Abstract
To improve DNA vaccination against Mycobacterium tuberculosis, we evaluated the effectiveness of a Sindbis virus-based DNA construct expressing the tuberculosis antigen 85B (Sin85B). The protective efficacy of Sin85B was initially assessed by aerogenically challenging immunized C57BL/6 mice with virulent Mycobacterium tuberculosis. At 1 and 7 months postinfection, the lung bacterial burdens were considerably reduced and the lung pathology was improved in vaccinated mice compared to naive controls. Furthermore, the mean survival period for Sin85B-immunized mice (305 ± 9 days) after the tuberculous challenge was extended 102 days relative to the naive mice (203 ± 13 days) and was essentially equivalent to the survival time of Mycobacterium bovis BCG-vaccinated mice (294 ± 15 days). The essential role of gamma interferon (IFN-γ) in Sin85B-mediated protection was established by showing that significantly increased levels of IFN-γ mRNA were present postinfection in lung cells from vaccinated mice relative to control mice and by demonstrating that IFN-γ depletion prior to challenge abolished the vaccine-induced protection. The substantial antituberculosis protective responses induced by Sin85B immunization of CD4−/− mice strongly suggested that CD8 cells partially mediate Sin85B-induced protective immunity. Interestingly, Sin85B vaccination did not protect RNase L−/− (a key enzyme in the innate antiviral response) mice while significant protection was detected in RNase L−/− mice immunized with either BCG or a conventional DNA plasmid expressing antigen 85B. These data show that immunization with Sin85B offers protection similar to BCG in a murine model of pulmonary tuberculosis and suggest that Sin85B-induced protection is dependent upon both innate and acquired immune mechanisms.
More than 100 years after the seminal studies of Robert Koch, tuberculosis (TB) remains one of the leading causes of death due to a single infectious agent. The World Health Organization has estimated there are 8 to 9 million new tuberculosis cases each year, and approximately 2 million individuals die annually from this devastating disease (16, 34). In the past decade, the increasing incidence of multiple-drug-resistant tuberculosis has amplified this public health problem in many countries (12). Furthermore, the spread of human immunodeficiency virus (HIV) infections into regions where TB is endemic has elevated the TB case rates in these areas to alarming numbers. The HIV-Mycobacterium tuberculosis coinfection rates exceed 5% in eight African countries, and in South Africa alone more than 2 million adults are coinfected with M. tuberculosis and HIV (6). The development of a new more effective TB vaccine likely holds the most promise for controlling the worldwide TB epidemic. Although the current vaccine against TB, Mycobacterium bovis BCG, has been widely used for decades, its efficacy has been highly variable in controlled clinical trials and its capacity to prevent adult pulmonary TB is limited (5, 13). Moreover, BCG immunization is contraindicated for HIV-infected persons because BCG can cause a life-threatening disease in immunocompromised individuals (17).
Most published evidence has indicated that cell-mediated immune responses are required to control TB infections and that both CD4 and CD8 T cells contribute to overall antituberculosis protective immunity (14, 15). Since DNA vaccines can induce substantial humoral and cellular immunity and can evoke both CD4 and CD8 T-cell responses, genetic immunization is a promising approach for developing vaccines against intracellular pathogens including M. tuberculosis (3, 29). In fact, several studies have shown that TB DNA vaccine preparations can generate considerable antituberculosis protective immunity in rodents (1, 18, 28). In our laboratory, immunization with either TB DNA vaccine cocktails or a TB DNA vaccine expressing a TB polyprotein induced long-term protection against a tuberculous challenge in a murine model of pulmonary TB (7, 8, 9). However, to date, DNA vaccines have been much less immunogenic in primates and humans than in rodents. Multiple injections with milligram quantities of plasmid DNA (up to 20 mg per dose) have been generally needed to produce only modest immune responses in humans (23, 30).
To improve the efficacy of conventional DNA immunization, we have been evaluating DNA vaccines that express target antigens under the control of alphavirus replicases. DNA vaccines encoding alphavirus-derived RNA replicases have been shown to be highly immunogenic in mouse models at doses about 50- to 100-fold lower than those used with conventional vectors (19, 25). However, the underlying mechanisms of the enhanced activity of the alphavirus-derived vectors remain uncertain. Originally, replicase-based vectors were developed to overproduce antigen using alphaviral molecules that constitute an extremely efficient replication system (31). However, for several alphavirus-derived vectors, immunogenicity did not correlate with increased antigen production (24). Alternatively, the superior efficiency of the Sindbis virus-based vaccines may also result from their activation of innate antiviral immune mechanisms. The injection of viral-replicase-based vectors induces the production of double-stranded RNA (dsRNA) in the host cell, and dsRNA triggers antiviral pathways including the RNase L and RNA-dependent protein kinase (PKR) pathways. The RNase L and PKR enzymes are responsible for the degradation of mRNA and the inhibition of protein translation, which eventually lead to apoptotic cell death. In addition to limiting viral spread during an infection, apoptosis can stimulate the immune system because engulfed apoptotic cell bodies activate antigen-presenting cells, including dendritic cells, via cross priming mechanisms (4, 25). Recent studies suggest that replicase-based vectors stimulate innate immune mechanisms. Leitner et al. have shown that the immunogenicity and antitumor activity of a Sindbis virus-based DNA vaccine were blocked in mice that had defective RNase L pathways (22). Subsequently, the same investigators demonstrated that the induction of apoptotic cell death of transfected cells in vivo was required for the increased effectiveness of their Sindbis virus-based vector (21).
In a previous study, we demonstrated that a Sindbis virus vector expressing mycobacterial antigen 85 was highly immunogenic and conferred short-term protection against a tuberculous challenge in mice (19). In this report, we show that immunization with low doses of a Sindbis virus vector expressing the TB protein antigen 85B (Sin85B) confers extended protection against tuberculosis after an aerosol challenge. Additionally, we demonstrate that the mechanisms of vaccine-induced protection likely involve the stimulation of CD8 cells and the activation of innate immunity.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free C57BL/6, RNase L-deficient (B6.C-H2<bM12>/khEgJ), CD4-deficient (B6.129S2-Cd4<tm1Mak>/J), and CD8-deficient (B6.129S2-Cd8atm1Mak002665) mice were obtained from the Jackson Laboratories (Bar Harbor, Maine). The mice were maintained under barrier conditions and fed commercial mouse chow and water ad libitum. The mice were 6 to 8 weeks old at the time of vaccination.
DNA vaccine construction.
The Sincp vector was a gift from Robert Seder, Vaccine Research Center, National Institutes of Health. A two-step process was utilized to clone Ag85B into the Sincp vector because an XhoI restriction site is the sole cloning site in this vector and the antigen 85B gene has an internal XhoI site. First, the Ag85B gene was PCR amplified from a pJW4303-tpa85B construct using Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) such that the tissue plasminogen activator (tpa) signal sequence was fused in frame to the 5′ end of the Ag85B gene. The following PCR primers were used: TPAFor (ACAAGCTTATCATGGATGCAATGAAGAGAGGG) and Ag85BRev (AGGGATCCTCAGCCGGCGCCTAACGAAC). The PCR product was ligated into the PCR 2.1 Topo vector (Invitrogen) and then amplified in Escherichia coli Top10 cells (Invitrogen) in Luria-Bertani medium containing 50 μg/ml kanamycin. The tpaAg85B insert was removed from the Topo vector by digestion with NotI and HindIII and was then gel purified. The tpaAg85B fragment was ligated to the Sincp vector that had been previously gel purified following digestion with XhoI and NotI. Digestion with these restriction enzymes removes the β-galactosidase gene from the Sincp plasmid. The ligation reaction products were then heated to 70°C for 20 min, washed, and concentrated over a Microcon YM-30 (Millipore, Bedford, MA) 30,000-molecular-weight-cutoff microcentrifuge filter. The free ends of the Ag85B gene and the Sincp vector were made blunt using T4 DNA polymerase, and, after a heat inactivation step, the blunt ends of the Ag85B gene and the Sincp vector were ligated together using T4 DNA ligase. Nucleotide sequencing was performed to verify that the tpaAg85B insert had the correct sequence. This construct was maintained and amplified in E. coli DH5α.
Construction of pVax-85B was accomplished by PCR amplifying Ag85B as described above using the same primers. After the tpaAg85B PCR product was cloned into the PCR 2.1 Topo vector and amplified in E. coli Top10 cells, the Topo-85B construct was digested with HindIII and BamHI. After the tpaAg85B insert was gel purified, it was ligated into a gel-purified pVax vector (Invitrogen) that was previously digested with HindIII and BamHI. This construct was amplified and maintained in E. coli DH5α.
In vitro evaluation of Ag85B gene expression using reverse transcription-PCR (RT-PCR).
Rhabdomyosarcoma (RD) cells (ATCC CCL 136) were grown in high-glucose Dulbecco's modified Eagle medium supplemented with 5% fetal bovine serum, 2 mM glutamine, 0.1 mM minimal essential medium (MEM)-nonessential amino acid solution, 1 mM MEM-sodium pyruvate, and 10 mM HEPES. At 90% confluence in wells of a six-well plate, the cells were transfected with 4 μg per well of Sin85B, pVax-85B, or empty vector using Lipofectamine 2000 reagent (Invitrogen). After 48 h, total RNA was harvested using a RNAqueous-4PCR kit (Ambion, Austin, TX) and treated with DNase I. Two micrograms of the RNA was reverse transcribed using a Superscript first-strand cDNA synthesis kit (Invitrogen). Two microliters of the cDNA was used in a 50-μl PCR mixture using Platinum Taq DNA polymerase and the primers described above.
Evaluation of vaccine-induced immunity.
For the DNA vaccine studies, plasmids were prepared using an EndoFree Plasmid Mega kit (QIAGEN, Valencia, CA). Mice were vaccinated either once with 106 CFU BCG Pasteur (TMC1011; obtained from Trudeau Mycobacterial Culture Collection, Saranac Lake, NY) subcutaneously or three times at 3-week intervals with 200 μg of pVax-85B or 5 μg of Sin85B intramuscularly (i.m.) in all four limbs. The 5-μg dose of the Sin85B vaccine was previously found to be an optimal dose for this vector for inducing protection against TB in mice (19). To evaluate anti-85B humoral responses, sera were collected 4 weeks after the third vaccination prior to challenge and analyzed using an enzyme-linked immunosorbent assay protocol designed to detect anti-85B antibodies (9). The antibody titer was defined as the highest dilution of serum that gave an absorbance value that exceeded an optical density of 0.050 and that was twofold greater than that of a matched dilution of sera from naive mice (26).
After the vaccinations, mice were infected by aerosol with M. tuberculosis Erdman (TMC 107; obtained from Trudeau Mycobacterial Culture Collection) 2 to 3 months after vaccination with BCG or 1 month following the last DNA vaccination using a Middlebrook chamber (Glas Col, Terre Haute, IN) at a concentration known to deliver 200 to 300 CFU in the lungs over a 30-min exposure time. For the bacterial burden experiments, five mice were evaluated for each group. In survival studies, five to eight mice per group were assessed. Mice in the survival experiment were maintained until they became moribund and had to be euthanized.
To determine the extent of bacterial growth in the lungs and spleens, mice were sacrificed 28 or 190 days postchallenge and their lungs and spleens were removed aseptically and homogenized separately in phosphate-buffered saline (PBS) with 0.04% Tween 80 using a Seward Stomacher 80 blender (Tekmar, Cincinnati, OH). The lung and spleen homogenates were diluted serially in PBS-Tween 80, and 50-μl aliquots were added to Middlebrook 7H11 agar (Difco, Detroit, MI) plates supplemented with 10% Middlebrook oleic acid-albumin-dextrose-catalase enrichment (Becton Dickinson, Sparks, MD), 10 μg/ml ampicillin (Sigma, St. Louis, MO), 50 μg/ml cycloheximide (Sigma), and 2 μg/ml 2-thiophenecarboxylic acid hydrazide (TCH) (Sigma). The addition of TCH to the growth medium inhibits the growth of BCG but not M. tuberculosis. All plates were incubated at 37°C for 14 to 17 days in sealed plastic bags, and the colonies were counted to determine the number of CFU per organ.
Evaluation of vaccine-induced cytokine responses.
Vaccine-induced cytokine gene expression was assayed 10 days after TB challenge and involved the utilization of real-time RT-PCR. Initially, lung cells were isolated from three to five mice within each experimental group by shredding the pooled lung tissue with razor blades and incubating the tissue in dispase (Gibco BRL, Gaithersburg, MD) at 2 mg/ml in PBS plus 10% fetal bovine serum for 1 h. The lung cells were passed through a cell strainer (BD Falcon, Bedford, MA) and pelleted, and the erythrocytes were removed using ACK lysing buffer (Quality Biological, Inc., Gaithersburg, MD). The RNA from the single cell suspension was isolated for RT-PCR assays using an RNAqueous-4PCR kit. For cDNA synthesis, the RNA was treated with DNase I and reverse transcribed using a Superscript first-strand synthesis system (Invitrogen). The same amount of RNA (1 μg) from each group was used for the cDNA synthesis reaction. The cDNA was used as a template for real-time PCR using the following probe and primers specific for gamma interferon (IFN-γ): forward primer 5′ AGCAACAGCAAGGCGAAAA, reverse primer 5′ CTGGACCTGTGGGTTGTTGA, and probe 5′ 6-carboxyfluorescein-CCTCAAACTTGGCAATACTCATGAATGCATCC-6-carboxy tetramethylrhodamine. TaqMan reagents for assessing the mRNA levels for tumor necrosis factor alpha, interleukin-2 (IL-2), IL-12, RANTES, and IL-10 were obtained from Applied Biosystems (Foster City, CA). The PCR amplifications were completed with an ABI Prism 7000 sequence detection system (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Taqman reagents (Applied Biosystems) were used to measure GAPDH mRNA levels as an internal standard, and the level of cytokine mRNA relative to GAPDH mRNA was calculated using the following equation: relative mRNA expression = 2−(Ct of cytokine − Ct of GAPDH), where Ct is the threshold cycle.
In vivo IFN-γ depletion was done by injecting 0.5 mg of anti-IFN-γ monoclonal antibody (mAb) (clone XMG) into the peritoneums of five mice 3 weeks after the third vaccination. The mice were treated three times before challenge and then once per week until the mice were sacrificed.
Histopathology assessments.
For histopathologic analysis of infected animals, lung tissues were perfused and fixed with formalin in PBS and then embedded in paraffin for sectioning. The lung sections were stained with hematoxylin and eosin (H&E) or with Ziehl-Neelsen acid-fast stain and were evaluated by light microscopy.
RESULTS
Expression of antigen 85B from Sindbis virus-based and conventional DNA vectors.
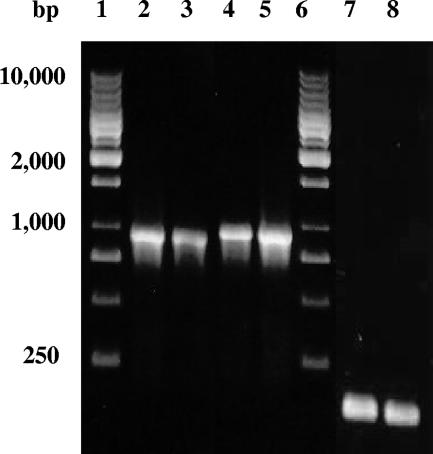
The relative levels of expression of antigen 85B from Sindbis virus-based and conventional DNA were characterized using in vitro transfection studies. Initially, the Sin85B plasmid, the conventional pVax-85B construct, and empty vector controls were transfected into RD cells. Forty-eight hours later, RNA was isolated from the transfectants and the levels of M. tuberculosis antigen 85B mRNA were characterized using RT-PCR. As controls, the levels of mRNA for the housekeeping GAPDH gene were evaluated. As seen in Fig. 1, similar levels of antigen 85B cDNA were generated from RNA isolated from RD cells transfected with either the Sin85B (lanes 2 and 3) or the pVax-85B (lanes 4 and 5) plasmids. Also, nearly identical levels of the GAPDH PCR product were produced, suggesting that essentially equivalent levels of RNA were used in the RT-PCR procedures (lanes 7 and 8). When RT-PCR was performed using mRNA from RD cells transfected with empty Sincp or pVax with antigen 85B-specific primers, no PCR product was observed (not shown). Overall, these transfection studies suggest that the levels of expression of the antigen 85B mRNA from the Sindbis virus-based plasmid and a conventional DNA vaccine vector were similar.
FIG. 1.
In vitro levels of expression of antigen 85B mRNA from the Sin85B and pVax-85B vectors were similar. RT-PCR analysis of mRNA from RD cells transfected with Sin85B (RD-Sin85B) (lanes 2 and 3, 10 and 5 μl of the PCR product, respectively) or pVax-85B (RD-pVax-85B) (lanes 4 and 5, 5 and 10 μl of the PCR product, respectively) using antigen 85B-specific primers was performed. Using the same mRNA, RT-PCR using GAPDH-specific primers was performed (lane 7, RD-Sin85B mRNA; lane 8, RD-pVax-85B mRNA) as controls. Lanes 1 and 6 contain DNA molecular size markers.
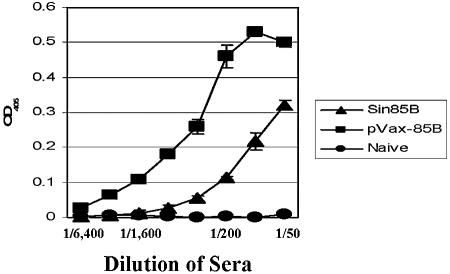
The relative levels of expression from the Sin85B and the pVax-85B constructs in vivo were assessed by measuring the titers of antibodies recognizing the 85B antigen four weeks after the third immunization with these plasmids. The humoral responses were evaluated using serial dilutions of the mouse sera and an enzyme-linked immunosorbent assay protocol designed to detect murine anti-85B antibodies (Fig. 2). The results of this testing indicate that the anti-85B antibody titer was higher for mice vaccinated with the pVax-85B plasmid (1:3,200) compared to mice immunized with the Sin85B construct (1:400). These data strongly suggest that the levels of antigen 85B expressed in vivo from the Sin85B construct are not elevated relative to expression from the pVax-85B plasmid.
FIG. 2.
Evaluation of antigen 85B antibody titers in mice vaccinated with either the Sin85B or pVax-85B constructs. Recombinant Ag85B was bound to wells of a 96-well plate, and twofold serial dilutions of sera from naive mice or mice vaccinated with either Sin85B or pVax-85B were added to the wells. The secondary antibody was alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G.
Immunization with the Sin85B DNA vaccine induces extended protection against tuberculosis after an aerosol challenge.
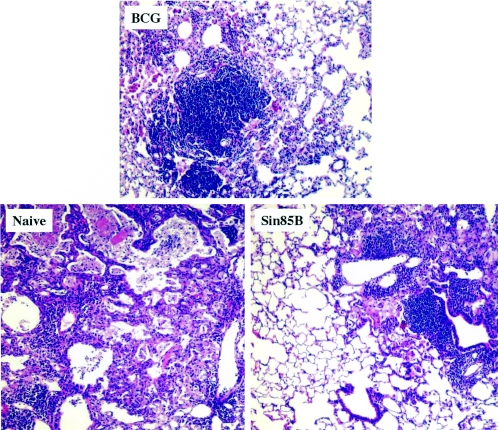
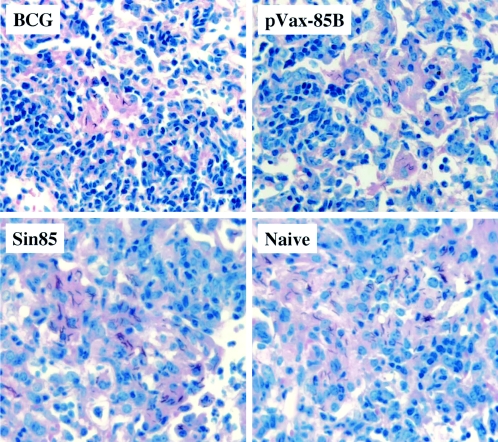
To evaluate the long-term effectiveness of the Sin85B vector, mice were vaccinated three times at 3-week intervals with 5 μg of Sin85B and then challenged by the aerosol route 4 weeks after the final vaccination with about 300 CFU of virulent M. tuberculosis Erdman. As controls, additional mice were vaccinated with 106 CFU of BCG Pasteur 2 months prior to the challenge. At 28 and 190 days postinfection, three groups of mice per time point were sacrificed and relative organ bacterial burden and lung pathology were assessed. The remaining groups of mice were maintained for survival studies. Twenty-eight days after the aerogenic challenge with TB, a significant reduction in mycobacterial CFU (relative to naive controls) was seen in both the lungs (−0.54 log10) and spleens (−0.73 log10) of mice immunized with 5 μg of Sin85B (Table 1). The 4-week-postchallenge results are similar to data obtained previously in immunization studies using 200 μg of a conventional DNA vaccine expressing antigen 85B (−0.5 log10 protection in the lungs; Table 2). As expected, larger relative decreases in organ mycobacterial CFU values were detected for BCG-vaccinated mice (lungs, −0.97 log10; spleens, −1.45 log10) at this 28-day time point. Importantly, the vaccine-induced protection was persistent because significant reductions in lung bacterial CFU compared to naive mice were also detected in Sin85B-vaccinated (−0.71 log10) and BCG-immunized (−1.09 log10) mice at 190 days postchallenge. In contrast, the organ CFU values for mice injected with the Sindbis vector alone were not significantly different than the naive-organ CFU at the 190-day time point. Consistent with the bacterial CFU values, the lung pathology at the 190-day time point was strikingly better for the vaccinated mice (Fig. 3). In the naive controls, considerable cellular infiltrate and necrosis, large areas of inflammation and consolidation, and extensive lung pneumonia were seen in lung sections. Conversely, substantially less inflammation, reduced cellular infiltrate, and more apparent healthy lung tissue suggested ongoing vigorous host pulmonary immune responses in the Sin85B- and BCG-immunized animals.
TABLE 1.
Antituberculosis protective immunity induced by immunization with the Sin85B construct
| Groupa | Mean log10 CFU ± SEMb at:
|
|||
|---|---|---|---|---|
| 1 mo in:
|
7 mo in:
|
|||
| Lung | Spleen | Lung | Spleen | |
| Naive | 6.66 ± 0.5 | 5.75 ± 0.20 | 6.60 ± 0.07 | 5.44 ± 0.05 |
| BCG | 5.67 ± 0.08 (−0.97)*** | 4.29 ± 0.15 (−1.45)* | 5.51 ± 0.24 (−1.09)** | 5.34 ± 0.08 |
| Sin85B | 6.12 ± 0.15 (−0.54)* | 5.01 ± 0.08 (−0.73)* | 5.89 ± 0.16 (−0.71)* | 5.32 ± 0.16 |
| SinVCc | NDd | ND | 6.39 ± 0.10 | 5.53 ± 0.15 |
C57BL/6 mice were immunized once with 106 CFU BCG subcutaneously or three times with 0.05 mg Sin85B i.m. at 3-week intervals. The mice were infected with a low-dose aerosol M. tuberculosis challenge 4 weeks after the last DNA immunization.
Mean bacterial CFU in the lungs and spleens 1 month or 7 months following an aerosol challenge with ∼300 CFU of M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the reductions in bacterial burden relative to naive controls. Statistical significance compared to controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Sindbis virus vector control.
ND, not done.
TABLE 2.
Sin85B-mediated protection is IFN-γ dependent
| Groupa | Mean log10 CFU of M. tuberculosis ± SEMb
|
|
|---|---|---|
| Lung | Spleen | |
| Naive | 6.42 ± 0.06 | 5.08 ± 0.10 |
| Sin85B | 5.79 ± 0.01 (−0.63)* | 4.64 ± 0.04 (−0.44) |
| Sin85B + αIFN-γc | 7.53 ± 0.13 (+1.11)*** | 6.25 ± 0.25 (+1.17)*** |
| pVax-85B | 5.88 ± 0.11 (−0.54)* | 4.96 ± 0.03 (−0.12) |
| pVax-85B + αIFN-γ | 7.74 ± 0.18 (+1.32)*** | 6.16 ± 0.23 (+1.08)** |
C57BL/6 mice were immunized three times with 0.2 mg pVax-85B or 0.05 mg Sin85B DNA constructs i.m. at 3-week intervals. The mice were aerogenically challenged 4 weeks after the last immunization.
Mean bacterial CFU in the lungs and spleens of mice 28 days following an aerosol infection with ∼300 CFU of M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the reductions in bacterial burden relative to naive control mice. Statistical significance compared to controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Anti-IFN-γ (αIFN-γ) mAb (0.5 mg; clone XMG) was injected intraperitoneally 2 days before, the day of, and every 7 days after the aerosol infection.
FIG. 3.
Histopathological analysis of lungs from vaccinated and naive mice obtained 190 days following an M. tuberculosis aerosol challenge. Lung snips were removed, fixed with formalin, and stained with H&E stain. Representative slides are shown for naive controls and BCG-vaccinated and Sin85B-vaccinated mice. Five mice per group and five sections per each lung were analyzed (magnification, ×172).
As seen in Fig. 4, the decreased mycobacterial growth and the improved lung pathology translated into significantly longer survival periods for the vaccinated mice (P < 0.05). In this study the mean time to death (MTD) for the naive mice was 203 ± 13 days after the low-dose tuberculous challenge. In contrast, the Sin85B-vaccinated mice survived an average of 102 days longer (MTD = 305 ± 9 days) while the survival period for the BCG-immunized mice was increased by 91 days (MTD = 294 ± 15 days).
FIG. 4.
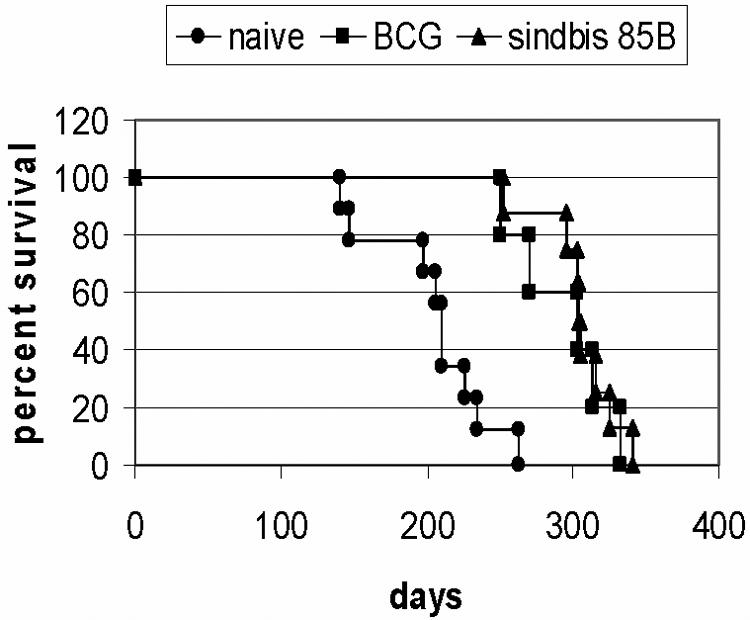
The survival of mice immunized with BCG or the Sin85B DNA vaccine following an aerogenic challenge with M. tuberculosis Erdman. BCG-vaccinated and naive mice served as controls. Six to eight mice per group were used in this analysis.
Immune mechanisms associated with the protection induced by immunization with the Sin85B plasmid—the role of gamma interferon in the protective response.
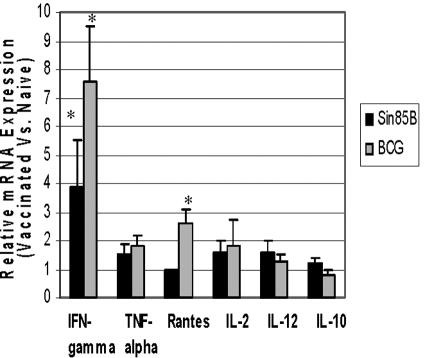
Protection against tuberculosis is largely mediated by cellular immune mechanisms, and IFN-γ has been shown to be a key cytokine involved in cellular response to tuberculous infections (14, 15). Therefore, initial mechanistic studies were designed to identify cytokines, including IFN-γ, that were induced in vaccinated mice after a low-dose aerogenic tuberculous challenge. Previous experiments had indicated that vaccine-induced protective responses could be directly detected at 10 days postchallenge in our mouse model of pulmonary TB by measuring relative cytokine mRNA levels in pulmonary cells (C. Goter-Robinson and S. Morris, unpublished data). For these studies, wild-type C57BL/6 mice were vaccinated either with the Sin85B construct or BCG and then challenged aerogenically with virulent M. tuberculosis. At 10 days postinfection, RNA was isolated from pulmonary lymphocytes and cytokine gene expression was subsequently evaluated using real-time RT-PCR. Among the six cytokines evaluated, only IFN-γ gene expression was substantially elevated (3.9-fold increase relative to naive mice) in the Sin85B-vaccinated mice at 10 days postchallenge (Fig. 5). For the BCG-immunized mice, only IFN-γ (7.6-fold elevation) and RANTES (2.6-fold increase) gene expression was consistently increased compared to naive mice.
FIG. 5.
Vaccine-induced cytokine mRNA responses. Ten days following an aerogenic challenge with M. tuberculosis Erdman, RNA was obtained from lung cells of BCG- and Sin85B-vaccinated mice and used for real-time RT-PCR analysis of IFN-γ, tumor necrosis factor alpha (TNF-alpha), RANTES, IL-2, IL-12, and IL-10 mRNA concentrations relative to mRNA levels of GAPDH. The cytokine levels are expressed as values relative to the mRNA concentrations of the naive controls. *, mRNA levels are significantly elevated compared to the naive controls (P < 0.05).
Since IFN-γ gene expression was elevated in Sin85B-vaccinated mice in response to a low-dose TB challenge, the role of this cytokine was further evaluated in antibody depletion experiments. Wild-type C57BL/6 mice were vaccinated with Sin85B and then, using an anti-IFN-γ mAb, were depleted of this cytokine immediately before an aerogenic TB challenge. As a control, mice that had been vaccinated with the conventional pVax-85B DNA vaccine were also depleted of IFN-γ with the same antibody and then challenged. Table 2 shows that the protective immunity induced by vaccination with either the Sin85B construct or the pVax-85B plasmid was completely ablated at 28 days postchallenge in mice treated with the anti-IFN-γ antibody. In this experiment, significant reductions in organ bacterial burdens (relative to naive mice) were detected in the lungs of untreated mice vaccinated with either Sin85B or pVax-85B. However, lung and spleen mycobacterial CFU were significantly increased (relative to naive and vaccinated animals) in the anti-IFN-γ-treated DNA-vaccinated mice.
Vaccination of CD4−/− and CD8−/− mice with the Sin85B construct.
To further dissect the antituberculosis protective mechanisms induced by vaccination with the Sin85B plasmid, mice deficient in CD4 cells were immunized with the Sin85B construct, the pVax-85B plasmid, or BCG and were then challenged with virulent TB. Mycobacterial growth assessments at 28 days postchallenge demonstrated that immunization with Sin85B induced substantial protective immunity in the lungs (−1.5 log10 CFU reduction relative to naive mice) and spleens (−0.7 log10 decrease) of CD4−/− mice (Table 3). Immunization with BCG also evoked a strong protective response in the organs (−2.5 log10, lungs; −2.0 log10, spleens) of CD4−/− mice. Conversely, immunization with the pVax-85B vaccine yielded significantly less protection in the lungs (−0.95 log10) than the other vaccines and no significant reduction in spleen growth in this model. The lung pathology seen in these CD4−/− mice at 28 days postinfection paralleled the bacterial-growth results. For the naive mice, extensive inflammation, necrosis, and pneumonia were detected in H&E-stained lung sections (data not shown). In contrast, smaller areas of inflammation, less cellular infiltrate, and no necrosis were seen in the lung sections taken from BCG- and Sin85B-vaccinated mice.
TABLE 3.
Vaccination with the Sin85B vaccine protects CD4−/− mice
| Groupa | Mean log10 of M. tuberculosis ± SEMb
|
|
|---|---|---|
| Lung | Spleen | |
| Naive | 8.28 ± 0.01 | 6.38 ± 0.10 |
| Sin85B | 6.72 ± 0.02 (−1.52)*** | 5.70 ± 0.04 (−0.68)* |
| pVax-85B | 7.32 ± 0.10 (−0.96)** | 6.03 ± 0.29 (−0.37) |
| BCG | 5.70 ± 0.08 (−2.58)*** | 4.38 ± 0.16 (−2.00)*** |
CD4−/− mice were immunized once with 106 CFU BCG or three times with 0.2 mg pVAX-85B or 0.05 mg Sin85B DNA i.m. at 3-week intervals. The mice were challenged by aerosol 4 weeks after the last DNA immunization.
Mean bacterial CFU in the lungs and spleens of mice 28 days following an aerosol infection with M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the reductions in bacterial burden relative to naive control mice. Statistical significance compared to controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Surprisingly, no protection was seen after CD8−/− mice were vaccinated with either the Sin85B construct or the pVax85B plasmid and then challenged with virulent TB. Similar bacterial burdens were detected in the lungs (naive, 6.32 ± 0.09 CFU; Sin85B, 6.25 ± 0.13 CFU; pVax85B, 6.28 ± 0.06 CFU) and spleens (naive, 5.54 ± 0.26 CFU; sin85B, 5.40 ± 0.26 CFU; pVax85B, 5.45 ± 0.04 CFU) of vaccinated and control CD8−/− mice at 28 days postinfection.
The absence of protection in RNase L−/− mice.
A recent study by Leitner et al. suggested that the activity of alphavirus-based DNA vaccines is partially linked to the activation of the RNase L innate immune pathway (22). To investigate the role of the RNase L pathway in antituberculosis protection elicited by the Sin85B plasmid, RNase L−/− mice were immunized with either Sin85B, pVax-85B, or BCG and then aerogenically challenged with virulent TB. Twenty-eight days later, significant reductions in mycobacterial growth were seen in the lungs and spleens of the BCG- and pVax-85B-immunized mice (Table 4). In contrast, the vaccine effect was abrogated in the Sin85B-immunized RNase L-deficient mice, with no significant decreases in mycobacterial burden detected relative to naive controls. Again, the lung pathology was consistent with the mycobacterial CFU assessments (Fig. 6). In acid-fast-stained lung sections of naive and Sin85B-immunized mice, substantial numbers of acid-fast bacilli were detected. In contrast, considerably less acid-fast rods were seen in pulmonary sections of animals vaccinated with either BCG or the pVax-85B plasmid. Moreover, in naive RNase L−/− mice, considerable interstitial pneumonia and inflammation were apparent after H&E staining (data not shown). Substantial interstitial swelling and cellular influx and inflammation were also seen in lung sections of the Sin85B-vaccinated RNase L-deficient mice. Conversely, reduced interstitial swelling and vaccine-induced lymphocyte-rich granulomatous structures were present in the lungs of the pVax-85B- and BCG-immunized RNase L−/− mice.
TABLE 4.
Sin85B-mediated protection is diminished in RNase L−/− mice
| Groupa | Mean log10 of M. tuberculosis ± SEMb
|
|
|---|---|---|
| Lung | Spleen | |
| Naive | 6.57 ± 0.11 | 4.97 ± 0.03 |
| Sin85B | 6.44 ± 0.12 | 5.21 ± 0.11 |
| pVax-85B | 5.90 ± 0.14 (−0.67)* | 4.60 ± 0.06 (−0.37)* |
| BCG | 4.95 ± 0.05 (−1.62)*** | 4.11 ± 0.10 (−0.86)*** |
RNase L−/− mice were immunized once with 106 CFU BCG or three times with 0.2 mg pVAX-85B or 0.05 mg Sin85B DNA i.m. at 3-week intervals. The mice were aerogenically challenged 4 weeks after the last DNA immunization.
Mean bacterial CFU in the lungs and spleens of mice 28 days following an aerosol infection with ∼300 CFU of M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the reductions in bacterial burden relative to naive control mice. Statistical significance compared to controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG. 6.
RNase L−/− mice were immunized with either BCG, pVax-85B, or Sin85B and then challenged aerogenically with virulent M. tuberculosis Erdman. Twenty-eight days postchallenge, the lungs were removed and lung snips were fixed with formalin and stained with Ziehl-Neelsen acid-fast stain. Naive mice served as controls. Magnification, ×186.
DISCUSSION
Generally, the results of clinical trials aimed at evaluating conventional DNA vaccines have been disappointing. Even when administered at mg doses, DNA vaccine constructs have usually induced only modest immune responses in humans (2, 10, 30). To enhance the immunogenicity of DNA vaccines, a new generation of nucleic acid vaccines has been developed, where expression of the encoded antigen involves the alphavirus RNA replication machinery, one of the most efficient replication systems in nature. We and others have previously demonstrated that these alphavirus-based vaccines are considerably more immunogenic than conventional DNA vaccines (19, 22, 24, 25). In this study, we extend these observations by showing that vaccination with low doses of a single Sindbis virus-based TB DNA vaccine encoding the highly immunogenic TB protein, antigen 85B, provides substantial protection after a virulent M. tuberculosis challenge. Importantly, the postchallenge survival period for mice immunized with only 5 μg of the single Sin85B plasmid was similar to the mean survival period of BCG-vaccinated mice. These long-term protection results indicate that Sindbis virus-based TB DNA vaccines are attractive human vaccine candidates. Their enhanced immunogenicity suggests that considerably lower doses of the Sindbis TB constructs may yield, in humans, more robust immune responses than conventional DNA vaccines. Additionally, while the effectiveness of other promising gene-based TB vaccines, the modified vaccinia virus- and adenovirus-vectored constructs, may be limited by either preexisting or postimmunization antiviral neutralizing antibodies, humoral responses to the Sindbis virus plasmid vectors have apparently not limited protective responses in mice and likely will not in humans (20, 27, 33). Clearly, a prime-boost protocol where vaccination with a Sindbis virus-based vector is followed by immunization with a single dose of a viral vector expressing the same TB antigen would be a potentially powerful new vaccination strategy that should be considered for testing in humans.
One mechanism that has been proposed to explain the enhanced immunogenicity of alphaviral vectors involves increased antigen production (25). While alphaviral-replicase-based vectors were originally designed to generate increased levels of antigen, recent studies have suggested that their enhanced efficacy may not be due to elevated antigen expression. For instance, Leitner and colleagues demonstrated in a tumor model that the enhanced immunogenicity of a Sindbis virus-based construct did not correlate with antigen production (22). Our results are consistent with this finding. Although the Sin85B plasmid was relatively more protective than the conventional pVax-85B construct, the levels of antigen 85B mRNA detected in cells transfected with these vectors were similar. Furthermore, the eightfold-higher antigen 85B antibody titers detected in mice vaccinated with the pVax-85B plasmid suggests that in vivo expression of antigen 85B from the conventional vector was similar or even slightly elevated compared to expression from the Sin85B plasmid.
The activation of antiviral innate immunity is an alternative immune mechanism that may explain the enhanced efficiency of alphaviral vectors. Alphavirus replicon-based vaccines, like RNA viruses, induce the production of dsRNA molecules which stimulate antiviral pathways. A key protein activated by dsRNA is RNase L, an enzyme responsible for the degradation of mRNA and inhibition of protein translation, which ultimately lead to apoptotic cell death (23). In support of this mechanism, a recent study demonstrated that activation of the RNase L pathway was required for the induction of immunity by an alphaviral vector (22). Our results are consistent with the activation of antiviral innate immunity contributing to the immunogenicity of the Sin85B plasmid. We observed an abrogation of protection when RNase L-deficient mice were vaccinated with the Sin85B construct. In contrast, the induction of protective immunity by vaccination with the conventional pVax-85B DNA vaccine and BCG was not significantly impacted in the RNase L−/− model, indicating that the efficacy of these vaccines is not dependent on stimulating this innate immune pathway for activity. Based on in vitro and in vivo studies, it has been recently reported that apoptosis resulting from the stimulation of antiviral innate immunity is essential for the increased immunogenicity of alphavirus-based vaccines (21). Similar studies are ongoing to determine whether the Sin85B construct induces increased apoptosis.
Our studies using CD4−/−, CD8−/−, and IFN-γ-depleted mice strongly suggest that CD8 cells and IFN-γ are also important mediators of the Sin85B-induced protective responses. Surprisingly, vaccination-challenge studies using CD4−/− mice demonstrated that the Sin85B plasmid evoked significantly better protective immunity than the pVax-85B construct. This result strongly suggests that the stimulation of CD8 cells by Sin85B vaccination contributes to the protection seen in CD4−/− mice. Previously, we had shown that the protection mediated by a TB DNA vaccine cocktail in this same model is dependent on CD8 T-cell activation (8). Interestingly, while we have demonstrated that CD4-deficient mice can be protected against a tuberculous challenge by a DNA vaccine expressing antigen 85B, D'Souza et al. reported that immunization with a 85A-expressing DNA vaccine induced immune responses that protected β2-microglobulin deficient mice (which lack functional CD8 cells) but not CD4−/− mice (11). Although the specific reasons for these disparate results are unclear, the use of different mouse strains, TB antigens, immunization schedules, and modes of challenge (intravenous versus aerosol) may have contributed to the differing outcomes. Additionally, a recent report indicating that CD4−/− mice retain major histocompatibility complex class II restricted CD8 cells suggests that data from these CD4-deficient mice should be interpreted with caution (32).
In summary, we have shown that vaccination with a highly immunogenic Sindbis virus-based vector expressing the M. tuberculosis protein antigen 85B induces extended antituberculosis protective responses after challenge. Our studies suggest that the Sin85B-induced protective immunity may result partially from the activation of innate immune pathways and may be mediated by CD8 T cells and IFN-γ. These findings suggest that targeting innate immune pathways with specific immunomodulators may allow the induction of powerful innate responses to complement the acquired immune responses induced by more conventional TB vaccines.
Editor: J. L. Flynn
REFERENCES
- 1.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. Kelly, A. Frank, M. Lui, J. Ulmer, K. Huygen, D. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzosky, J. A., M. Treube, S. Oh, I. Belyakov, J. Ahlers, J. Janik, and J. Morris. 2004. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J. Clin. Investig. 113:1515-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton, W. J., and U. Palendra. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34-45. [DOI] [PubMed] [Google Scholar]
- 4.Castelli, J., K. W. Wood, and R. J. Youle. 1998. The 2-5A system in viral infection and apoptosis. Biomed. Phamacother. 52:386-390. [DOI] [PubMed] [Google Scholar]
- 5.Colditz, G., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. Fineberg, and F. Mosteler. 1994. Efficacy of BCG vaccine against tuberculosis. JAMA 271:698-702. [PubMed] [Google Scholar]
- 6.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. Williams, M. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 7.Delogu, G., A. Li, C. Repique, F. Collins, and S. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrick, S. C., C. Repique, P. Snoy, A. L. Yang, and S. Morris. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect. Immun. 72:1685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrick, S. C., A. L. Yang, and S. Morris. 2004. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 23:780-788. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly, J., K. Berry, and J. Ulmer. 2003. Technical and regulatory hurdles for DNA vaccines. Int. J. Parasitol. 33:457-467. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza, S., O. Denis, T. Scorza, F. Nzaabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 12.Dye, C., M. Espinal, C. Watt, C. Mbianga, and B. G. Williams. 2002. World-wide incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 185:1197-1202. [DOI] [PubMed] [Google Scholar]
- 13.Fine, P. E. M. 2001. BCG: the challenge continues. Scand. J. Infect. Dis. 33:243-245. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis 84:93-101. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 16.Frieden, T. R., T. Sterling, S. Munsiff, C. Watt, and C. Dye. 2003. Tuberculosis. Lancet 362:887-899. [DOI] [PubMed] [Google Scholar]
- 17.Hesseling, A. C., H. Schaaf, W. Hanekom, N. Beyers, M. Cotton, R. Gie, B. Marais, P. van Helden, and R. Warren. 2003. Danish bacille Calmette-Guerin vaccine—induced disease in human immunodeficiency virus-infected children. Clin. Infect. Dis. 37:1226-1233. [DOI] [PubMed] [Google Scholar]
- 18.Huygen, K., J. Content, O. Denis, D. Montgomery, A. Yawman, R. Deck, C. DeWitt, I. Orme, S. Baldwin, C. D'Souza, A. Drowert, E. Lozes, P. Vandenbussche, J. Van Vooren, M. Liu, and J. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 19.Kirman, J., T. Turon, H. Su, C. Kraus, J. Polo, J. Belisle, S. Morris, and R. Seder. 2003. Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect. Immun. 71:575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostense, S., W. Koudstaal, M. Sprangers, G. J. Weverling, G. Penders, N. Helmus, R. Vogels, M. Bakker, B. Berkhout, M. Havenga, J. Goudsmit. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 18:1213-1216. [DOI] [PubMed] [Google Scholar]
- 21.Leitner, W., L. Hwang, E. Bergman-Leitner, S. Finklestein, A. Frank, and N. Restifo. 2004. Apoptosis is essential for the increased efficacy of alphaviral replicase-based DNA vaccines. Vaccine 29:1537-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitner, W., L. Hwang, M. DeVeer, A. Zhou, R. Silverman, B. Williams, T. Dubensky, H. Ying, and N. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitner, W., and N. Restifo. 2003. DNA vaccines and apoptosis: to kill or not to kill? J. Clin. Investig. 112:22-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitner, W., H. Ying, D. Driver, T. Dubensky, and N. Restifo. 2000. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 60:51-55. [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner, W., H. Ying, and N. Restifo. 2000. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 28.Morris, S., C. Kelley, A. Howard, Z. Li, and F. Collins. 2000. The immunogenicity of single and combination vaccines against tuberculosis. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 29.Olsen, A., and P. Andersen. 2003. A novel TB vaccine: strategies to combat a complex pathogen. Immunol. Lett. 85:207-211. [DOI] [PubMed] [Google Scholar]
- 30.Powell, K. 2004. DNA vaccines—back in the saddle again? Nat. Biotechnol. 22:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlesinger, S. 2001. Alphavirus vectors: development and potential therapeutic applications. Expert Opin. Biol. Ther. 1:177-191. [DOI] [PubMed] [Google Scholar]
- 32.Tyznik, A., J. C. Sun, and M. J. Bevan. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J. Exp. Med. 199:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J., L. Therson, R. Stokes, M. Samuelson, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parental, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173:6357-6365. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2004. Tuberculosis. Infection and transmission. Fact sheet 104. http://www.who.int/mediacentre/factsheets/fs104/en/.