Abstract
Cellular immunity mediated by T lymphocytes, in particular CD4+ and CD8+ type 1 cells, is the main defense against pathogenic fungi. Here, CD28-deficient (CD28−/−) mice were used to study the role of costimulation for the generation and maintenance of T-cell-mediated, type 1 cytokine-dependent mechanisms of vaccine immunity to Blastomyces dermatitidis infection. Disruption of CD28 costimulation reduced the number of type 1 CD4 and CD8 cells generated and impaired resistance to infection. Type 1 T-cell subsets generated in vaccinated CD28−/− mice were durable and protected mice for at least 3 months after vaccination. Our findings suggest that CD28 is required for the induction of optimal, protective T-cell responses to B. dermatitidis infection but may be dispensable for the maintenance of T-cell memory.
Protective immunity to infections with endemic fungi, including Blastomyces dermatitidis, is thought to require type 1-dependent cell-mediated immunity (CMI) responses (10, 11, 33, 35) and can be mediated by both vaccine-induced CD4+ (35) and CD8+ T cells (34) that produce type 1 cytokines, particularly gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). To uncover the molecular requirement that lead to effective generation and maintenance of memory T cells, we investigated the role of B7:CD28 costimulation for the activation of CD4 and CD8 T cells in vaccine-induced immunity to B. dermatitidis infection.
It is now accepted that the engagement of the T-cell receptor (TCR) by peptide/major histocompatibility complex (signal 1) is generally not sufficient to trigger a T-cell response and that ligation of a costimulatory receptor(s) (signal 2) is required (17, 19, 24, 31). Indeed, signaling via the TCR alone can induce a state of anergy in naive CD4+ T cells in certain situations (4, 5). The most-studied costimulatory molecules are B7-1 (CD80) and B7-2 (CD86), which bind to the counter-receptors CD28 and CTLA-4 at the T-cell surface. The B7-1/B7-2:CD28/CTLA-4 costimulatory pathway has received considerable attention as a regulatory control point in T-cell responses. The low levels of B7-1 and B7-2 on immature antigen-presenting cells (APC) are upregulated after activation and interact with positive (CD28) or negative (CTLA-4) receptors on T cells. Both B7 molecules are considered essential for induction of major histocompatibility complex class II-restricted T-cell responses (4). In support of this view, CD4 T-cell responses to lymphocytic choriomeningitis virus (9, 20), herpes simplex virus type 1 (13), and influenza virus (3) are greatly reduced in CD28−/− mice. Conversely, parasitic infection with Leishmania major (7), Toxoplasma gondii (30), and Heligmosomoides polygyrus (15) activates CD4 cells independently of CD28/B7 interaction.
The role of B7 costimulation in the initiation of CD8 responses in vivo is less clear. In cases where the CD8 response occurs through a CD4-dependent pathway of APC activation, it is held that APC conditioning involves costimulatory signaling via CD40-CD40L and B7-CD28. Consequently, both B7-1/B7-2 and CD28 are required for cytotoxic-T-lymphocyte (CTL) cross-priming by CD40-activated APC. This indicates that CD28 can function as a molecular rheostat by which upregulation of B7 molecules on APC is perceived as “help” by CTL, leading to their primary activation and functional development (25). In cases where the CD8 T-cell response occurs through an alternative, CD4-independent pathway of dendritic cells activation, it is held that only the B7/CD28 costimulation, downstream of APC activation, is essential for induction of naive CD8 T cells. In support of this model, CD8 T-cell responses during L. monocytogenes infection is normal in CD40L−/− mice but defective in CD28−/− mice (27). Similarly, the generation of CD4 T cell-independent CTL to both replicating vaccinia viruses and exogenous antigens depend upon the direct interaction of the CD8 T-cell with the APC displaying the B7 molecules (28). Further, cross-priming of CD8 CTL by immunostimulatory sequence DNA vaccines occurs by directly upregulating B7 on APC and thereby bypassing CD40-dependent T-cell help (8).
Here, we assessed T-cell responses in wild-type and CD28−/− mice during vaccine induced immunity to B. dermatitidis infection. Our findings demonstrate that B7:CD28 costimulation is required for the optimal induction of vaccine-induced CD4+ and CD8+ T cells and resistance. On the other hand, although reduced, significant numbers of type 1 cytokine producing T cells and resistance to B. dermatitidis infection were generated and maintained independent of CD28/B7 interaction. Hence, our studies suggest that CD28 is required for the optimal induction of protective memory T-cell responses but may not be required for their long-term maintenance.
MATERIALS AND METHODS
Fungi.
Strains used were ATCC 26199 (16), and the isogenic, attenuated mutant lacking BAD1, designated strain 55 (6). Isolates of B. dermatitidis were maintained as yeast on Middlebrook 7H10 agar with oleic acid-albumin complex (Sigma Chemical Co., St. Louis, MO) at 39°C.
Mouse strains.
Inbred strains of mice, including C57BL/6 and CD28 deficient B6.129S2-Cd28tm1Mak stock 2666 (26), were obtained from Jackson Laboratories, Bar Harbor, ME. Male mice 6 to 7 weeks of age at the time of purchase were housed and cared for throughout these experiments according to the guidelines of the University of Wisconsin Animal Care Committee, who approved all aspects of the present study.
In vivo cell depletion.
CD4+ and CD8+ T cells were depleted by monoclonal antibody (MAb) treatment. MAb GK1.5 (rat immunoglobulin G2b [IgG2b] anti-CD4) was purchased from the American Type Culture Collection, and MAb 2.43 (rat IgG2b anti-CD8) was provided by A. Rakhmilevich, University of Wisconsin-Madison, WI. Ascites was made in BALB/c Nu/Nu males. Rat IgG in ascites was ammonium-sulfate precipitated. For quantitation, an aliquot of precipitated MAb was purified over an ImmunoPure Immobilized Protein A/G column (Pierce, Rockford, IL), and the optical density at 280 nm (OD280) of the purified protein was measured. For depletion, mice received 100 μg of anti-CD4 MAb or anti-CD8 MAb intravenously 1 day before vaccination and/or infection and weekly afterward. Cell depletion analyzed by fluorescence-activated cell sorting showed >95% depletion of desired subsets in the peripheral blood and lung (data not shown).
Real time reverse transcription-PCR.
Lung cells were harvested at day 2 postinfection with 2 × 103 26199 yeast by crushing the organs from individual mice separately (n = three to five mice/group) in 40 μM cell strainers (Becton Dickinson, Lincoln Park, N.J.) to obtain single-cell suspensions. Erythrocytes were lysed with NH4Cl-Tris solution and washed twice. Total RNA was isolated from 1-5 × 106 lung cells by using the RNeasy Minikit (QIAGEN, Valencia, CA). RNA was purified over RNeasy minicolumns and treated with the RNase-free DNase Set (QIAGEN). A total of 0.5 to 1 μg of RNA in a final volume of 20 μl was reverse transcribed by using random hexamers and the TaqMan reverse transcription-PCR kit (Applied Biosystems, Foster City, CA). A total of 5 μl of a 1:10 dilution of cDNA was amplified in a final volume of 25 μl PCR using SYBR Green SuperMix (Bio-Rad, Hercules, CA). The following intron-spanning primers were used at a final concentration of 100 nM: IFN-γ (forward, TGCATTCATGAGTATTGCCAAGT; reverse, CGCTTCCTGAGGCTGGATT) spans 2.3-kb intron, TNF-α (forward, TGGCCTCCCTCTCATCAGTT; reverse, TCCTCCACTTGGTGGTTTGC) spans 470-bp intron, granulocyte-macrophage colony-stimulating factor (GM-CSF; forward, GGGCGCCTTGAACATGAC; reverse, TTGTGTTTCACAGTCCGTTTCC) spans 869-bp intron, and 18S rRNA as an endogenous control gene (forward, CGCCGCTAGAGGTGAAATTCT; reverse, CGAACCTCCGACTTTCGTTCT). Amplification was performed in an iCycler iQ real-time PCR detection system (Bio-Rad) and assayed under the same conditions for all targets: 5 min at 95°C, followed by 40 cycles of 15 s at 95°C and 45 s at 60°C. Transcript quantity was calculated by using the comparative CT method (18) and reported as the n-fold difference relative to a calibrator cDNA (i.e., sample from unvaccinated mice). The data represent an average of two independent experiments.
Intracellular cytokine staining.
Lung cells from individual mice were harvested at day 4 postinfection with 2 × 103 26199 yeast as described above. The isolated lung cells (0.5 × 106 cells/ml) were stimulated for 4 h with anti-CD3 (clone 145-2C11; 1 μg/ml) in the presence of 2 μM monensin (Sigma) to halt the egress of cytokines from the cells. After cells were washed and stained for surface CD4 and CD8 with anti-CD4 fluorescein isothiocyanate, anti-CD8 CyChrome, and CD44-APC MAbs (clones H129.19, 53-6.7, and IM7; Pharmingen, San Diego, CA), they were fixed in 2% paraformaldehyde at 4°C overnight and the next day were permeabilized with 0.1% saponin in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% sodium azide. Permeabilized cells were stained with phycoerythrin-conjugated MAbs and isotype controls (Pharmingen) for IFN-γ (clone XMG1.2), TNF-α (clone MP6-XT22), and GM-CSF (clone MP1-22E9) in 20% mouse serum for 30 min at 4°C, washed, and analyzed by fluorescence-activated cell sorting. Lymphocytes were gated on CD4 or CD8 and CD44-high, and cytokine expression within each gate analyzed. The number of cytokine producing CD4+ and CD8+ T cells per lung was calculated by generating the product of % cytokine producing cells, and the number of CD4+ and CD8+ cells in the lung.
Vaccination and experimental infection with B. dermatitidis.
Mice were vaccinated as described previously (33) twice, two weeks apart, each time receiving a subcutaneous injection of 104 strain 55 yeast at each of two sites, dorsally and at the base of the tail, unless otherwise stated. Mice were infected intratracheally with 2 × 103 wild-type strain 26199 yeast as described previously (33). Infected mice were analyzed 2 weeks after infection for extent of lung infection, as determined by plating homogenized lung and enumeration of yeast CFU on brain heart infusion (Difco, Detroit, MI) agar. To distinguish wild-type strain 26199 yeast from vaccine strain 55 (containing a selectable marker) in tissue homogenates, samples were plated on brain heart infusion with or without 100 μg of hygromycin B/ml (6).
Histological analysis.
Lung and skin tissue was fixed in 10% neutral formalin and embedded in paraffin wax. Sections 5 μm thick were stained with hematoxylin-eosin and Gomori's methenamine silver. Skin lesions were scored for size necrosis, fibroblasts, collagen content, macrophages, polymorphonuclear leukocytes, lymphocytes, number of organisms, penetration of connective tissue capsule, and smaller satellite lesions. To quantify the number of yeast and extent of inflammation, a randomly collected tissue section was analyzed for each animal in a group. The number of yeast was counted with a ×60 objective lens, in 20 fields projected on a TV screen, and the data were expressed as yeast/high-power field. The extent of pneumonic consolidation was measured at a final projected magnification of 8.8 and expressed as a percentage of the total lung area in a section. The Delesse principle of morphometry was applied to quantify the relative amount of granulomatous inflammation among groups (32). Comparative involvement can be validly inferred since tissue samples were collected randomly from each member of the group, a sample was randomly oriented “en face” in the tissue block and thus the tissue section, and the granulomas in the lungs of the animals should be distributed randomly given the method of introduction of fungi into a dichotomously branching bronchial tree.
Statistical analysis.
The number of cytokine-producing CD4+ and CD8+ T cells, the relative changes in cytokine transcripts, and the differences in the number of CFU were analyzed by using the Wilcoxon rank test for nonparametric data (14). A P value of <0.05 is considered statistically significant.
RESULTS
Induction of type 1 cytokine responses in the absence of CD28/B7 interaction.
Since CD28 costimulation can enhance the initial activation of T cells, promote cell division, augment cell survival and induce effector functions such as cytokine secretion, we explored whether vaccinated CD28−/− mice failed to undergo priming and acquire type 1 cytokine-producing T cells. We vaccinated CD28−/− mice and wild-type mice either in the presence of CD4 cells (drives vaccine resistance mediated by CD4 cells) (33, 35) or in the absence of CD4 cells (drives resistance mediated by CD8 cells) (34). By day 4 postinfection, the number of primed (CD44+) T cells was sharply reduced in the lungs of vaccinated CD28−/− mice versus the corresponding wild-type controls (Fig. 1, top panel).
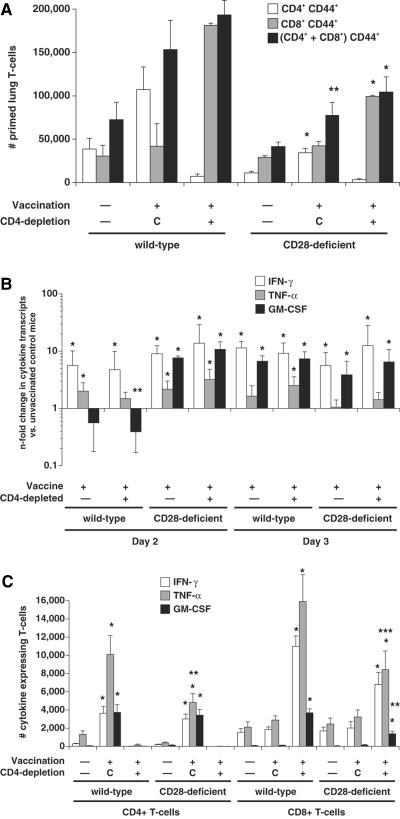
FIG. 1.
T-cell priming and production of IFN-γ, TNF-α, and GM-CSF transcript and protein by lung T cells after B. dermatitidis infection in vaccinated and unvaccinated wild-type and CD28−/− mice. (Top panel) Priming of lung T cells as measured by surface expression of CD44 on cells analyzed 4 days postinfection. The data are an average of six to nine mice/group. Mice were infected with 2 × 103 yeast. *, P < 0.05; **, P < 0.1 (versus vaccinated wild-type controls). Controls (C) represent vaccinated mice treated with rat IgG. (Middle panel) n-fold changes in lung cytokine transcripts in vaccinated wild-type and CD28−/− mice versus unvaccinated controls as measured by real-time PCR. The data represent an average of three to five mice/group. Mice were infected with 2 × 103 yeast and analyzed 2 and 3 days after infection. *, P < 0.05 (versus unvaccinated controls). Cytokine transcripts did not differ significantly in vaccinated CD28−/− mice versus wild-type controls, except for a marginal difference in GM-CSF transcript at day 2. **, P = 0.06. (Bottom panel) Intracellular cytokine production by CD44+ CD4+ and CD44+ CD8+ cells. The data represent an average of six to nine mice per group. Mice were infected with 2 × 103 yeast and analyzed 4 days after infection. *, P < 0.008 (versus corresponding unvaccinated mice); **, P ≤ 0.02; ***, P < 0.07 (versus corresponding vaccinated wild-type controls). Controls (C) represent vaccinated mice treated with rat IgG.
In parallel, we monitored lung T cells ex vivo for transcript expression and intracellular cytokine protein. We measured lung cell cytokine transcripts 48 and 72 h postinfection, since IFN-γ mRNA levels peak at this time point in association with vaccine resistance (34). Here, IFN-γ and GM-CSF transcript levels increased significantly by 2 to 3 days postinfection in vaccinated wild-type mice (CD4-depleted and nondepleted) versus unvaccinated controls (Fig. 1, middle panel). IFN-γ and GM-CSF transcript levels rose significantly and to a similar extent in vaccinated CD28−/− mice (CD4-depleted and nondepleted mice) versus unvaccinated controls. Thus, no defect in type 1 cytokine differentiation was evident in vaccinated CD28-deficient mice at the level of cytokine transcript.
We assayed intracellular cytokine protein in lung T cells 4 days after infection since the influx of cytokine-producing T cells in vaccinated mice peaks at day 4 postinfection, and this event coincides with a reduction in lung CFU (34). Here, on day 4 postinfection, the number of lung CD4+ and CD8+ T cells producing type 1 cytokines IFN-γ, TNF-α, and GM-CSF increased significantly in both vaccinated wild-type and CD28−/− mice compared to unvaccinated controls (Fig. 1, bottom panel). However, the number of lung CD4+ T cells producing TNF-α was sharply reduced in vaccinated CD28−/− mice compared to vaccinated wild-type controls (Fig. 1, bottom panel). Similarly, in CD4-depleted mice, the number of CD8 cells producing GM-CSF was sharply reduced and the number producing TNF-α showed a similar trend toward reduction in vaccinated CD28−/− mice compared to wild-type controls. Thus, in the absence of CD28 costimulation, mice evinced a defect in T-cell priming and generated fewer CD4 cells that express TNF-α and fewer CD8 cells that produce GM-CSF and possibly TNF-α. Even though the levels of type 1 cytokine transcript were unimpaired by the absence of CD28 costimulation, the numbers of type 1 cytokine-producing CD4+ and CD8+ T cells were moderately reduced.
The number of IFN-γ- and GM-CSF-producing CD4 cells in vaccinated CD28−/− mice was not significantly reduced compared to corresponding wild-type mice despite the reduced number of primed CD4 cells in CD28−/− mice versus the wild type. This discrepancy could be explained by the fact that a higher percentage of lung CD4 cells produced IFN-γ and GM-CSF in vaccinated CD28−/− mice than in wild-type mice (8.9 ± 1.0 and 10.1 ± 1.0 versus 4.3 ± 0.5 and 4.5 ± 0.6, respectively).
Protective immunity in CD28-deficient mice.
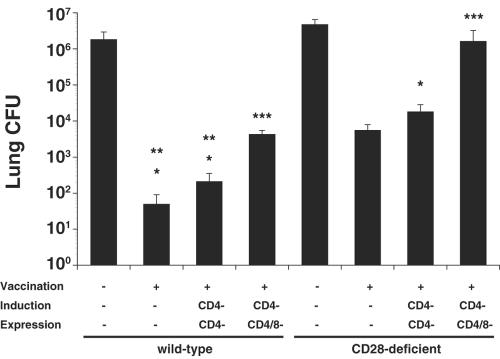
To explore whether CD28 is necessary to acquire functional resistance against B. dermatitidis infection, we challenged mice 4 weeks after vaccination. Vaccinated CD28−/− mice (with CD4 T cells) reduced lung CFU 850-fold versus nonvaccinated controls, whereas vaccinated CD28−/− mice depleted of CD4 cells reduced lung CFU by 260-fold (Fig. 2). In contrast, vaccinated wild-type mice reduced lung CFU 36,000-fold when CD4 cells were present and 8,700-fold when CD4 cells were depleted compared to unvaccinated controls. CD4-depleted CD28−/− mice that were depleted of CD8 cells during vaccine expression had a 380-fold-higher lung burden than the corresponding wild-type control mice, suggesting that elements of adaptive immunity besides CD4 and CD8 cells might also be impaired by the loss of CD28 costimulation. Overall, in the absence of CD28 costimulation, vaccine-induced CD4 and CD8 T cells did confer immunity, but the lung CFU values were, respectively, 42 and 33 times higher in vaccinated CD28−/− mice than in wild-type mice, indicating impaired function.
FIG. 2.
Role of CD28 costimulation for the generation of protective CD4 and CD8 T-cell responses. Mice were vaccinated subcutaneously twice with 104 live yeast at each site of injection. To induce protective CD8 T-cell immunity, CD28−/− and wild-type mice were depleted by anti-CD4 MAb throughout the experiment. Mice were infected with 2 × 103 wild-type yeast and analyzed for lung CFU 14 to 16 days after infection. The data are geometric mean CFU ± the standard error of the mean of two independent experiments; n = 8 to 12 mice/group. *, P < 0.001 (versus unvaccinated controls); **, P < 0.001 (versus corresponding vaccinated CD28−/− mice); ***, P < 0.005 (versus corresponding CD4-depleted mice).
We also examined the histological appearance of inflammation in the lungs of infected mice at the time that burden of lung infection was assessed. The extent of lung consolidation was much higher in CD28−/− mice than in corresponding groups of wild-type mice (Table 1), indicating that the amount of granulomatous lung inflammation correlated inversely with the resistance phenotype. Hence, disruption of the CD28/B7 costimulatory pathway impairs vaccine resistance mediated by CD4 and CD8 cells to B. dermatitidis; however, CD28−/− mice are still partially resistant to infection.
TABLE 1.
Extent of granulomatous inflammation in the lungs of wild-type and CD28−/− mice after B. dermatitidis infection
| Expt and mouse type | Extent (%) of granumatous inflammationa
|
|||
|---|---|---|---|---|
| Unvaccinated | Vaccinated | Vaccinated, CD4 depletedb | Vaccinated, CD4/CD8 depletedc | |
| Expt 1 | ||||
| Wild type | 28.9 | 4.4 | 2.2 | ND |
| CD28−/− | 67.0 | 27.8 | 20.0 | ND |
| Expt 2 | ||||
| Wild type | 71.8 | 0.0 | 4.6 | 14.6 |
| CD28−/− | 88.0 | 15.6 | 40.2 | 64.4 |
That is, the percentage of microscopic field inflamed with granulomas, quantified in 10 to 12 mice per group. The Delesse principle (32), described in Materials and Methods, was applied to systematically assess the extent of granulomatous inflammation in randomly collected and analyzed tissue of all the mice. ND, not determined.
C57BL/6 mice (8 to 10/group) were depleted of CD4+ T cells during the induction and expression phase of vaccine immunity.
In addition to CD4+-T-cell depletion as described above, CD8+ T cells were depleted during the expression phase of vaccine immunity.
CD8 cells require CD28 costimulation for optimal control of the vaccine.
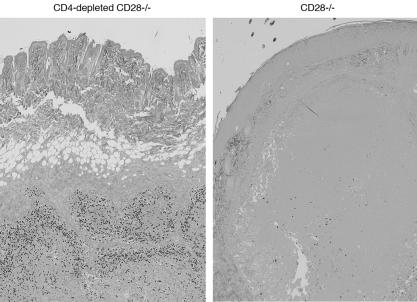
At the time mice were sacrificed to assess the burden of lung infection (4 weeks after booster vaccination), 5 of 12 vaccinated CD4-depleted CD28−/− mice had large, necrotic skin lesions, which consisted of caseous foci harboring abundant organisms (Fig. 3). The lesions were large (3.5 to 7 mm in diameter) and contained 250 to >300 yeasts per high-power field, which were mainly concentrated along the periphery of the lesions. Of 12 mice in that group, 2 had small numbers of vaccine yeast in their lungs (10 and 160 CFU, respectively). In contrast, vaccinated CD28−/− mice (with CD4 cells) had fewer and smaller (1.5 to 2 mm in diameter) lesions with 10 times less yeast per high-power field (Fig. 3), and there was no evidence of dissemination in that group. Wild-type mice that were vaccinated in the presence or absence of CD4 cells all cleared live vaccine yeast from the injection site and showed no dissemination to other organs. Thus, in the absence of CD28 costimulation, vaccine yeast at the injection site were better controlled when CD4 cells were present versus absent, implying that under these conditions CD4 cells were better than CD8 cells in controlling vaccine yeast.
FIG. 3.
Histological appearance of skin tissue at the site of vaccination of vaccinated CD28−/− and wild-type mice. Tissue was analyzed 3 weeks postvaccination when CD28−/− mice were harvested to determine lung CFU. Skin tissue was stained with Gomori's methenamine silver; all images are at ×40 magnification. Yeast cells were 10-fold more plentiful in CD4-depleted CD28−/− mice than in nondepleted CD28−/− mice and were concentrated mainly along the periphery of the lesions.
To investigate further whether CD8 cells primed in the absence of CD28 costimulation ultimately clear vaccine strain yeast from the skin or, alternatively, succumb to dissemination, we vaccinated mice with 100 times more yeast (106 yeast/site) and observed them for dissemination of the vaccine and death. Three of nine CD4-depleted CD28−/− mice died after vaccination, one with dissemination of vaccine yeast in the lung (4.98 × 106 CFU); the remaining six mice in that group and all CD28−/− mice with CD4 cells present cleared the vaccine yeast from the skin. Thus, CD28−/− mice again demonstrated impaired clearance of vaccine yeast when CD4 cells were absent versus present, although both groups of CD28−/− mice ultimately cleared vaccine yeast from the skin. In contrast, wild-type mice vaccinated with or without CD4 cells cleared vaccine yeast from the skin, lacked dissemination of vaccine yeast in the lung, and showed no mortality.
Maintenance of memory CD4 and CD8 T cells in the absence of CD28 costimulation.
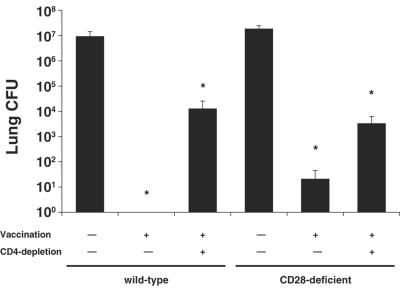
Since T cells can be activated independent of CD28 costimulation during a primary infection with T. gondii but fail to mount a protective secondary response in the absence of CD28 (30), we explored whether antifungal T-cell responses generated in the absence of CD28:B7 interaction are durable. After a resting period of 3 months postvaccination, we infected mice and 3 weeks later analyzed their burden of lung infection. Vaccination of nondepleted wild-type and CD28−/− mice (where immunity is largely mediated by CD4 cells [34]) led to a reduction of lung CFU by 7 and 5.9 logs, respectively, compared to unvaccinated controls (Fig. 4). In vaccinated CD4-depleted CD28−/− mice, residual CD8 cells reduced lung CFU as efficiently as did CD8 cells from the corresponding CD4-depleted wild-type mice compared to unvaccinated controls (5,500- versus 740-fold, respectively). Hence, CD28 costimulation is dispensable for the maintenance of protective memory CD4 and CD8 T cells.
FIG. 4.
Maintenance of vaccine induced resistance by CD4 and CD8 cells in the absence of CD28. Mice were vaccinated subcutaneously twice with 106 live yeast at each site of injection. After a resting period of 3 months, mice were infected intratracheally with 2 × 103 wild-type yeast. The data are geometric mean CFU ± the standard error of the mean; n = six to nine mice/group. *, P < 0.001 (versus unvaccinated control mice).
DISCUSSION
We have engineered a live attenuated vaccine that induces sterilizing immunity against lethal experimental B. dermatitidis infection (33). In immune competent mice, CD4+-T-cell cytokines TNF-α and IFN-γ mediate vaccine immunity (35). However, these factors, and even CD4 cells themselves (34), are dispensable in immunodeficient mice, illustrating the plasticity of vaccine immunity at both the cellular and the molecular levels (34, 35). We have recently observed that interleukin-12 (IL-12) is required for the induction, but not maintenance, of protective memory responses against B. dermatitidis infection (36). Dispensability of IL-12 during the maintenance phase of antifungal memory immunity was unexpected and, to our knowledge, was not previously established during CMI to infectious diseases. Thus, investigating mechanisms of vaccine-induced immunity has uncovered multiple layers of plasticity or redundancy at the cellular and molecular level. Since interactions between costimulatory ligands and their receptors are crucial for the activation of T cells and their development of memory, we sought to test the role of costimulation in vivo during various phases of the immune response.
Here, we explored the requirement for CD28 costimulation during control of the vaccine strain, induction of type 1 CD4 and CD8 T cells, and maintenance of durable T-cell memory during vaccine immunity to experimental B. dermatitidis infection. We show that the generation of antifungal type 1 CD4 and CD8 T cells, and acquisition of vaccine resistance is partially dependent on CD28 costimulation. Disruption of the CD28/B7 costimulatory pathway reduced vaccine resistance mediated by CD4 and CD8 T cells.
Reduced resistance correlated with a modest reduction in T-cell priming and in the numbers of type 1 cytokine protein-producing T cells in the lung during a recall response (CD4 and CD8 cells producing TNF-α and CD8 cells producing GM-CSF) but not with alterations in cytokine transcript. The discrepancy between protein and transcript data may be explained by the observation that stimulation through CD28 increases the stability of TNF-α and GM-CSF mRNA, which enhances production of lymphokine protein (22). Thus, the reduced numbers of TNF-α and GM-CSF producing T cells in CD28−/− mice in our study may be due to decreased stability of transcripts.
CD8 cells, but not CD4 cells, required CD28 costimulation for optimal control of the vaccine at the injection site, whereas maintenance of both memory T-cell subsets did not require CD28-B7 interactions. These results indicate that CD4 and CD8 cells differ in their requirement for CD28 costimulation during the initial phase of control of the vaccine but that the requirement for costimulation also changes over time. Differential requirements of T-cell subsets for CD28 costimulation at any given time, and during distinct phases of the immune response, have been described in a murine model of toxoplasmosis and during infection with LCMV. CD28 was not necessary to resolve a primary infection with Toxoplasma gondii, but CD28−/− mice failed to resist reinfection (30). The susceptible mice lacked IL-2 and IFN-γ in recall responses and had reduced numbers of CD4+ T cells with a memory phenotype; CD8+ memory T cells were not reduced, indicating differential requirements of T-cell subsets for CD28 costimulation. Similarly, CD4 responses to LCMV were greatly reduced in CD28−/− mice, as measured by IL-2 production and cell numbers (9, 20), whereas loss of CD28/B7 interactions did not affect the generation and maintenance of CD8 T-cell memory (26, 29). Dispensability of CD28 costimulation for the activation of CD8 cells was attributed to the high levels of viral replication, allowing sustained T-cell signaling in the absence of costimulation (21). However, it is unlikely that viral replication alone explains differential requirements for costimulation, since CD28 costimulation was obligatory to generate optimal CD8 CTL responses after influenza virus and vesicular stomatitis virus infections (21, 23).
In our model, CD8 cells show delayed control of the vaccine strain. This suggests a more stringent requirement for CD28 costimulation in the activation of CD8 cells than CD4 cells, which contrasts with the findings described in experimental T. gondii and LCMV infections. On the other hand, CD4-depleted wild-type mice also take longer to control the vaccine strain than wild-type mice with CD4 cells (unpublished observation). Thus, there may be an inherent hierarchy in functional differences between CD4 and CD8 T-cell subsets, independent of CD28 costimulation.
Although vaccine resistance mediated by CD4 and CD8 T cells was impaired in CD28−/− mice in our study, both T-cell subsets still sharply reduced lung CFU by 2 to 3 logs. Moreover, maintenance of both memory T-cell subsets did not require CD28-B7 interactions. Our results can be interpreted in the following light. First, replication of live organisms may increase the level and duration of antigenic stimulation, which can greatly increase signaling through the TCR and thereby lower the requirements for costimulation (1). Second, CD28/B7 costimulatory interaction alone cannot fully account for the development of T-cell immunity. Many receptor-ligand interactions have been identified as costimulatory and positive regulators of T cells. ICOS as a member of the CD28 family and several members of the tumor necrosis factor receptor superfamily, including CD40, OX40, 4-1BB, CD27, CD30, and herpesvirus entry mediator, deliver costimulatory signals both early and late after encounter with antigen (2, 12). Since there are multiple costimulatory receptors, several costimulatory signals might be required for the development of effective T-cell immunity in a given situation. Acknowledging that redundancy exists, the requirement for one or all of these molecular interactions probably reflects two things: (i) that several signals are required simultaneously at any one time during the life of a T cell and (ii) that different signals are required at different times and/or stages of the T-cell response, e.g., to promote alternative effector functions or to allow the antigen-specific T-cell population to persist, creating long-lasting immunity. Such a model helps explain either requisite or dispensable roles for CD28 at different stages or for different T cells subsets in the model of antifungal immunity described here.
Acknowledgments
This study was supported by USPHS grants AI40996 and AI35681 and a Burroughs Wellcome Fund Scholar Award in Molecular Pathogenic Mycology (B.S.K).
We thank Jens Eickhoff (Department of Biostatistics and Medical Informatics) for statistical assistance and Robert Gordon (Department of Pediatrics) for assistance with illustrations.
Editor: T. R. Kozel
REFERENCES
- 1.Bachmann, M. F., R. M. Zinkernagel, and A. Oxenius. 1998. Immune responses in the absence of costimulation: viruses know the trick. J. Immunol. 161:5791-5794. [PubMed] [Google Scholar]
- 2.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T-cell costimulation in antiviral immunity. Semin. Immunol. 16:185-196. [DOI] [PubMed] [Google Scholar]
- 3.Bertram, E. M., P. Lau, and T. H. Watts. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T-cell numbers late in the primary response and regulates the size of the T-cell memory response following influenza infection. J. Immunol. 168:3777-3785. [DOI] [PubMed] [Google Scholar]
- 4.Boussiotis, V. A., G. J. Freeman, J. G. Gribben, and L. M. Nadler. 1996. The role of B7-1/B7-2:CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol. Rev. 153:5-26. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis, V. A., J. G. Gribben, G. J. Freeman, and L. M. Nadler. 1994. Blockade of the CD28 costimulatory pathway: a means to induce tolerance. Curr. Opin. Immunol. 6:797-807. [DOI] [PubMed] [Google Scholar]
- 6.Brandhorst, T. T., M. Wüthrich, T. Warner, and B. Klein. 1999. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. J. Exp. Med. 189:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, D. R., J. M. Green, N. H. Moskowitz, M. Davis, C. B. Thompson, and S. L. Reiner. 1996. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 184:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, H. J., T. Hayashi, S. K. Datta, K. Takabayashi, J. H. Van Uden, A. Horner, M. Corr, and E. Raz. 2002. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 168:4907-4913. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, J. E., J. P. Christensen, N. N. Kristensen, N. J. Hansen, A. Stryhn, and A. R. Thomsen. 2002. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int. Immunol. 14:701-711. [DOI] [PubMed] [Google Scholar]
- 10.Cozad, G. C. 1992. Experimental blastomycosis, p. 221-236. In Y. Al-Doorey and A. F. DiSalvo (ed.), Blastomycosis. Plenum Publishing Corp., New York, N.Y.
- 11.Cozad, G. C., and C. T. Chang. 1980. Cell-mediated immunoprotection in blastomycosis. Infect. Immun. 28:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft, M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609-620. [DOI] [PubMed] [Google Scholar]
- 13.Edelmann, K. H., and C. B. Wilson. 2001. Role of CD28/CD80-86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher, L. D., and G. van Belle. 1993. Biostatistics: a methodology for the health sciences, p. 611-613. John Wiley & Sons, Inc., New York, N.Y.
- 15.Gause, W. C., S. J. Chen, R. J. Greenwald, M. J. Halvorson, P. Lu, X. D. Zhou, S. C. Morris, K. P. Lee, C. H. June, F. D. Finkelman, J. F. Urban, and R. Abe. 1997. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J. Immunol. 158:4082-4087. [PubMed] [Google Scholar]
- 16.Harvey, R. P., E. S. Schmid, C. C. Carrington, and D. A. Stevens. 1978. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am. Rev. Respir. Dis. 117:695-703. [DOI] [PubMed] [Google Scholar]
- 17.Hathcock, K. S., and R. J. Hodes. 1996. Role of the CD28-B7 costimulatory pathways in T cell-dependent B cell responses. Adv. Immunol. 62:131-166. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, M. R., K. Wang, J. B. Smith, M. J. Heslin, and R. B. Diasio. 2000. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal. Biochem. 278:175-184. [DOI] [PubMed] [Google Scholar]
- 19.Kaye, P. M. 1995. Costimulation and the regulation of antimicrobial immunity. Immunol. Today 16:423-427. [DOI] [PubMed] [Google Scholar]
- 20.Kopf, M., A. J. Coyle, N. Schmitz, M. Barner, A. Oxenius, A. Gallimore, J. C. Gutierrez-Ramos, and M. F. Bachmann. 2000. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kundig, T. M., A. Shahinian, K. Kawai, H. W. Mittrucker, E. Sebzda, M. F. Bachmann, T. W. Mak, and P. S. Ohashi. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5:41-52. [DOI] [PubMed] [Google Scholar]
- 22.Lindstein, T., C. H. June, J. A. Ledbetter, G. Stella, and C. B. Thompson. 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T-cell activation pathway. Science 244:339-343. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., R. H. Wenger, M. Zhao, and P. J. Nielsen. 1997. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J. Exp. Med. 185:251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel, P. J., L. H. Boise, J. M. Green, and C. B. Thompson. 1996. CD28 costimulation prevents cell death during primary T-cell activation. J. Immunol. 157:636-642. [PubMed] [Google Scholar]
- 25.Prilliman, K. R., E. E. Lemmens, G. Palioungas, T. G. Wolfe, J. P. Allison, A. H. Sharpe, and S. P. Schoenberger. 2002. Cutting edge: a crucial role for B7-CD28 in transmitting T help from APC to CTL. J. Immunol. 169:4094-4097. [DOI] [PubMed] [Google Scholar]
- 26.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T-cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 27.Shedlock, D. J., J. K. Whitmire, J. Tan, A. S. MacDonald, R. Ahmed, and H. Shen. 2003. Role of CD4 T-cell help and costimulation in CD8 T-cell responses during Listeria monocytogenes infection. J. Immunol. 170:2053-2063. [DOI] [PubMed] [Google Scholar]
- 28.Sigal, L. J., H. Reiser, and K. L. Rock. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J. Immunol. 161:2740-2745. [PubMed] [Google Scholar]
- 29.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T-cell memory. J. Immunol. 167:5565-5573. [DOI] [PubMed] [Google Scholar]
- 30.Villegas, E. N., M. M. Elloso, G. Reichmann, R. Peach, and C. A. Hunter. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J. Immunol. 163:3344-3353. [PubMed] [Google Scholar]
- 31.Walunas, T. L., D. J. Lenschow, C. Y. Bakker, P. S. Linsley, G. J. Freeman, J. M. Green, C. B. Thompson, and J. A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T-cell activation. Immunity 1:405-413. [DOI] [PubMed] [Google Scholar]
- 32.Weibel, E. R., G. S. Kistler, and W. F. Scherle. 1966. Practical stereological methods for morphometric cytology. J. Cell Biol. 30:23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wüthrich, M., H. I. Filutowicz, and B. S. Klein. 2000. Mutation of the WI-1 gene yields an attenuated Blastomyces dermatitidis strain that induces host resistance. J. Clin. Investig. 106:1381-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wüthrich, M., H. I. Filutowicz, T. Warner, G. S. Deepe, Jr., and B. S. Klein. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wüthrich, M., H. I. Filutowicz, T. Warner, and B. S. Klein. 2002. Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts. J. Immunol. 169:6969-6976. [DOI] [PubMed] [Google Scholar]
- 36.Wüthrich, M., T. Warner, and B. S. Klein. IL-12 is required for induction but not maintenance of protective, memory responses to Blastomyces dermatitidis: implications for vaccine development in immune-deficient hosts. J. Immunol., in press. [DOI] [PubMed]