Abstract
During stress conditions, such as infection, the synthesis of heat shock proteins (HSPs) in microorganisms is upregulated. Since a high degree of homology exists within each HSP family, we postulated that exposure to microorganisms could prime the immune system for evolutionarily diverse HSPs. We tested this hypothesis by priming mice with three microorganisms, namely, Mycobacterium bovis BCG, Mycobacterium vaccae, and Chlamydia pneumoniae. After this, mice received a dose of the various HSPs. We found that BCG and M. vaccae but not C. pneumoniae primed the immune system for the induction of secondary immunoglobulin G (IgG) responses to most of the HSPs tested. Analysis of the IgG1 and IgG2a profile and gamma interferon production induced against the HSPs revealed the induction of a mixture of responses. We also observed that sera from mice treated with M. vaccae and HSP70 were cross-reactive, but no antibody complexes were observed in their kidneys, which frequently are targets for autoantibody reactions. Our findings add further support for the use of HSPs as effective vaccine adjuvants.
Heat shock proteins (HSPs) are some of the most conserved proteins present in all prokaryotes and eukaryotes (13, 16, 22). They undertake crucial functions in maintaining cell homeostasis. From an immunological point of view, HSPs have attracted increasing interest, since they serve as carriers of antigens and effectively induce antigen-specific B- and T-cell responses without the need of adjuvant help (32, 33, 42, 43). These immunomodulatory functions of HSPs are based on various properties: (i) HSPs stimulate the production of chemokines which attract immunological cells; (ii) HSPs possess the ability to activate dendritic cells, thus initiating innate immune responses; and (iii) HSPs are capable of delivering peptides to major histocompatibility complex molecules for the priming of adaptive immunity.
The HSP70 family is one of the best studied among the HSPs and is endowed with crucial immunological functions because of their ability to interact with professional antigen-presenting cells through different receptors (6, 8, 40, 51). Many cytokines (interleukin-12, tumor necrosis factor alpha, and gamma interferon [IFN-γ]) and CC chemokines are elicited by HSP70. Sequences in the HSP70 C-terminal portion have been identified as responsible for the induction of such cytokines (21, 52). We have previously shown that the C-terminal fragment of HSP70 (Pf70C) acted as a carrier in mice when conjugated to the malarial antigen EB200 (Pf70C-EB200) and delivered both as a chimeric protein and as a DNA construct (31).
Mycobacterium bovis bacillus Calmette-Guérin (BCG) and Mycobacterium vaccae, a saprophytic mycobacterium, have been found to possess strong immunostimulatory properties. It has been reported that preexisting inflammation due to BCG can facilitate T-cell priming for inert antigens (11). Different studies have revealed that priming with BCG followed by immunization with the synthetic malaria peptide (NANP)40 conjugated to mycobacterial antigens, such as purified protein derivatives (PPDs), or HSPs resulted in enhanced (NANP)40-specific immune response in mice or monkeys (5, 29). Also, the potent immunomodulatory effect of M. vaccae has been found to be associated with HSPs (38). It has been proposed that during infection, the expression of microbial HSPs is upregulated, which sensitizes the T cells in the infected host and enhances the ability of the bacteria to activate the immune system (20, 26).
In the present study, we aimed to investigate whether exposure to different infectious agents would prime the immune system to evolutionarily diverse HSPs and to any subunit antigen coupled to them. Considering that (i) HSPs are extremely conserved proteins, (ii) all microorganisms express HSPs, and (iii) microbial HSP production is increased during infection, it is conceivable that infection would facilitate T-cell sensitization to HSPs. We hypothesize that T cells induced after priming will cross-react with HSPs of different origin.
Since BCG is extensively used as a vaccine against tuberculosis (44), this approach is particularly interesting for the development of efficacious vaccination strategies. Also, humans and animals are sensitized to mycobacteria or other parasites through natural infection. In this study, we first tested our hypothesis by exposing mice to BCG followed by boosting with the recombinant fusion protein Pf70C-EB200 and with various HSPs. Later on, we evaluated the same protocol using M. vaccae and Chlamydia pneumoniae. We found that exposure of mice to BCG and M. vaccae but not to C. pneumoniae induced secondary responses to Pf70C as well as to other HSPs of different families and origins. Also, Pf70C behaved as a carrier molecule in the induction of EB200-specific antibody responses.
MATERIALS AND METHODS
BCG and M. vaccae.
Lyophilized BCG was obtained from Statens Serum Institute, Copenhagen, Denmark. It was resuspended in phosphate-buffered saline (PBS) at a concentration of 2.5 × 108 CFU/ml. For the preparation of heat-killed BCG, the BCG suspension was autoclaved at 121°C for 15 min at 15 lb. M. vaccae strain ATCC 15483 was grown on tryptone soy peptone agar medium at 37°C for 4 to 5 days. Heat-killed M. vaccae was prepared by scraping the colonies from the culture plate, autoclaved for 15 min at 121°C, and suspended in PBS at 10 mg/ml (equivalent to 1010 organisms per ml) (38).
Recombinant protein immunogens.
The C-terminal portion from Plasmodium falciparum HSP70, which is 142 amino acids long, was included in the present study (30). Expression of the His6-Pf70C-EB200 fusion protein from the plasmid pAff10cPf70CEB200 was performed in Escherichia coli strain BL21(DE3) (Novagen, Madison, WI) as previously described (36). The fusion protein was affinity purified using immobilized metal ion affinity chromatography on TALON metal affinity resin columns (ClonTech Laboratories Inc., Palo Alto, CA), as previously described (36), followed by buffer change to PBS, pH 7.2, by overnight (ON) dialysis at 4°C. The fusion protein glutathione S-transferase-Pf70C was expressed and affinity purified on glutathione media. For the enzymatic cleavage of Pf70C from glutathione S-transferase, specific protease factor Xa (Amersham Pharmacia Biotech, Uppsala, Sweden) was used. Factor Xa was removed with p-aminobenzamidine beads (Sigma Aldrich Chemie, Steinheim, Germany). Protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, and the gels were either stained with Coomassie brilliant blue or used in a Western blotting procedure.
Recombinant M. bovis HSP65 (MB65), Mycobacterium leprae HSP65 (ML65), Mycobacterium tuberculosis HSP65 (MTB65), E. coli HSP65 (EC65), M. tuberculosis HSP70 (MTB70), human HSP60 (Hu60), M. tuberculosis HSP10 (MTB10), and M. tuberculosis HSP16 (MTB16) were provided by LIONEX (Braunschweig, Germany). These are representatives of high- and low-molecular-weight HSPs of various origins related (MB65, ML65, MTB65, MTB70, MTB10, and MTB16) and unrelated (EC65 and Hu60) to BCG, M. vaccae, and Chlamydia pneumoniae.
Sequence alignments were done with Clustal X version 1.8.1 (45) and are shown in Fig. S1a and S1b of the supplemental material. Percentages of identity between the proteins are shown in Table 1.
TABLE 1.
Percentages of sequence identity between evolutionarily diverse HSPsa
| HSP | MTB65 | ML65 | EC65 | Hu60 | Mouse60 |
|---|---|---|---|---|---|
| MTB65 | 100 | 94.8 | 58.6 | 44.8 | 45.2 |
| ML65 | 100 | 58.3 | 44.6 | 45 | |
| EC65 | 100 | 48.6 | 48.6 | ||
| Hu60 | 100 | 97.6 | |||
| Mouse60 | 100 |
MTB65, M. tuberculosis HSP65 or M. bovis HSP65; Mouse60, mouse HSP60. The percent identity between mouse HSP70 and MTB70 is 46.4.
Immunization of mice.
Female 6- to 8-week-old BALB/c mice were purchased from Taconic M & B (Ry, Denmark) and kept in the animal facilities of Stockholm University. The priming dose was 106 CFU of BCG per mouse. For M. vaccae priming, 1 μg (wet weight), equivalent to 106 M. vaccae bacilli, was given. Mycoplasma-free C. pneumoniae isolate Kajaani was propagated in HL cells, and 106 infection-forming units were prepared as described before for immunization (4). Bacteria were delivered intraperitoneally (i.p.) in 200 μl of balanced salt solution (Life Technologies, Paisley, Scotland, United Kingdom). Priming was done once, followed 3 weeks later by boosting with 25 μg per mouse of recombinant HSPs. Animals were bled by retro-orbital plexus puncture 7 days after the last immunization. Sera were stored at −20°C until assayed.
Determination of serum antibody levels by enzyme-linked immunosorbent assay (ELISA).
To determine specific antibody responses, microtiter plates (Costar, Corning Incorporated, Corning, NY) were coated with 2 μg/ml of each protein in carbonate buffer, pH 9.4. A cocktail of six synthetic peptides (P-1, P-6, P-7, P-10, P-13, and P-16) (1, 2) representing sequences in EB200 was used to coat the plates (1 μg/ml of each peptide) for the determination of EB200-specific antibodies. P-1 and P-13 were synthesized by 9-fluorenylmethoxy carbonyl chemistry (35), and the rest of the peptides were synthesized by Neo systems (Strasbourg, France).
For the detection of serum antibodies against different cross-reactive antigens, plates were coated with 10 μg/ml of DNA (in PBS), collagen III (in PBS), trinitrophenyl-bovine serum albumin (TNP-BSA) (in carbonate buffer), and histone (in carbonate buffer). Plates were incubated ON at room temperature (RT) followed by incubation ON with serial dilutions of sera. Sera were initially diluted to 1:100 to determine specific antibodies and to 1:20 for the detection of cross-reactive antibodies. Isotypes were determined by incubating the plates for 2 h at RT with alkaline phosphatase-conjugated goat immunoglobulin specific for mouse total immunoglobulins (Igs), IgM, total IgG, IgG1, and IgG2a (Southern Biotechnology Inc., Birmingham, AL). Color was developed at RT with p-nitrophenyl phosphate disodium (Sigma Diagnostics, St. Louis, MO), and the absorbance was measured at 405 nm at different time points.
IFN-γ detection.
Immunized mice were sacrificed 2 weeks after the last immunization, and spleens were removed aseptically. A cell suspension from the splenocytes was prepared from individual spleens and adjusted to a cell concentration of 5 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.01 M HEPES (Invitrogen Corporation, Paisley, Scotland, United Kingdom). One hundred microliters of the cell suspension was added to each well of 96-well flat-bottomed microtiter plates (Costar) with or without 10 μg/ml of each HSP, PPD, or 2 μg/ml of concanavalin A (ConA) as a positive control. After incubating the cultures for 72 h at 37°C in 5% CO2, pooled supernatants from triplicate cultures were analyzed for the presence of IFN-γ using a commercially available ELISA kit (Mabtech AB, Stockholm, Sweden).
Detection of immune complex deposits in kidneys.
The presence of antibody deposits in kidney sections was detected by direct immunofluorescence. Kidneys from HSP70-treated or nonimmunized mice were horizontally cut into two slices and embedded in OCT compound (Miles Scientific, Nunc, Naperville, IL) to make composite blocks. Sections (5 μm thick) were cut from the composite in a Jung cryostat (Leica Instruments GmbH, Heidelberg, Germany) chilled to −20°C. The sections were fixed in acetone for 5 min, air dried, incubated with serial dilutions of fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse Ig antibodies (DakoCytomation, Norden A/S, Denmark) for 30 min at RT, washed three times with PBS, and air dried. After covering the sections with antifading solution, they were viewed with a Reichard-Jung (Vienna, Austria) Polyvar microscope equipped with a 200-W mercury lamp and a filter set for FITC. The initial dilution for FITC-conjugated antibody was 1/40. Kidney sections of a mercuric chloride (HgCl2)-treated SJL mouse (3, 14) were used as positive controls.
Statistical analyses.
Three mice were included in each experimental group, and the results were expressed as means ± standard deviations (SD) of triplicate samples (sera and spleen) from individual mice from each group. A two-tailed Student's t test was performed to identify significant differences between experimental groups.
RESULTS
Both live and heat-killed BCG prime the immune system for induction of antibodies specific for the C-terminal fragment of HSP70.
To assess whether BCG could provide the sensitization of T cells for the induction of secondary responses to HSPs, we tested for the ability of both live and heat-killed BCG to prime an immune response to Pf70C-EB200.
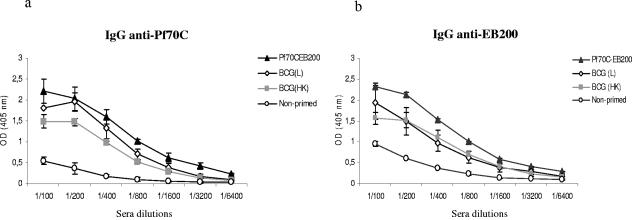
BALB/c mice were exposed either to live or to heat-killed BCG and 3 weeks later immunized with the fusion protein Pf70C-EB200. As negative and positive controls, we immunized mice once (primary response) and twice (secondary response) in a 3-week interval with Pf70C-EB200, respectively. As shown in Fig. 1a, when mice were primed with either live or heat-killed BCG, followed by immunization with Pf70C-EB200 once, anti-Pf70C IgG antibodies were produced. The antibody titer in live-BCG-primed mice attained similar levels as that obtained from the group of mice immunized twice (secondary response) with Pf70C-EB200 (Fig. 1a). The control group, immunized with Pf70C-EB200 once, produced negligible amounts of IgG anti-Pf70C antibodies (Fig. 1a).
FIG. 1.
Induction of IgG antibodies specific to Pf70C (a) and EB200 (b) in BALB/c mice primed with live (L) or heat-killed (HK) BCG or with the recombinant protein Pf70C-EB200. On day 0, mice were primed i.p. either with Pf70C-EB200 (▴) or with 106 CFU live (⋄) or heat-killed (░⃞) BCG followed by boosting with 25 μg Pf70C-EB200 given i.p. 3 weeks later. Mice in the control group were immunized with only Pf70C-EB200 (○) once. Bleeding was done 7 days after boosting with the protein. The production of IgG antibodies specific for Pf70C and EB200 was evaluated by ELISA using 2.0 μg/ml of recombinant Pf70C and a cocktail of six synthetic peptides (1 μg/ml of each peptide) to coat the plates. Data are the mean optical density (OD) values (A405 nm) and SD of individual sera of three mice per group, serially diluted in the wells. We show one representative result of three independent experiments. Background reactivity of the sera with BSA at the corresponding dilution was subtracted.
We further investigated the ability of Pf70C to exert a carrier effect on EB200 in mice primed with BCG. The sera from immunized mice were analyzed for the presence of EB200-specific IgG antibodies by ELISA. The results revealed that exposure of mice to live or heat-killed BCG generated EB200-specific secondary antibodies (Fig. 1b). Control mice immunized with only Pf70C-EB200 without previous priming exhibited negligible amounts of IgG anti-EB200 antibodies (Fig. 1b).
Priming with BCG promotes both Th1 and Th2 type immune responses against Pf70C.
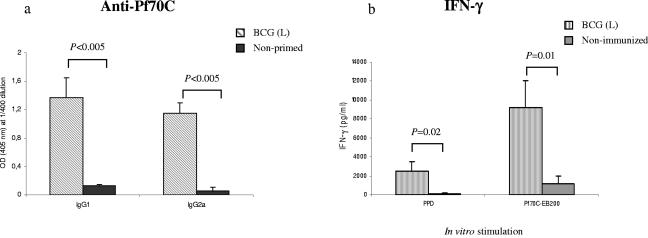
To determine whether priming with live BCG followed by Pf70C-EB200 boosting affected the pattern of the Pf70C-specific humoral response, the IgG1 and IgG2a isotype profile was analyzed. Priming with live BCG elicited both Pf70C-specific IgG1 and IgG2a antibodies, indicating that a mixed Th1/Th2 type of immune response was induced (Fig. 2a). The effect of BCG priming on IFN-γ production was also analyzed using splenocytes derived from mice treated or not treated with live BCG and 5 weeks later stimulated in vitro with Pf70C-EB200 and PPD for 72 h. PPD was chosen because it is commonly used to measure the T-cell reactivity in humans previously exposed to Mycobacterium (23). Moreover, it has been reported that BCG vaccination induced an increased production of IFN-γ in response to PPD in infants (19). As illustrated in Fig. 2b, splenocytes from mice primed with live BCG responded to Pf70C-EB200 with significantly higher IFN-γ levels than that observed in splenocytes from nontreated mice. This correlates with the generation of Pf70C-specific IgG2a antibodies in live-BCG-primed mice. Even if lower, the production of IFN-γ in response to PPD was also significantly higher in the BCG-primed animals than that induced in the control group (Fig. 2b). PPD is a mixture of many different proteins, and possibly the concentration of HSPs is rather low. This may explain the lower activating capacity compared to Pf70C-EB200.
FIG. 2.
(a) Analysis of Pf70C-specific IgG1 and IgG2a isotype levels in sera of mice primed with live (L) BCG. Serial dilutions of sera of individual mice were studied. The results from the 1:400 dilutions are presented, showing the mean OD values (A405 nm) and SD of results from three mice. Statistical analysis showed significant differences between the groups at P < 0.005 compared with the respective control group (Student's t test). Background reactivity of the sera with BSA at the corresponding dilution was subtracted. (b) In vitro production of IFN-γ by splenocytes derived from mice in response to Pf70C-EB200 or PPD. Spleens from live BCG-primed (not boosted) mice or nonimmunized mice were collected 5 weeks after priming. Splenocytes from individual mice were harvested. Single-cell preparations were stimulated separately with 10 μg/ml of Pf70C-EB200 or PPD, cultured (5 × 105 cells/well) in triplicate in 96-well cell culture plates, and incubated for 72 h at 37°C in 5% CO2. Cell supernatants were collected, pooled, and assayed for the presence of IFN-γ by ELISA. Values are the means and SD of results derived from three different spleens.
BCG has the ability to prime for various evolutionarily diverse HSPs.
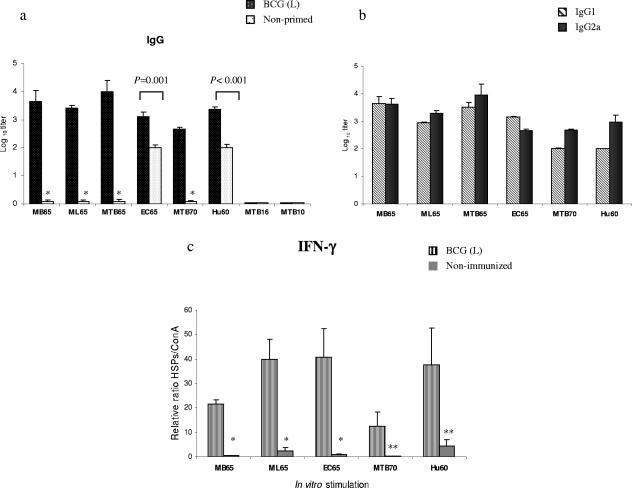
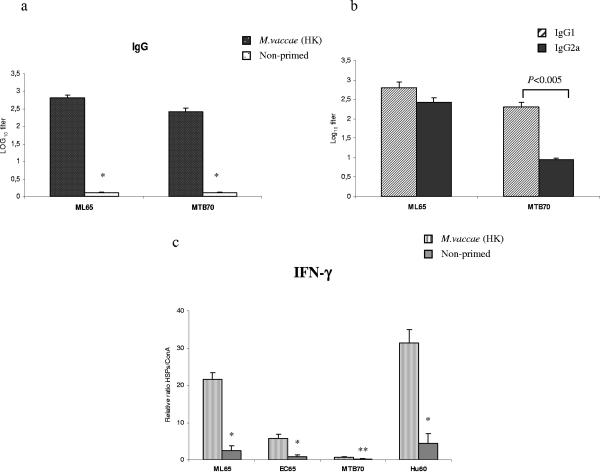
To further investigate the priming effect of BCG on other phylogenetically diverse HSPs, BALB/c mice were primed once with BCG i.p., and 3 weeks later each group received high- and low-molecular-weight HSPs of various origins related (MB65, ML65, MTB65, MTB70, MTB10, and MTB16) and unrelated (EC65 and Hu60) to BCG. Control groups were immunized only once with the above-mentioned HSPs. The antigen-specific IgG antibodies were measured by ELISA 7 days after the last immunization. As illustrated in Fig. 3a, BCG-primed mice developed secondary antibody responses against all HSPs except MTB16 and MTB10. The level of antibodies generated in all primed groups was significantly higher than the levels induced in nonprimed mice, but the magnitude of the response varied. While negligible amounts of antibodies were generated in control groups immunized with MB65, ML65, MTB65, and MTB70, the primary response to EC65 and Hu60 was comparatively high. MB65, ML65, MTB65, and Hu60 elicited stronger responses than EC65 or MTB70. The detection of HSP-specific IgG1 and IgG2a antibodies demonstrated the induction of a mixture of Th1 and Th2 types of response (Fig. 3b).
FIG. 3.
(a) HSP-specific IgG responses induced in BALB/c mice primed with live (L) BCG and boosted with various HSPs once. Control mice received only HSPs once. Serum samples were obtained from the mice 7 days after the protein immunization. The presence of HSP-specific antibodies was detected by ELISA using serial dilutions of individual sera from three mice. Significant differences between groups following a Student's t test are depicted with asterisks (*, P < 0.005 with the respective control group). (b) HSP-specific IgG1/IgG2a profile. Titers are expressed as the log10 of the reciprocal of the highest dilution at an OD of 1.0. Background reactivity of the sera with BSA at the corresponding dilution was subtracted. (c) In vitro IFN-γ production by the splenocytes stimulated with various HSPs from mice primed with live BCG or not primed. Splenocytes of individual mice from each group were stimulated separately in triplicate with 10 μg/ml of each HSP and with 2 μg/ml of ConA (positive control) in 96-well cell culture plates and incubated for 72 h at 37°C in 5% CO2. Cell supernatants were collected, pooled, and assayed for the presence of IFN-γ by ELISA. Since the absolute values varied from experiment to experiment, to facilitate comparisons we normalized all values in relation to ConA stimulation. Data are expressed as the ratio between HSP and ConA stimulation multiplied by 100. Values represent means of triplicate samples with SD. *, P = 0.05; **, P < 0.01 with the respective controls.
To ascribe the priming effect of BCG in IFN-γ production in vitro, splenocytes derived from nonimmunized mice or mice primed with BCG were stimulated with various HSPs or with ConA as a positive control. After 72 h of incubation, cell supernatants were analyzed for the presence of IFN-γ by ELISA. BCG priming induced increased production of IFN-γ, but the magnitude was different for each HSP (Fig. 3c). The levels of IFN-γ were higher upon stimulation with ML65, EC65, and Hu60 than upon stimulation with MB65 or MTB70 (Fig. 3c). Nearly undetectable amounts of IFN-γ were produced by splenocytes derived from mice not primed with BCG (Fig. 3c).
M. vaccae but not C. pneumoniae primes for the induction of secondary responses to HSPs.
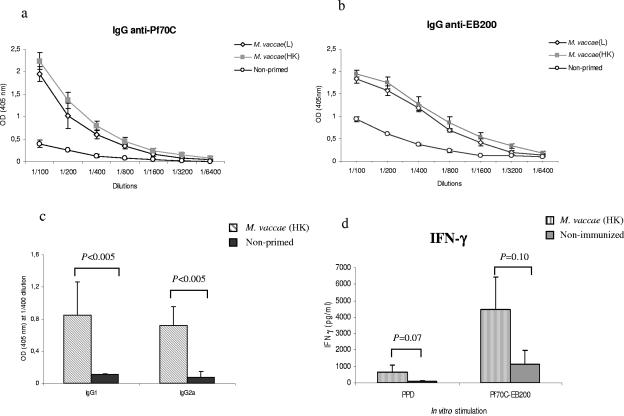
As HSPs are present in other microorganisms, not only in BCG, we tested the ability of M. vaccae and C. pneumoniae to prime the immune system against HSPs. We followed the same protocol as for BCG. First, we evaluated the priming effect of M. vaccae and C. pneumoniae for the induction of a Pf70C-specific secondary response. As illustrated in Fig. 4a, both live and heat-killed M. vaccae but not C. pneumoniae (data not shown) could mediate the induction of anti-Pf70C IgG antibodies. We also observed that Pf70C acted as a carrier for the production of EB200-specific IgG antibodies in M. vaccae-primed mice (Fig. 4b).
FIG. 4.
(a) Pf70C- and (b) EB200-specific IgG response elicited in mice primed with live (L) or heat-killed (HK) M. vaccae followed by boosting with Pf70C-EB200 3 weeks later. Immunization and bleeding were done as previously described for BCG. (c) Analysis of Pf70C-specific serum IgG1 and IgG2a isotype levels in mice primed with heat-killed M. vaccae. Serial dilutions of sera from individual mice (three mice per group) were studied. The results from the 1:400 dilutions are presented, with mean OD (A405 nm) and SD shown. Statistical analysis showed significant differences between the groups. Background reactivity of the sera with BSA at the corresponding dilution was subtracted. (d) In vitro production of IFN-γ by splenocytes stimulated with Pf70C-EB200 or PPD derived from mice primed with M. vaccae or not primed. Values are the means and SD derived from three spleens. Values represent means of triplicate samples with SD. *, P = 0.05; **, P < 0.01 with the respective controls.
Since M. vaccae has been used in a heat-killed form in diverse vaccination trials (39), we continued the rest of our experiments here using heat-killed bacteria. M. vaccae-primed mice generated both IgG1- and IgG2a-specific antibodies against Pf70C (Fig. 4c). Also, elevated levels of IFN-γ were produced by splenocytes derived from M. vaccae-primed mice stimulated in vitro with Pf70C-EB200 (Fig. 4d). However, low levels of IFN-γ production were observed upon PPD stimulation, which correlates with the observation of von Reyn et al. (50).
We further investigated whether heat-killed M. vaccae and C. pneumoniae could prime for other HSPs, such as ML65 or MTB70. No priming effect was promoted by C. pneumoniae (not shown), but priming with heat-killed M. vaccae induced increased levels of anti-ML65 and anti-MTB70 IgG antibodies compared to mice immunized with ML65 or MTB70 alone. Analysis of the IgG subclasses revealed that heat-killed M. vaccae priming promoted a mixture of IgG1 and IgG2a responses in mice boosted with ML65. In the MTB70-boosted group, a predominant IgG1 response was induced (Fig. 5b).
FIG. 5.
(a) ELISA measurement of IgG anti-ML65 and anti-MTB70 antibody response elicited in mice after priming with heat-killed (HK) M. vaccae and boosting with ML65 and MTB70, respectively. Statistical analysis showed significant differences at P values of <0.005 compared with the respective control group by Student's t test (*). (b) ML65- and MTB70-specific IgG1 and IgG2a antibody responses were measured from individual sera, and titers are expressed as the log10 of the reciprocal of the highest dilution giving an OD of 1.0. (c) In vitro IFN-γ production by the splenocytes stimulated with various HSPs from mice primed with heat-killed M. vaccae. Splenocytes of individual mice from each group were stimulated separately in triplicate with 10 μg/ml of each HSP and with 2 μg/ml of ConA (positive control) in 96-well cell culture plates and incubated for 72 h at 37°C in 5% CO2. Cell supernatants were collected, pooled, and assayed for the presence of IFN-γ by ELISA. Data are expressed as the ratio between HSP and ConA stimulation multiplied by 100. Values represent means of triplicate samples (three spleens) with SD. *, P < 0.005; **, P = 0.07 with the respective controls.
Finally, we assessed the priming effect of heat-killed M. vaccae on IFN-γ production. Splenocytes isolated from M. vaccae-primed mice secreted high levels of IFN-γ in response to ML65 and Hu60, whereas lower levels of IFN-γ were released in response to stimulation with EC65 (Fig. 5c). Splenocytes from the untreated control group stimulated in vitro produced significantly lower levels of IFN-γ in response to HSPs. A negligible amount of IFN-γ was induced by MTB70 in splenocytes from primed mice (Fig. 5c).
The induction of autoreactive antibodies by HSPs.
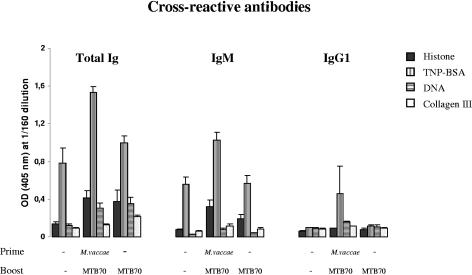
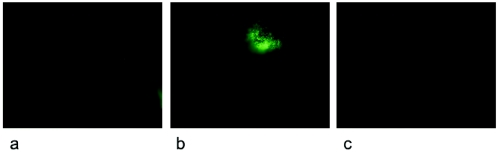
One of the concerns for the use of HSPs as adjuvants in human vaccines is the association of HSPs with the induction of autoimmune conditions. To resolve this issue, we asked whether immunization with HSPs could result in the generation of autoantibodies. We first analyzed sera from immunized and untreated mice for their capacity to bind to a number of antigens, namely, histone, TNP-BSA, DNA, and collagen III, commonly used to detect autoantibodies. The highest degree of cross-reactivity was observed in the sera from mice primed with M. vaccae and boosted twice with MTB70 (Fig. 6). No major cross-reactivity was observed in mice immunized only with HSPs. Antibody deposits are frequently observed in the kidneys of mice suffering from autoimmune diseases caused by autoantibodies (3, 15, 41). Thus, to evaluate the possible correlation between the observed cross-reactivity and autoimmunity, we tested for the presence of antibody deposits in kidneys from M. vaccae- and HSP-treated mice. Kidneys were examined by using a direct immunofluorescence technique. As a positive and negative control, we used kidneys from an HgCl2-treated SJL mouse and nonimmunized mice, respectively. The kidney sections from the HgCl2-treated SJL mouse showed a strong deposit of immunoglobulins, while the M. vaccae- and HSP-treated mice exhibited no such immune antibody complex deposition and were comparable to the negative control (Fig. 7). Only one of the kidneys is shown.
FIG. 6.
Cross-reactivity of total Igs, IgM, and IgG1 antibodies against histone, TNP-BSA, DNA, and collagen III elicited in mice primed or not primed with heat-killed M. vaccae followed by boosting twice with MTB70. Antibody responses were measured from pooled sera of three mice per group. The sera were diluted in consecutive twofold dilutions, starting from 1:20 up to 1:640. ODs from 1:160 dilutions are presented here.
FIG. 7.
Analysis for the presence of renal immune complex deposits of total Igs by using a direct immunofluorescence method. A direct immunofluorescence assay was performed with FITC-conjugated rabbit anti-mouse Igs incubated on a kidney cryostat section from (a) M. vaccae-primed and MTB70 (two times)-boosted mice, (b) HgCl2-treated SJL mice, and (c) nonimmunized mice. Staining of a glomerulus is seen at 63× magnification. We show here the staining with a 1:40 dilution of the FITC-conjugated rabbit anti-mouse Igs.
DISCUSSION
HSPs are highly conserved molecules and widely distributed in prokaryotic and eukaryotic cells. It has been reported that upon vaccination with BCG and other microorganisms, the synthesis of bacterial HSPs is upregulated, which sensitizes the T cells in the infected host (10, 20, 26). This phenomenon could be advantageous for the improvement of vaccine strategies. In the present study, we investigated BCG, M. vaccae, and C. pneumoniae for the ability to prime for their induction of secondary immune responses to evolutionarily diverse HSPs and to the malarial antigen EB200 coupled to a fragment of HSP70 (Pf70C).
We have demonstrated that BCG and M. vaccae are capable of priming the immune system to respond to Pf70C and to the peptide EB200 coupled to it. This is in agreement with previous reports showing that mycobacterial HSP70 and HSP65 contain strong T-cell epitopes and exert a helper effect in vivo when conjugated to synthetic peptides or bacterial oligosaccharides (5, 29). We also describe here that both BCG and M. vaccae primed the immune system to induce memory responses to phylogenetically diverse HSPs of different families with high molecular weights (MW). No priming was observed against the low-MW MTB16 and MTB10 HSPs. Priming of mice with BCG or M. vaccae might lead to the induction of a pool of memory T cells able to undergo clonal expansion upon boosting with evolutionarily diverse HSPs by recognizing conserved (cross-recognized) epitopes. Whether T cells can recognize conserved epitopes present only on high-MW HSPs needs to be assessed.
Interestingly, two of the high-MW HSPs, namely, Hu60 and EC65, induced a very high “primary” immune response compared to the other HSPs tested. A possible explanation could be that mice had been previously exposed to these two HSPs. Mice are exposed to their own HSP60, which displays a high degree of homology (97.6%) to human HSP60 (Table 1). Regarding the high “primary” response to EC65, it can be speculated that mice may also be exposed to E. coli-derived HSPs, considering the fact that E. coli contributes to the intestinal flora.
In contrast to BCG and M. vaccae, priming with C. pneumoniae was ineffective in inducing secondary responses to HSPs. One explanation could be that the HSPs from C. pneumoniae are not well conserved. Alternatively, the mode of infection with chlamydia may be different from that of mycobacteria, and the expression of chlamydial HSPs during infection may not be sufficiently upregulated to prime a memory response.
Priming with heat-killed BCG or M. vaccae was also effective in inducing both Pf70C- and EB200-specific antibodies to HSP molecules. Proteins are usually denatured by heat treatment. Therefore, the HSP priming effect induced by heat-killed bacteria was surprising. One explanation for the effectiveness of heat-killed BCG and M. vaccae for priming the humoral response may be that immunodominant HSP T-cell epitopes remain intact after heat denaturation of the bacteria. Alternatively, HSPs may be protected from heat denaturation inside the cell. We found that even if the priming effect was reduced, heat-denatured Pf70C could efficiently prime the immune system (data not shown).
The involvement of bacterial HSPs in autoimmune phenomena may be considered as a potential caveat for including HSPs in human vaccines due to the homologies between bacterial and human HSPs (18). Also, considering the risk of inducing autoimmune disorders between HSPs and certain host tissue antigens, we investigated whether antibodies induced in mice immunized with MTB70 would cross-react with a panel of autoreactive antigens. We found that cross-reactive antibodies against TNP were preferentially induced in the M. vaccae- and MTB70-treated mice. However, these cross-reactive antibodies are also predominant in the control group, indicating that cross-reactivity to TNP is possibly more related to the nature of the hapten as such than to the stimulation by M. vaccae.
It has to be pointed out that the presence of cross-reactive antibodies does not necessarily have to be correlated with autoimmunity. Cross-reactive antibodies are frequently detected in sera from healthy individuals (7, 28, 46) and commonly induced in primary immune responses shortly after challenge with the antigens. However, to evaluate the possible involvement of the observed cross-reactive antibodies in autoimmune reactions, we looked for the presence of antibody deposits in the kidneys of the mice displaying the highest degree of cross-reactivity in their serum. The rationale behind this experiment was that deposits are frequently observed in the kidneys of mice suffering from autoimmune diseases caused by autoantibodies (3, 15, 41). No immune complex was detected in the kidneys of these mice. Thus, even if not formally proven, this observation suggests that the observed cross-reactive antibodies were probably not pathological.
Moreover, the concept of HSPs as a cause for autoimmunity is not clearly established. For instance, it has been shown that the prevention of certain autoimmune conditions could be achieved by pretreatment of animals with mycobacterial HSP70 or with some defined epitopes in the HSP sequences. T-cell immunity to HSP70 and to HSP65 has been shown to modulate the arthritogenic response in adjuvant-induced arthritis and to protect these animals from such autoimmune reaction (47, 54). It has also been shown that mycobacterial HSP70 triggers self-HSP cross-reactive T cells with the potential to downregulate arthritis (48, 53).
Most interestingly, a new concept suggesting a new role for HSPs as sensors for internal and external danger is emerging and may explain the presence of HSPs in the site of injury more as a consequence than as the cause of the reaction. Different experimental data strongly support the view that conserved HSPs (self or foreign) are indeed negotiators between danger and control mechanisms of autoimmunity (49). Thus, the priming to microbial HSP could be regarded more as a regulatory effect than an enhancing event for autoimmunity. More studies have to be performed to clarify this issue, but our findings that the HSP70-induced cross-reactive antibodies do not accumulate in the kidney and thus are not apparently pathogenic support this idea.
There is widespread recognition of the need for improved vaccines for control of infectious diseases, and scientists are searching for appropriate combinations of antigens and adjuvants or suitable carrier molecules for inclusion in subunit vaccines. The use of high-MW HSPs as carriers is particularly interesting for the development of vaccine strategies, since most humans have been in contact with microbial HSPs. BCG is widely used as a vaccine against tuberculosis, and a large number of people are sensitized to mycobacteria or other parasites through natural contacts. Moreover, sensitization to HSPs has been reported to occur in individuals naturally exposed to a wide variety of infections, such as malaria (24), filariasis (34, 37), schistosomiasis (27), American trypanosomiasis (12), leprosy (9, 25), and candidiasis (17). It has also been reported that vaccination against pertussis using a whole-cell vaccine is capable of priming the immune system to respond to microbial HSPs (10). Collectively, our results provide support and offered rationale for the utility of HSPs in vaccine design.
Supplementary Material
Acknowledgments
Martin Rottenberg is acknowledged for providing C. pneumoniae. We also sincerely thank Klavs Berzins for providing us all of the synthetic peptides. We also thank Eduard Torrents for his help in sequence analysis.
This study was supported by grants from the European Union (QLK2-CT-2002-00846) and from Magnus Bergvalls Stiftelse. E.J. is supported by a postdoctoral fellowship from the Fundación Ramón Areces (Spain).
Editor: J. L. Flynn
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ahlborg, N., F. Sterky, D. Haddad, P. Perlmann, P. Å. Nygren, R. Andersson, and K. Berzins. 1997. Predominance of H-2d- and H-2k-restricted T-cell epitopes in the highly repetitive Plasmodium falciparum antigen Pf332. Mol. Immunol. 34:379-389. [DOI] [PubMed] [Google Scholar]
- 2.Ahlborg, N., D. Haddad, A. B. Siddique, C. Roussilhon, C. Rogier, J. F. Trape, M. Troye-Blomberg, and K. Berzins. 2002. Antibody responses to the repetitive Plasmodium falciparum antigen Pf332 in humans naturally primed to the parasite. Clin. Exp. Immunol. 129:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Balaghi, S., E. Moller, G. Moller, and M. Abedi-Valugerdi. 1996. Mercury induces polyclonal B cell activation, autoantibody production and renal immune complex deposits in young (NZB x NZW)F1 hybrids. Eur. J. Immunol. 26:1519-1526. [DOI] [PubMed] [Google Scholar]
- 4.Bandholtz, L., M. R. Kreuger, C. Svanholm, H. Wigzell, and M. E. Rottenberg. 2002. Adjuvant modulation of the immune responses and the outcome of infection with Chlamydia pneumoniae. Clin. Exp. Immunol. 130:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrios, C., A. R. Lussow, J. Van Embden, R. Van der Zee, R. Rappuoli, P. Costantino, J. A. Louis, P. H. Lambert, and G. Del Giudice. 1992. Mycobacterial heat-shock proteins as carrier molecules. II. The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette-Guérin priming. Eur. J. Immunol. 22:1365-1372. [DOI] [PubMed] [Google Scholar]
- 6.Basu, S., R. J. Binder, T. Ramalingam, and P. K. Srivastava. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303-313. [DOI] [PubMed] [Google Scholar]
- 7.Berneman, A., B. Guilbert, S. Eschrich, and S. Avrameas. 1993. IgG auto- and polyreactivities of normal human sera. Mol. Immunol. 30:1499-1510. [DOI] [PubMed] [Google Scholar]
- 8.Binder, R. J., R. Vatner, and P. Srivastava. 2004. The heat-shock protein receptors: some answers and more questions. Tissue Antigens 64:442-451. [DOI] [PubMed] [Google Scholar]
- 9.Britton, W. J., L. Hellqvist, A. Basten, and R. L. Raison. 1985. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135:4171-4177. [PubMed] [Google Scholar]
- 10.Del Giudice, G., A. Gervaix, P. Costantino, C. A. Wyler, C. Tougne, E. R. de Graeff-Meeder, J. van Embden, R. van der Zee, L. Nencioni, R. Rappuoli, et al. 1993. Priming to heat shock proteins in infants vaccinated against pertussis. J. Immunol. 150:2025-2032. [PubMed] [Google Scholar]
- 11.Dudani, R., Y. Chapdelaine, H. van Faassen, D. K. Smith, H. Shen, L. Krishnan, and S. Sad. 2002. Preexisting inflammation due to Mycobacterium bovis BCG infection differentially modulates T-cell priming against a replicating or nonreplicating immunogen. Infect. Immun. 70:1957-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engman, D. M., E. A. Dragon, and J. E. Donelson. 1990. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J. Immunol. 144:3987-3991. [PubMed] [Google Scholar]
- 13.Fink, A. L. 1999. Chaperone-mediated protein folding. Physiol. Rev. 79: 425-449. [DOI] [PubMed] [Google Scholar]
- 14.Hansson, M., M. Djerbi, H. Rabbani, H. Mellstedt, F. Gharibdoost, M. Hassan, J. W. Depierre, and M. Abedi-Valugerdi. 2005. Exposure to mercuric chloride during the induction phase and after the onset of collagen-induced arthritis enhances immune/autoimmune responses and exacerbates the disease in DBA/1 mice. Immunology 114:428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haraldsson, M. K., N. G. de la Paz, J. G. Kuan, G. S. Gilkeson, A. N. Theofilopoulos, and D. H. Kono. 2005. Autoimmune alterations induced by the New Zealand Black Lbw2 locus in BWF1 mice. J. Immunol. 174: 5065-5073. [DOI] [PubMed] [Google Scholar]
- 16.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 17.Ivanyi, L., and J. Ivanyi. 1990. Elevated antibody levels to mycobacterial 65-kDa stress protein in patients with superficial candidiasis. J. Infect. Dis. 162:519-522. [DOI] [PubMed] [Google Scholar]
- 18.Jindal, S., A. K. Dudani, B. Singh, C. B. Harley, and R. S. Gupta. 1989. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol. Cell. Biol. 9:2279-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kampmann, B., G. N. Tena, S. Mzazi, B. Eley, D. B. Young, and M. Levin. 2004. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect. Immun. 72:6401-6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. 1990. Heat shock proteins and the immune response. Immunol. Today 11:129-136. [DOI] [PubMed] [Google Scholar]
- 21.Lehner, T., Y. Wang, T. Whittall, E. McGowan, C. G. Kelly, and M. Singh. 2004. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem. Soc. Trans. 32:629-632. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist, S., and E. A. Craig. 1988. The heat-shock proteins. Annu. Rev. Genet. 22:631-677. [DOI] [PubMed] [Google Scholar]
- 23.Lussow, A. R., G. Del Giudice, L. Renia, D. Mazier, J. P. Verhave, A. S. Verdini, A. Pessi, J. A. Louis, and P. H. Lambert. 1990. Use of a tuberculin purified protein derivative-Asn-Ala-Asn-Pro conjugate in bacillus Calmette-Guérin primed mice overcomes H-2 restriction of the antibody response and avoids the need for adjuvants. Proc. Natl. Acad. Sci. USA 87:2960-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattei, D., A. Scherf, O. Bensaude, and L. P. da Silva. 1989. A heat shock-like protein from the human malaria parasite Plasmodium falciparum induces autoantibodies. Eur. J. Immunol. 19:1823-1828. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie, K. R., E. Adams, W. J. Britton, R. J. Garseia, and A. Basten. 1991. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J. Immunol. 147:312-319. [PubMed] [Google Scholar]
- 26.Milon, G., M. Lebastard, and G. Marchal. 1985. T-dependent production and activation of mononuclear phagocytes during murine BCG infection. Immunol. Lett. 11:189-194. [DOI] [PubMed] [Google Scholar]
- 27.Moser, D., O. Doumbo, and M. Q. Klinkert. 1990. The humoral response to heat shock protein 70 in human and murine Schistosoma mansoni. Parasite Immunol. 12:341-352. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen, C. H., R. G. Leslie, B. S. Jepsen, M. D. Kazatchkine, S. V. Kaveri, and E. Fischer. 2001. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur. J. Immunol. 31:2660-2668. [DOI] [PubMed] [Google Scholar]
- 29.Perraut, R., A. R. Lussow, S. Gavoille, O. Garraud, H. Matile, C. Tougne, J. van Embden, R. van der Zee, P. H. Lambert, J. Gysin, et al. 1993. Successful primate immunization with peptides conjugated to purified protein derivative or mycobacterial heat shock proteins in the absence of adjuvants. Clin. Exp. Immunol. 93:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perraut, R., O. Mercereau-Puijalon, D. Mattei, E. Bourreau, O. Garraud, B. Bonnemains, L. Pereia de Silva, and J. C. Michel. 1995. Induction of opsonizing antibodies after injection of recombinant Plasmodium falciparum vaccine candidate antigens in preimmune Saimiri sciureus monkeys. Infect. Immun. 63:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qazi, K. R., M. Wikman, N. M. Vasconcelos, K. Berzins, S. Ståhl, and C. Fernández. 2005. Enhancement of DNA vaccine potency by linkage of Plasmodium falciparum malarial antigen gene fused with a fragment of HSP70 gene. Vaccine 23:1114-1125. [DOI] [PubMed] [Google Scholar]
- 32.Rico, A. I., S. O. Angel, C. Alonso, and J. M. Requena. 1999. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol. Immunol. 36:1131-1139. [DOI] [PubMed] [Google Scholar]
- 33.Roman, E., and C. Moreno. 1996. Synthetic peptides non-covalently bound to bacterial hsp 70 elicit peptide-specific T-cell responses in vivo. Immunology 88:487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothstein, N. M., G. Higashi, J. Yates, and T. V. Rajan. 1989. Onchocerca volvulus heat shock protein 70 is a major immunogen in amicrofilaremic individuals from a filariasis-endemic area. Mol. Biochem. Parasitol. 33:229-235. [DOI] [PubMed] [Google Scholar]
- 35.Sallberg, M., U. Ruden, L. O. Magnius, E. Norrby, and B. Wahren. 1991. Rapid “tea-bag” peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol. Lett. 30:59-68. [DOI] [PubMed] [Google Scholar]
- 36.Selden, R. 1995. Transfection and expression of cloned DNA. Curr. Protocols Immunol. 2:10-13. [Google Scholar]
- 37.Selkirk, M. E., D. A. Denham, F. Partono, and R. M. Maizels. 1989. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J. Immunol. 143:299-308. [PubMed] [Google Scholar]
- 38.Skinner, M. A., R. Prestidge, S. Yuan, T. J. Strabala, and P. L. Tan. 2001. The ability of heat-killed Mycobacterium vaccae to stimulate a cytotoxic T-cell response to an unrelated protein is associated with a 65 kilodalton heat-shock protein. Immunology 2:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinner, M. A., D. L. Keen, N. A. Parlane, G. F. Yates, and B. M. Buddle. 2002. Increased protection against bovine tuberculosis in the brushtail possum (Trichosurus vulpecula) when BCG is administered with killed Mycobacterium vaccae. Tuberculosis 82:15-22. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava, P. 2002. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2:185-194. [DOI] [PubMed] [Google Scholar]
- 41.Stohl, W., D. Xu, K. S. Kim, M. N. Koss, T. N. Jorgensen, B. Deocharan, T. E. Metzger, S. A. Bixler, Y. S. Hong, C. M. Ambrose, et al. 2005. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 52:2080-2091. [DOI] [PubMed] [Google Scholar]
- 42.Suzue, K., and R. A. Young. 1996. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J. Immunol. 156:873-879. [PubMed] [Google Scholar]
- 43.Suzue, K., and R. A. Young. 1996. Heat shock proteins as immunological carriers and vaccines. EXS 77:451-465. [DOI] [PubMed] [Google Scholar]
- 44.ten Dam, H. G. 1986. W.H.O.-sponsored research in BCG vaccination. Dev. Biol. Stand. 58:9-14. [PubMed] [Google Scholar]
- 45.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utiyama, S. R., J. Guardiano, M. L. Petzl-Erler, V. Mocelim, and I. J. de Messias-Reason. 2000. Autoantibody profile among Kaingang and Guarani tribe Indians in Southern Brazil. Rev. Panam. Salud Publica 7:371-376. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 47.van Eden, W., J. E. Thole, R. van der Zee, A. Noordzij, J. D. van Embden, E. J. Hensen, and I. R. Cohen. 1988. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331:171-173. [DOI] [PubMed] [Google Scholar]
- 48.van Eden, W., U. Wendling, L. Paul, B. Prakken, P. van Kooten, and R. van der Zee. 2000. Arthritis protective regulatory potential of self-heat shock protein cross-reactive T cells. Cell Stress Chaperones 5:452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Eden, W., A. Koets, P. van Kooten, B. Prakken, and R. van der Zee. 2003. Immunopotentiating heat shock proteins: negotiators between innate danger and control of autoimmunity. Vaccine 21:897-901. [DOI] [PubMed] [Google Scholar]
- 50.von Reyn, C. F., R. D. Arbeit, G. Yeaman, R. D. Waddell, B. J. Marsh, P. Morin, J. F. Modlin, and H. G. Remold. 1997. Immunization of healthy adult subjects in the United States with inactivated Mycobacterium vaccae administered in a three-dose series. Clin. Infect. Dis. 24:843-848. [DOI] [PubMed] [Google Scholar]
- 51.Wang, Y., C. G. Kelly, J. T. Karttunen, T. Whittall, P. J. Lehner, L. Duncan, P. MacAry, J. S. Younson, M. Singh, W. Oehlmann, G. Cheng, L. Bergmeier, and T. Lehner. 2001. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971-983. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Y., C. G. Kelly, M. Singh, E. G. McGowan, A. S. Carrara, L. A. Bergmeier, and T. Lehner. 2002. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J. Immunol. 169: 2422-2429. [DOI] [PubMed] [Google Scholar]
- 53.Wendling, U., L. Paul, R. van der Zee, B. Prakken, M. Singh, and W. van Eden. 2000. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J. Immunol. 164:2711-2717. [DOI] [PubMed] [Google Scholar]
- 54.Yang, X. D., J. Gasser, B. Riniker, and U. Feige. 1990. Treatment of adjuvant arthritis in rats: vaccination potential of a synthetic nonapeptide from the 65 kDa heat shock protein of mycobacteria. J. Autoimmun. 3:11-23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.