), all the green fluorescence was observed in the upper right quadrant, suggesting that all Y. pestis cells present within the spleen were located either intracellularly or tightly adhered to host cells. Extracellular, non-cell-associated bacteria were observed only in the lower right quadrant on day 5 postinfection, during the late phase of infection (Fig. 2E). These data suggest that Y. pestis is predominantly a cell-associated pathogen over the first 4 days postinfection and escapes host cell association only during the very late stages of infection.
FIG. 2.
Presence of Y. pestis(pACGFP) in the spleens of BALB/c mice from day 2 postinfection onward. Groups of five BALB/c mice were infected with approximately 100 MLD of Y. pestis(pACGFP), and their spleens were removed from day 2 postinfection onward. At each time spleens were removed, and cell suspensions were prepared and fixed in 4% paraformaldehyde. Cells were acquired after 24 h of incubation at 4°C in the dark. The y axis indicates cell size (FSC), while the x axis indicates green fluorescence.
Intracellular niche of Y. pestis(pACGFP).
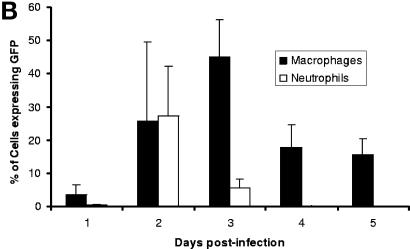
In order to find the cell-associated niche of Y. pestis(pACGFP) in the spleens of BALB/c mice, a further set of time course experiments was performed. Preliminary experiments, in which spleen homogenates were stained with fluorescently labeled antibodies, proved to be unsatisfactory, as too few cells were acquired to get statistically significant results by flow cytometry. In order to enhance the sensitivity, entire CD11b+ macrophage and Ly-6G+ neutrophil populations were separated from spleen homogenates. The three populations obtained (CD11b+, Ly-6G+, and CD11b− Ly-6G−) were stained with fluorescently labeled Ly-6G (PE) and CD11b (Cy-chrome) antibodies and analyzed by flow cytometry (Fig. 3). Analysis gates were set up based on established size and granularity characteristics of neutrophils and macrophages (Fig. 3A). Over the course of infection, a consistent defined population of CD11b+ macrophages was associated with Y. pestis(pACGFP) (Fig. 3A). In contrast, fluorescent bacteria were consistently observed in the isolated Ly-6G+ neutrophil fraction on days 1 and 2 postinfection (Fig. 3A). Fluorescent bacteria were not observed in the CD11b− Ly-6G− population at any time during infection (data not shown). No expression of CD11b was observed in the Ly-6G+ target population, nor was there any Ly-6G expression in the CD11b+ target population (data not shown), indicating that none of the intracellular bacteria attributed to macrophages were actually present in the neutrophil population (and vice versa). Quantitative analysis of cells positive for GFP indicated that CD11b+ macrophages were consistently infected over the course of infection with mean infection levels between 4 and 27% (Fig. 3B). These levels were significantly greater than those in the Ly-6G+ neutrophil population from day 3 postinfection onward (P < 0.001). Intracellular bacteria were observed within Ly-6G+ neutrophils on days 1 and 2 postinfection; there was a mean of 27.2% Y. pestis(pACGFP)+ cells on day 2 postinfection, but this value decreased rapidly as infection progressed (Fig. 3B). These data indicate that CD11b+ macrophages are the main intracellular hosts of Y. pestis throughout infection in the host spleen. They also suggest that Ly-6G+ neutrophils are unable to phagocytose Y. pestis from day 3 postinfection onward.
FIG. 3.
Dissemination of Y. pestis(pACGFP) into cells of the innate immune system following subcutaneous infection of BALB/c mice. The spleens from groups of five BALB/c mice were removed on days 1, 2, 3, 4, and 5 postinfection. Neutrophils (Ly-6G+ cells) and macrophages (CD11b+ cells) were isolated using Minimacs beads, stained for the presence of Ly-6G and CD11b, fixed in 4% paraformaldehyde for 24 h at 4°C, and analyzed by flow cytometry. (A) Dot plots from CD11b+ and Ly-6G+ populations on each day postinfection, indicating the presence of GFP fluorescence. Regions from which CD11b+ and Ly-6G+ cell data were gated are also indicated. (B) Percentages of CD11b+ (solid bars) and Ly-6G+ (open bars) cells expressing GFP over the course of infection. The data are means with 95% confidence limits.
Laser confocal microscopy was used to assess whether the cell-associated bacteria observed as described above were either intracellular or attached to the cell surface. Samples from BALB/c mice infected with Y. pestis(pACGFP) were obtained from day 2 postinfection onward, and CD11b+, Ly-6G+, and CD11b− Ly-6G− fractions were stained as described above with fluorescent markers. Analysis of the Ly-6G+ and CD11b− Ly-6G− fractions revealed that there were no discernible cell-associated bacteria on the days examined. Cell-associated bacteria were observed with increasing frequency in the CD11b+ fractions throughout the infection. Figure 4A to D shows extended views of individual CD11b+ cells with bacteria clearly enclosed within the cellular membrane. This observation is further supported by Fig. 4E, which shows vertical and horizontal Z-projections taken from the extended view indicated in Fig 4D. This analysis was repeated for all images, and none of the samples showed bacteria attached to the external cell surfaces. Interestingly, intracellular replication was observed within CD11b+ cells (Fig. 4A and B), indicating that CD11b+ macrophages are a site of Y. pestis replication.
FIG. 4.
Laser confocal microscope images of day 5 and 6 postinfection CD11b+ cell fractions from the spleens of BALB/c mice infected with Y. pestis(pACGFP). (A to D) Extended views of macrophages containing a number of single (C and D) or replicating (A and B) intracellular bacteria isolated on day 5 (A and B) and day 6 (C and D) postinfection. (E) Single view from panel D, accompanied by horizontal and vertical Z-projections indicating the presence of a bacterium within the macrophage and not on its surface. Bars = 10 μm.
Apoptosis of macrophages and neutrophils during the early stages of Y. pestis GB infection.
Having established the distribution of plague organisms in the splenic cell population of infected mice using fluorescent bacteria, we used virulent wild-type Y. pestis GB to study the induction of apoptosis in cells of the innate immune system. Spleen homogenates were prepared on days 1, 2, and 3 postinfection from groups of five BALB/c mice infected subcutaneously with 100 MLD of Y. pestis GB. The induction of apoptosis as a result of infection was assessed by determining the binding of fluorescent Annexin V to surface-expressed phosphatidylserine on target cells in the absence of staining by vital dyes, such as propidium iodide (CD11b+ cells) or 7AA-D (Ly-6G+ cells) (Fig. 5). In apoptotic cells, the membrane phospholipid phosphatidylserine is translocated to the outer leaflet of the plasma membrane. Expression of phosphatidylserine in the absence of staining by vital dyes like propidium iodide is indicative of the early stages of apoptosis, whereas costaining with propidium iodide is indicative of necrotic cells. Plague infection induced some apoptosis among splenic CD11b+ macrophages (Fig. 5A) on days 1 and 2 postinfection. In contrast, nearly all Ly-6G+ neutrophils were singly positive for Annexin V on days 1 and 2 postinfection (Fig. 5B). The increase in Annexin V expression was significantly more than that in baseline apoptosis in noninfected BALB/c mice (P < 0.05). The increase in apoptosis in both populations coincided with the detection of culturable bacteria on day 2 postinfection during Y. pestis(pACGFP) infection.
FIG. 5.
Apoptosis in splenic macrophages (A) and neutrophils (B) following infection of BALB/c mice with Y. pestis. BALB/c mice were infected subcutaneously with approximately 100 MLD of Y. pestis GB. Groups of five mice were humanely culled on days 1, 2, and 3 postinfection. Spleens were removed and stained for the presence of Annexin V (an early cell surface-expressed marker of apoptosis) on CD11b+ (A) or Ly-6G+ (B) cells (open bars). In addition, the vital dyes propidium iodide (PI) (for CD11b+ cells) and 7AA-D (for Ly-6G+ cells) were added to assess cell necrosis (solid bars). Following staining, cells were washed and fixed in 4% paraformaldehyde overnight and acquired the next day. The bars indicate mean percentages of target cells (CD11b+ or Ly-6G+), and the error bars indicate standard deviations. An asterisk indicates that there is a significant difference between apoptosis on day 0 and apoptosis following infection.
Immunomodulation of macrophage function in vitro.
The data presented above strongly suggest that the interaction between host macrophages and Y. pestis is an important factor in determining the outcome of infection. In vitro studies using the J774 macrophage cell line were undertaken to determine the effect of cytokine-driven immunomodulation on the innate response to plague. The macrophage-activating cytokines IFN-γ and TNF-α were used as pre- and postexposure treatments for macrophage cultures containing Y. pestis at a multiplicity of infection of 10 bacteria to 1 cell (Fig. 6). After 30 min of exposure to Y. pestis, treatment with either IFN-γ alone, TNF-α alone, or a combination of IFN-γ and TNF-α caused a significant increase in the rate of infection of macrophages compared to untreated controls (P < 0.002). At 3 and 5 h postinfection, only macrophages treated with both IFN-γ and TNF-α had bacterial loads that were significantly lower than those of the untreated controls (Fig. 6). At 24 h postinfection all three treatments caused significant decreases in intracellular loads (P < 0.04). The largest decrease was induced by the combined treatment with IFN-γ and TNF-α, which caused an almost 1,000-fold decrease in the intracellular load compared to that of untreated control macrophages (Fig. 6). IFN-γ and TNF-α, therefore, appear to act synergistically to induce optimal Y. pestis clearance by macrophages in vitro.
FIG. 6.
Synergistic effect of IFN-γ and TNF-α on intracellular killing of Y. pestis GB by J774 macrophages. Approximately 106 J774 macrophages were pretreated with 50 μg IFN-γ (striped bars), 40 μg TNF-α (dotted bars), both IFN-γ and TNF-α (open bars), or medium alone (solid bars) 12 h before infection with 107 Y. pestis (multiplicity of infection, 10:1) for 30 min. Cells were washed and maintained in gentamicin media with the relevant cytokine treatments and were lysed at 0, 3, 5, and 24 h postinfection. The bars indicate the geometric means for plate counts, and the error bars indicate the standard errors of the means.
DISCUSSION
The precise pathogenesis of Y. pestis infection is yet to be resolved. The extracellular nature of Yersinia pseudotuberculosis in vivo has led to speculation that Y. pestis behaves in a similar manner (4). The expression of a sophisticated immunomodulatory type III secretion mechanism by Y. pestis has been widely accepted as evidence that the plague organism is mainly an extracellular pathogen (4, 6). However, some studies have indicated that a strong cell-mediated response, induced by cytokines normally associated with the clearance of intracellular pathogens, can cure infection with Y. pestis (17). Our studies support the concept that the plague organism is an intracellular pathogen that resides and grows within the macrophage population until the terminal stages of infection (5, 14, 30).
We demonstrated that there are three stages of Y. pestis infection in the spleen (Fig. 1): early, plateau, and late. This finding is supported by previous studies (19). On day 1 postinfection Y. pestis bacilli are present within a few splenic macrophages and neutrophils, but Y. pestis is not culturable from spleen homogenates for a further 24 h. This suggests that, at least in the spleen, the innate immune system is initially able to limit the growth of Y. pestis either by direct killing or by induced stasis.
Bacterial growth in the spleen occurs between days 1 and 2 postinfection. Our studies indicate that this growth coincides with an increase in the proportion of splenic macrophages and neutrophils that contain Y. pestis(pACGFP) and an increase in neutrophil apoptosis. It is therefore possible that the neutrophil population is responsible for suppressing bacterial growth in the spleen until day 2 postinfection and that this suppression is broken by the development of bacterial immunomodulatory mechanisms that induce neutrophil cell death. As a consequence, Y. pestis may become resistant to phagocytosis by neutrophils by inducing their cell death during the early stages of infection, although it may take some time (1 to 2 days) for Y. pestis to do this. The most likely explanation for these observations is that during the initial stages of infection, plague bacilli replicate within macrophages until they escape and infect other macrophages or circulate to other host organs. The expression of F1 antigen and a functional type III secretion system by these bacteria may account both for our inability to find Y. pestis-infected neutrophils after day 2 postinfection and for the observed resistance to phagocytosis by neutrophils, as has been demonstrated for other Yersinia species (13, 23). Furthermore, the expression of a functioning type III secretion system by the escaping bacteria may induce the apoptosis observed in splenic neutrophils and macrophages that is usually associated with other Yersinia species infections (25). Our results also indicate that neutrophils may kill all extracellular bacteria in the spleen during the early stage of infection and that the high degree of apoptosis and comparatively low bacterial counts observed during the early and plateau phases of infection are indicative of efficient neutrophil activity.
Relatively little is known about the expression of virulence determinants during Y. pestis infection in vivo. Studies of Y. pseudotuberculosis infection in mice have demonstrated that YopJ is essential for the establishment of a systemic infection in vivo, via the induction of macrophage apoptosis and suppression of TNF-α production (16). Many in vitro studies of Yersinia species have demonstrated that there is rapid expression of virulence markers like Yops, F1 antigen, and V antigen 1 to 4 h postinfection in macrophage cell lines. These markers have been shown to induce apoptosis (15, 24), suppress the production of proinflammatory cytokines like TNF-α (3, 26), inhibit Fc receptor-mediated phagocytosis (10), and prevent neutrophil chemotaxis (34). Our data indirectly suggest that while these mechanisms may be expressed early during infection, they do not become effective in the spleen until between days 1 and 2 postinfection.
Once Y. pestis has overcome the suppressive effect of the host innate response, it establishes a relatively constant level of infection during the plateau phase of infection between days 2 and 4 postinfection. During this period, we observed that plague bacilli were predominantly detected only in the macrophage population. It is possible that Y. pestis preferentially infects host macrophages through recognition of specific surface-associated molecules. It has been suggested that expression of the CCR5 receptor may aid entry into host monocytes by plague (9, 29). Interestingly, the amount of apoptosis in macrophages was considerably less than that in neutrophils, particularly during the early to plateau phase of infection. These observations support those of Cavanaugh and Randall (5) and suggest that the macrophage is a favorable intracellular host for plague bacilli that provides a site which avoids contact with other components of the host's immune system. The compliant nature of macrophages during Y. pestis infection is in direct contrast to its aggressive response to other intracellular pathogens. This suggests that it is being immunomodulated by the plague organism. Since no Y. pestis(pACGFP) cells were observed on the surface of host macrophages, modulation of macrophage behavior may occur from within the cell and not via the classical type III secretory mechanisms commonly associated with Yersinia infections. Our in vitro studies with the J774 macrophage cell line indicate that this immunomodulation can be overcome via pretreatment with the proinflammatory cytokines IFN-γ and TNF-α. These cytokines have been strongly implicated in the resolution of infections with Yersinia spp. (2, 17) and act synergistically to overcome immunosuppression by the agent. Our in vitro assays demonstrated that a combination of IFN-γ and TNF-α was most effective for activating J774 macrophages to kill the plague organism. This may explain the ability of this cytokine combination to protect mice against plague infection in vivo (17).
In our model, the late stage of Y. pestis infection is characterized by a rapid increase in the number of bacteria within the spleen and escape of bacteria from macrophages into the extracellular compartment of the spleen. The cause of this escape is unclear but is likely to be related to the macrophage necrosis or apoptosis observed during in vitro studies (30, 33). It is likely that the endotoxin-induced systemic inflammatory response syndrome associated with the terminal stages of Y. pestis infection (5) is linked to this escape of bacilli from thee intracellular compartment (Fig. 2E).
In conclusion, our in vivo studies suggest that in the spleen, Y. pestis resides within host macrophages for the majority of its infection of the murine host. Furthermore, we present some evidence which suggests that in vivo, as well as in vitro (30), Y. pestis replicates within macrophages. We also suggest that the immunomodulatory behavior of Y. pestis is different for the two main cells of the innate immune system. In neutrophils Y. pestis infection results in rapid apoptosis during the early stages of infection that is followed by bacterial resistance to phagocytosis during the later stages of infection. In contrast, host macrophages seem to be used as a site of intracellular replication and relative safety from other immune effector cells. Many studies of Yersinia species have suggested that these bacteria have evolved highly efficient immunomodulatory machinery that is able to suppress the host's macrophage-mediated proinflammatory response (6, 11). Recent studies of Yersinia enterocolitica have suggested that this anti-inflammatory state could be caused by V antigen-induced interleukin-10 production, and this may also be true for this model of Y. pestis infection (28). Our in vitro data support these studies since pretreatment of macrophages with strong proinflammatory stimuli (IFN-γ and TNF-α) seems to overcome this plague-induced immunosuppression.
Editor: D. L. Burns
REFERENCES
- 1.Andrew, P. W., J. P. S. Jackett, and D. B. Lowrie. 1985. Killing and degradation of microorganisms by macrophages, p. 311-335. In R. T. Dean, W. Jessop, and J. W. Dean (ed.), Mononuclear phagocytes: physiology and pathology. Elsevier Biomedical Press, Amsterdam, The Netherlands.
- 2.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland, A., and G. R Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brubaker, R. 1991. Factors promoting acute and chronic disease caused by yersinae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanaugh, D., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 83:348-363. [PubMed] [Google Scholar]
- 6.Cornelis, G. R. 1998. The Yersinia deadly kiss. J. Bacteriol. 180:5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elvin, S., and E. D. Williamson. 2000. The F1 and V subunit vaccine protects against plague in the absence of IL-4 driven immune responses. Microb. Pathog. 29:223-230. [DOI] [PubMed] [Google Scholar]
- 9.Elvin, S. J., E. D. Williamson, J. C. Scott, J. N. Smith, G. Perez de Lema, S. Chilla, P. Clapham, K. Pfeffer, D. Schlondorff, and B. Luckow. 2004. Ambiguous role of CCR5 in Y. pestis infection. Nature 430:417. [DOI] [PubMed] [Google Scholar]
- 10.Fallman, M., K. Andersson, S. Hakansson, K.-E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields, K., M. L Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacteria surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, M., D. Rogers, P. Russell, A. J. Stagg, D. L. Bell, S. M. Eley, R. W. Titball, and E. D. Williamson. 1999. The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol. Med. Microbiol. 23:107-113. [DOI] [PubMed] [Google Scholar]
- 13.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarner, J., W. J. Shieh, P. W. Greer, J. M. Gabastou, M. Chu, E. Hayes, K. B. Nolte, and S. R. Zaki. 2002. Immunohistochemical detection of Yersinia pestis in formalin-fixed, paraffin-embedded tissue. Am. J. Clin. Pathol. 117:205-209. [DOI] [PubMed] [Google Scholar]
- 15.Mills, S., A. D. Boland, M.-P. Sary, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monack, D., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyston, P., N. Dorrell, K. Williams, S.-R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyston, P., P. Russell, K. Williams, E. D. Williamson, and R. W. Titball. 1996. An aroA mutant of Yersinia pestis is attenuated in guinea-pigs, but virulent in mice. Microbiology 142:1847-1853. [DOI] [PubMed] [Google Scholar]
- 20.Perry, R., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poland, J., and A. M. Barnes. 1979. Plague, p. 515-559. In J. Steele, H. Stoenner, W. Kaplan, and M. Torten (ed.), CRC handbook series in zoonoses. CRC Press, Boca Raton, Fla.
- 22.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 23.Ruckdeschel, K., A. Roggenkamp, S. Schuert, and J. Heesemann,. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat J. Heesemann, and B. Rouout. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouout, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signalling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 26.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouout. 1998. Yersinia enterocolitica impairs activation of transcription of factor NF-kB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumour necrosis factor α production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine USP and EV76 vaccine induced protection against Yersinia pestis. Vaccine 13:1551-1556. [DOI] [PubMed] [Google Scholar]
- 27a.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 29.Stephens, J. C., D. E. Reich, D. B. Goldstein, H. D. Shin, M. W. Smith, M. Carrington, C. Winkler, G. A. Huttley, R. Allikmets, L. Schriml, B. Gerrard, M. Malasky, M. D. Ramos, S. Morlot, M. Tzetis, C. Oddoux, F. S. di Giovine, G. Nasioulas, D. Chandler, M. Aseev, M. Hanson, L. Kalaydjieva, D. Glavac, P. Gasparini, E. Kanavakis, M. Claustres, M. Kambouris, H. Ostrer, G. Duff, V. Baranov, H. Sibul, A. Metspalu, D. Goldman, N. Martin, D. Duffy, J. Schmidtke, X. Estivill, S. J. O'Brien, and M. Dean. 1998. Dating the origin of the CCR5-Delta 32 AIDS-resistance allele by the coalescence of haplotypes. Am. J. Hum. Genetics 62:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straley, S. P. H. 1984. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 45:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Titball, R., and S. E. C. Leary. 1998. Plague. Br. Med. Bull. 54:625-633. [DOI] [PubMed] [Google Scholar]
- 32.Wake, A., and Y. Sutoh. 1983. Mechanisms of protection against virulent Yersinia pestis infection without participation of humoral antibody: H-2 restriction in athymic mouse model. Curr. Microbiol. 8:79-84. [Google Scholar]
- 33.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32:227-237. [DOI] [PubMed] [Google Scholar]
- 34.Welkos, S., A. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24:185-196. [DOI] [PubMed] [Google Scholar]
- 35.Williamson, E., P. M. Vesey, K. J. Gillhespy, S. M. Eley, M. Green, and R. W. Titball. 1999. An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin. Exp. Immunol. 116:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong, J., and S. S. Elberg. 1977. Cellular responses to Yersinia pestis modulated by product(s) from thymus-derived lymphocytes. J. Infect. Dis. 135:67-78. [DOI] [PubMed] [Google Scholar]