Expression library immunization (ELI) is a novel protocol for the systemic screening of any given genome to identify potential vaccine candidates. The basic concept of ELI introduced by Barry et al. is simple yet powerful (9) and can be applied to screen sequenced or unsequenced genomes of infectious agents. In principle, ELI can reduce any pathogen's genome in a relatively unbiased way to only a few antigens that provide protective immune responses. The essential concept in this approach is to employ the host immune system to select the best vaccine candidates against a particular disease. The essence of this approach is that the entire genome of a pathogen (bacterial, viral, parasitic, or fungal) can be cloned into genetic immunization vectors under the control of a eukaryotic promoter to create a library that would express all the open reading frames (ORFs) of a pathogen. Purified plasmid DNA from this library is inoculated into animals, usually in subgenomic pools of clones, to induce immune responses against the cloned antigens. The immunized animals are then challenged with the pathogenic organism to see which clones induced protective immunity and therefore which pool of antigens should be further deconvoluted to individual protective antigens. The main advantage of ELI is twofold; it provides a rapid screening protocol for an entire genome and the readout of screening is often protection, an end goal for vaccine development. In this review, we will focus our discussion on the technical merits of ELI compared to those of other vaccine discovery systems directed against infectious diseases. We will also explain the advantage and limitations of different ELI protocols and their application to search the genomes of bacterial, viral, and eukaryotic parasites. Finally, we will discuss the future of ELI in light of the current genomic revolution.
Worldwide statistics of infectious disease (46) indicate an increase in the spreading of infectious agents. The best possible hope to combat these infections is to develop new vaccines. Scientists are in a fierce race against these pathogens to reduce the death toll of nearly 13 million estimated each year from infectious diseases (33, 91). Among the 578 outbreaks verified by the WHO between 1998 and 2001, diseases such as cholera, meningitis, hemorrhagic fever, anthrax, and viral encephalitis were the most frequently reported (46). In recent years, the threat of bioterrorism heightened the global health concerns regarding infectious diseases. With little technological maneuvers, virulent strains of infectious agents can be generated in a common laboratory setting using well-established chemical synthesis protocols (e.g., poliomyelitis) (16). Moreover, with the emergence of several animal pathogens crossing to the human population (e.g., avian influenza virus and coronavirus) (36, 82), the need for strategies to control such emerging diseases is greatly heightened.
Vaccines are considered the cornerstone of our defense against bioweapons and emerging infectious agents (38), a strategy that is meant to disarm potential biothreats. Annually, 3 million children are saved by vaccination, while 2 million more are expected to die because of the lack of effective vaccines (11). The need for effective vaccines intensified the efforts to screen for novel vaccine candidates that can be readily available and delivered safely to a large number of afflicted individuals. For decades, the approach of single-antigen screening to identify vaccine candidates provided effective vaccines; however, the paramount need to combat emerging and reemerging infectious agents necessitates finding alternative approaches that screen whole genomes. Fortunately, ELI promises to fill this gap (9).
With all of the available genetic and recombination technologies developed in the past 50 years to screen vaccine candidates, we have just started to scratch the surface of the vaccinome (all potential vaccine candidates encoded in a given genome). Historically, vaccine development is a random process of discovery (empirical vaccines) where naturally attenuated strains of viruses or bacteria are found to protect humans after trials in animals (live attenuated vaccines). Classical examples are the use of cowpox vaccinia to protect against smallpox in humans (71) and the attenuated strain of Mycobacterium bovis BCG (bacillus Calmette-Guérin) to protect against tuberculosis (20). In most of these cases, an unknown mutation or mutations occurred during serial passage of the organism that led to its attenuation. Recent advances in the fields of bacterial genetics and recombinant DNA technology introduced the concept of “rational” vaccine design, where certain genes or their products are targeted for deletion. This approach increased our arsenal of vaccines against diseases such as cholera and typhoid fever (e.g., Vibrio cholerae CVD103-HgR and Salmonella enterica serovar Typhi CVD 908htrA, respectively) (49, 86). A potential problem of live attenuated vaccines has been shown in vaccinated individuals who become immunocompromised, as has been reported for human immunodeficiency virus (HIV) patients vaccinated with M. bovis BCG (18). A safer strategy than using attenuating pathogens is to design vaccines that include only protective antigens (subunit vaccine). Using this strategy, factors detrimental to the host (e.g., immunosuppressive elements) could be easily avoided. Unfortunately, strong adjuvants are needed to construct effective subunit vaccines, as was shown in the case of Mycobacterium tuberculosis and other infectious agents (17, 37, 42).
Recently, genetic immunization became a leading technology for screening potential vaccine candidates against both human and animal diseases which can eventually be used as subunit vaccines (35, 92). Genetic vaccines have several advantages over traditional methods. Like any subunit vaccine, only the antigen or antigens that produce an effective immune response are included, which could reduce any unintended responses. However, since the inoculating agent is DNA, the purification and handling of the genetic vaccines are simpler than those for traditional vaccines. The purification of any genetic vaccine can be standardized with any lot completely sequenced and therefore completely known. Additionally, there is no need for a “cold chain” to keep the gene vaccine viable, which is not true for live attenuated vaccines (8, 30). Finally, the mode of entry of genetic vaccines via direct cell delivery mimics the natural infection process, where pathogens can enter the host cells but without the potential for unwanted replication that has been seen with live attenuated vaccines. Even better, the delivery of a very small amount of a vaccine (nearly 100 ng) can elicit a strong protective immune response (8).
Plasmid DNA-expressing antigens encoded in pathogen genomes were successful in inducing humoral and cell-mediated immunity in laboratory and large animals as well as nonhuman primates (51). In animals, by employing various genetic immunization protocols, protective immunity was obtained against bacterial (e.g., M. tuberculosis) (53, 63), viral (e.g., influenza) (29), and parasitic (e.g., malaria and leishmania) (44) infestation. Even when combined infections of multiple pathogens were used in a challenge, genetic immunization proved to be a very effective strategy to combat the infection (89). Detailed analysis of genetic immunization indicated that it can induce both cellular and humoral immune responses in neonate animals with mixed Th1/Th2 responses, an advantage over polysaccharide subunit vaccines (12, 39). Additionally, the type of immune responses generated by genetic immunization can be manipulated towards either cellular or humoral responses or even combined responses when needed (see below). A discussion on the details of the immunological basis of genetic immunization, and in particular ELI, was recently reviewed by Barry et al. (7) and will be discussed briefly in the subsequent sections.
In several animals (e.g., mice, fowl, and fish), genetic immunizations proved to be successful against several diseases (5, 6). Unfortunately, such success was not matched in primates. In experiments conducted on primates and humans, genetic immunization induced low levels of antibodies, and large doses of DNA were needed to elicit suitable immune responses (21, 41, 94). We proposed to call the inability of genetic immunization to induce sufficient immune responses in primates the “simian barrier” (80). More recently (45), a reliable and effective cytotoxic-T-lymphocyte response in nonhuman primates was generated by including genetic adjuvants such as interleukin-2 (IL-2), IL-4, and gamma interferon (IFN-γ) with the use of lower doses of gene vaccines directed against HIV. Additionally, a regimen of priming with a gene vaccine followed by subunit vaccine boosting (93) could circumvent the “simian barrier” by stimulating both Th1 and Th2 immune responses. Introducing improvements on the genetic immunization vectors (by including a robust promoter sequence, for example) could also overcome the “simian barrier.”
SPECIAL CONSIDERATIONS BEFORE CHOOSING AN ELI PROTOCOL
Because of time, effort, and the large number of animals associated with ELI protocols, key aspects of the experimental approach are necessary to examine before deciding on a particular ELI protocol. Considerations related to the library construction, library deconvolution, and animal screening need to be examined carefully. In the following section, we will discuss key aspects of ELI and provide alternative solutions to shortcomings, when possible.
Animal models.
First and foremost, the presence of a suitable animal model to screen vaccine candidates in a high-throughput format is important for the success of any ELI protocol. Problems associated with the model readout for protection (e.g., only minor differences in organ colonization) will require a greater number of animals to discern differences between the experimental groups. Also, in diseases where the readout of protection is dependent on changes in organ pathology, it will be difficult to generate quantitative measures that can be evenly applied in a high-throughput format that is required to distinguish between vaccine pools. Such readouts can be applied for small genomes (e.g., viruses) or when semiquantitative measures of lesion severity in tissues are developed.
Traditionally, the best possible choice for a disease protection model would be the natural host. For some diseases, this is feasible, as has been demonstrated by Moore et al. with Mycoplasma hyopneumoniae (61), but for most diseases, this is not a practical solution. Because of the expense and availability of reagents to analyze the outcome of animal infections, it is not surprising that most of the bacterial and parasitic genomes are screened in mice, a salient laboratory animal model. Despite the difference in the genetic background of inbred mice, mice infected with different human and animal pathogens can consistently reproduce important aspects of infectious diseases (15). In one ELI experiment, a mouse model was used to screen Chlamydophila abortus, and the final set of antigens was tested in the natural host, dairy cows. The vaccine delivered either as a genetic vaccine or as protein provided partial protection from the natural herd-induced chlamydial infections (79).
Technologies available to design humanized or transgenic mice could also be used to provide specific aspects of an infection that could not be produced in intact mice (27). Humanized or transgenic mice could provide good models for vaccine screening. For most pathogens, the mouse model is used as a first screen for the library pools before fewer numbers of plasmids are selected for testing in a more expensive or sophisticated animal model. Needless to say, genetic immunizations were successful in protecting different animals when tested in a challenge system, indicating the suitability to screen ELI in animal models other than mice, as reviewed previously by van den Hurk et al. (92). Finally, it is always necessary to screen an ELI library for the particular disease phenotype or form that is targeted for vaccine development. Use of the appropriate route of infection and parameters that mimic the disease condition in the target host will maximize the chances to identify protective vaccine candidates (35). In some of the ELI experiments, readouts other than animal protection are employed. This is especially necessary when a suitable laboratory animal model is not available or feasible, such as for hepatitis viruses and HIV, respectively. In these cases, care must be taken to choose a readout that is immunologically relevant to the disease, if that is known. Immune correlates such as cytokine levels, antibody response, and cell proliferation assays have been used to identify potential vaccine candidates (1, 85).
Vector design and delivery.
The choice of a vector for library construction in an ELI screen is very important, since vector design can skew the immune response to either Th1- or Th2-dependent responses. If a more humoral response is desired, the gene fragments are often fused to a secretory leader sequence (e.g., tissue plasminogen activator [TPA]) so as to direct the antigen out of the cell. Alternatively, if a cellular response is desired, gene fragments are often fused to the mouse ubiquitin gene so as to direct the potential antigen to the proteasome for degradation and major histocompatibility complex (MHC) class I loading (83). Other vector modifications to improve ELI performance included gene fusion to HSP (heat shock protein sequence), monocyte chemotactic protein 3, and cytotoxic-T-lymphocyte antigen 4 and fusion to dendritic cell-targeting moieties (40, 67). The choice of a suitable targeting strategy depends on the assumption of which type of immune response is necessary for a vaccine to be protective, although researchers have shown that a cellular response can sometimes be generated with a secretory leader sequence and that antibodies can be raised against a target even if fused to ubiquitin (83). In most cases, the desired immune response is known, but in some cases, both types of immune response are required.
Molecular targeting is usually a term that refers to targeting antigens to specific cellular compartments for appropriate processing or to using cytokine adjuvants to improve the immune responses following vaccination. An example of molecular targeting is to fuse bacterial antigens (e.g., mycobacterial ESAT-6 or MPT-64) to TPA signal sequences to elicit higher levels of both cellular and humoral responses (50). Additionally, using ubiquitin sequence in the DNA constructs of several mycobacterial antigens in a DNA “cocktail” of TPA constructs generated protective immune responses in mice against an aerosol challenge with the virulent strain of M. tuberculosis (24). So far, molecular adjuvants (e.g., granulocyte-macrophage colony-stimulating factor or IL-12) are not widely used to augment the immune responses generated by ELI. Nonetheless, a higher level of protection against different disease models such as tuberculosis (26, 56), malaria, and HIV (31, 72) was reported when cytokines were coadministered with single- or multiple-gene vaccines. Whether the inclusion of cytokines in ELI screening will improve the overall performance of ELI or not remains to be determined.
Another key step in ELI screening is the efficient delivery of plasmid DNA to elicit the desired immune responses. In one study that examined the route of vaccination on the elicited immune responses, immunization of horses via skin and mucosal injection of plasmid DNA encoding the hemagglutinin antigen of equine influenza provided protective responses superior to only skin immunization (54). Additionally, intradermal gene gun delivery of vaccines resulted in predominantly immunoglobulin G2 (IgG2) antibody responses even when small doses of hemagglutinin-encoding plasmids were used, while intramuscular immunization against the same antigen in mice raised a predominantly IgG1 isotype (69). In another report, intramuscular gene expression of influenza nucleoprotein antigen was sufficient to elicit protective cellular immunity against influenza (90). It is noteworthy that gene gun immunization induced superior immune responses to the same antigens compared to intradermal or intramuscular injections (10). Taken together, deciding on the appropriate vector design combined with a suitable delivery protocol can affect the outcome of ELI screening. In our hands, we found that plasmid DNA delivery via gene gun combined with intramuscular injection is a safe choice to elicit protective immunity against pathogens, regardless of their preference of immune responses (89).
Library size and genome coverage.
Another consideration for ELI design is the suitable size of the genomic library to evaluate most of the encoded antigens. The number of clones in the library can vary depending on the desired insert length, the desired coverage level, the size of the genome, and the type of ELI method chosen (random versus directed) (see below). For a modest-size bacterial genome of ∼2 Mb with an average 1-kb ORF size, 2,000 vaccine candidates need to be assayed for their potential protection. Previously, both dose and route of immunization were shown to direct the elicited immune response to a specific arm of the immune system (34). With the increase of genome size targeted for ELI screening, there will definitely be an increase in the number of plasmids encoding antigens which need deconvolution (7). For example, for eukaryotic parasites (such as Plasmodium spp.), an ELI screen using only encoded antigens could be more appropriate than a typical random ELI (rELI) protocol. Unfortunately, in most cases, the exact pool dose and size needed to identify protective plasmids could not be completely predicted and need to be estimated empirically. To test for the appropriate pool dose and size in the animal model of choice, it is recommended to do a dose-response curve using a known antigen, if available, and dilute it in a pool of 50 to 100 plasmids with an equimolar amount of plasmid DNA. Usually, mice can tolerate up to 2 μg or 50 μg of plasmid DNA with untoward reactions when gene gun or intramuscular injections are used, respectively (34). The ability to induce protection from this pool usually implies the suitability of both the dose and number of plasmids used to inoculate animals. A similar approach can be applied to test the reproducibility of detecting vaccine candidates using a particular model system.
MAIN APPROACHES FOR ELI
Although ELI can be applied to both infectious and noninfectious diseases such as cancer, we will focus our discussion on ELI screening for vaccines against infectious agents. In the following section, we will discuss the various approaches for conducting ELI screening supported by examples from published work on bacterial, viral, and parasitic infections. Several steps of rELI, directed ELI (dELI), and cDNA-directed ELI (cDELI) protocols are shared and will be discussed only in the rELI section. The rest of the steps will be discussed to delineate the differences and feasibility of each approach.
(i) rELI protocol.
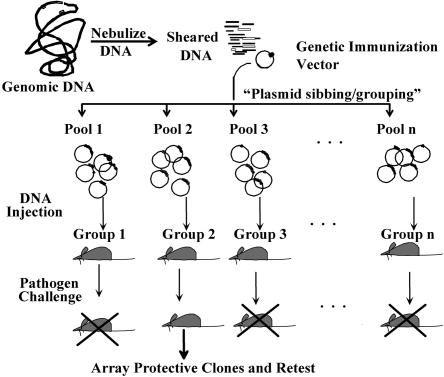
The concept for rELI is to randomly shear genomic DNA and to clone the fragments into an appropriate mammalian expression vector. Use of genomic DNA as starting material further enhances the unbiased approach for which ELI was created; no assumptions are made as to what is or is not an open reading frame. Additionally, all genes in the genome are expressed in approximately the same ratio throughout the library (Fig. 1). In the following sections, we will describe the necessary steps to use rELI in the search for vaccine targets.
FIG. 1.
An outline of ELI protocol. The entire genome of a pathogen is fractioned into small fragments before cloning into a mammalian expression vector creating a library that would express all the ORFs of a pathogen. Groups of animals can be immunized with separate plasmid pools followed by a pathogen challenge using a suitable route of infection. Plasmids from a protected mouse group(s) will be further divided into smaller pools and retested in other mice groups to identify single protective antigens.
(a) Library construction.
In a typical rELI, each library will contain at most one-sixth of its plasmids encoding in-frame ORFs (assuming equal cloning of evenly digested genomic DNA). Accordingly, to cover 100% of each genome, six libraries of at least 1.2 × 104 plasmids/library (2,000 × 6 reading frames) need to be generated. In the original publication of ELI screening a genome of ∼1,000 genes, nine pools of random genomic libraries of Mycoplasma pulmonis with ∼27,000 plasmids were screened in mouse groups. Each of the random libraries was fused in frame with human growth hormone sequences to direct the encoded antigens for extracellular secretion and the production of humoral immune responses. Each fused ORF was represented in three libraries to ensure full genome expression in each reading frame. Full protection against subsequent challenge with the virulent strain of M. pulmonis was achieved in two out of the nine libraries. On the other hand, six other libraries of M. pulmonis gave different levels of protection, indicating differences in the library composition and hence the ability to generate protective immunity. Higher numbers of libraries and clones per library were used when the genomes of Leishmania major (66) and Plasmodium chabaudi were screened in mice tissues with peptides encoding an average of 25 amino acids/peptide (77). Unfortunately, with the increase of library complexity, more cycles of deconvulsion are required to identify single antigens. For example, to screen the genome of L. major, three rounds of screening in mice were employed to reduce the library complexity from 105 to 103 clones to select a protective genomic library (66). However, when the dELI approach is employed, the library complexity can be greatly reduced because of the cloning of individual ORFs in separate plasmids.
To generate a representative genomic DNA (gDNA) for library construction, researchers have taken different approaches to fractionate the gDNA into “clonable” fragments. In some cases, the gDNA is sonicated or nebulized followed by a fill-in reaction or adaptor ligation to facilitate subsequent cloning into a mammalian expression vector. Depending on the expected gene size in a genome, fragmented gDNA could undergo size selection before cloning. In other cases, the gDNA is digested with suitable restriction enzyme before ligation into a set of vectors that will allow fusions in all three reading frames. In most of the experiments where genomic DNA is used as starting material for ELI, the pCMV vector (or one of its derivative) is employed, similar to the process reported in the original publication describing ELI (9). In this vector, antigen-encoding sequences are inserted between the mouse cytomegalovirus promoter and the human growth hormone terminator sequences. In some reports, the pcDNA3.1 vector (Invitrogen) has been used successfully, especially when cDNA was used as starting material for ELI. A major difference between the two vector systems is the presence of a simian virus 40 origin of replication in the pcDNA3.1, which could be an undesired feature once a final vaccine is tested in a mammalian host.
(b) Library deconvolution.
Once constructed, the ELI libraries need to be screened in animals using pools of plasmids in a process termed “library deconvolution.” This process involves the screening of successively smaller pools of clones in animals to identify clones that induce protective immunity. Employing such a protocol will reduce the complexity of the library and eventually identify clones that provide protective immunity. Most of the genome tested so far in an ELI protocol (Table 1) employed an initial large pool or set of pools. This is because the task of deconvoluting the protective pool is often complicated. For those projects that attempted to find the protective clone, two different methods were used for systematic screening of the library. The first approach is to split the protective pool into smaller subpools, with no overlapping clones in each pool. In another approach, the protective pools can be arranged in a matrix to reduce the number of animals used in the challenge trials (79). In the matrix screening, all of the clones from the protective pools should be picked into 96-well plates and arrayed into a virtual two-dimensional grid. By this pooling method, each clone is located in two unique pools, corresponding to one in each of the axes. When two intersecting pools of the matrix are protective, a single clone could be rapidly identified as the protective antigen. This approach can be expanded to a three-dimensional grid, which reduces the number of challenge rounds even more.
TABLE 1.
List of microbial systems where ELI was applied
| Pathogen | Animal model | Outcome | Genome size/library size | Library type | Reference |
|---|---|---|---|---|---|
| Eukaryotic parasites | |||||
| Coccidioides immitis | Mouse | Reduced to single clone | 29 Mb/10 pools of 80 genes | cDELI | 43 |
| Ixodes scapularis | Mouse | Reduction to defined pools | 2,100 Mb | cDELI | 2 |
| L. donovani | Mouse | Reduced to sequenced pools | ∼34 Mb/15 pools of 2,000 clones | cDELI | 58 |
| L. major | Mouse | Partial decovolution | 50 Mb/3 pools with 105 clones each | rELI | 66 |
| P. chabaudi adami | Mouse | Reduction from 3,000 to 616 clones | 25-30 Mb/10 pools of 3,000 clones | rELI | 77 |
| P. chabaudi adami | Mouse | Test different fusion vectors | 25-30 Mb/3 large pools of 30,000 clones | rELI | 67 |
| Taenia crassiceps | Mouse | Partial protection | 8,000 clones | cDELI | 55 |
| Toxoplamsa gondii | Mouse | Partial protection | 65 Mb/1 large library | rELI | 32 |
| Trypanosoma cruzi | Mouse | Test immune correlate | 40 Mb/1 large library pool | rELI | 1 |
| Bacteria | |||||
| B. abortus | Mouse | Partial protection | 3 Mb/1 library with 20,000 clones | rELI | 48 |
| C. abortus | Mouse | Reduced to single clone | 1.1 Mb | rELI | 79 |
| C. ruminantim (Ehrlichia ruminantium) | Mouse | Partial protection | 1.57 Mb/22 pools with 3,000 clones each | rELI | 14 |
| M. hyopneumoniae | Pig | Used a new in-frame cloning vector | 0.9Mb | rELI | 61 |
| M. pulmonis | Mouse | Complete protection | 1 Mb/3,000 clones | rELI | 9 |
| Piscirickettsia salmonis | Coho salmon | Partial protection | Unkonwn/22,000-28,000 clones | rELI | 60 |
| Viruses | |||||
| HIV-1 | Mouse | Complete protection | 9.7 kb/32 clones | dELI | 74 |
| HIV-2 | Baboon | Partial protection | 9.7 kb/38 clones | dELI | 52 |
| SIVa | Macaque | Partial protection | 9 kbp/rELI vs dELI libraries | rELI and dDELI | 85 |
SIV, simian immunodeficiency virus.
(c) Identification of vaccine candidates.
The result of each screening round of ELI pools in animals is protection. In some cases, protection can be manifested as a reduction in pathogen count such as in the mouse model of Mycoplasma pneumonia (9) or Cowdria ruminantium (14). In another system, a reduction of mortality rate or elongation of the survival time is the assay readout (13, 60). Once a protective pool with a fairly small number of plasmids is identified, the encoded DNA is usually sequenced to allow more detailed analysis of the protective antigens. At this stage, clones containing noncoding sequences or with clear reasons that prevent their expression (e.g., the presence of a stop codon at the 5′ end of the insert) are excluded from further analysis. Of those trials that refined the pool to a single gene, many did not cover the entire genome (43). At this point, there is a trade-off between pooling plasmids and testing them as single antigens. If the plasmid pool is too large, a potential antigen may be at too a low a level to confer protection; however, a library of subpools that are too small may miss antigenic fragments. An advantage of the ELI approach is that a set of vaccine candidates is discovered often; if one proves ineffective or partially protective, a combination of antigens can be employed instead.
(d) Challenges of rELI.
In the rELI approach, many rounds of screening are often necessary to completely deconvolute a protective pool. This deconvolution also assumes that a single gene is responsible for the protection seen in a library pool. If two proteins can act synergistically to provide protection, either by forming a complex, one providing T-cell help for the other, or by invoking multiple protective responses, then subsequent dividing into subpools will reduce or eliminate a protective response. This effect has been reported previously by Almazan et al. (2) and Melby et al. (58), where subpools were less protective than the larger pool. Another concern is that the library may contain genes that make the disease worse or that somehow interfere with the protection readout. In some cases, researchers have reported that some of the pools have had a negative effect on protection, even over a mock-vaccinated control (2). In other cases, no negative effects are seen (74); the difference will always be pathogen and model specific. Nonetheless, in an attempt to boost the immune responses generated by genomic libraries of ELI, a live attenuated strain of Neisseria meningitidis was inoculated after DNA immunization (95). In this experiment, 9 out of 10 sublibraries induced bactericidal activity against a challenge with Neisseria, reflecting the ability of DNA genomic libraries to prime the immune system.
Another challenge for the rELI genomic library approach is that only ∼1/6 of the clones will be in the correct reading frame and one-third of the clones will be able to be used for a cDELI library. The number of in-frame gene fragments can be even less if the pathogen contains introns or large noncoding regions. For example, when a 20,000-clone genomic library of Brucella abortus was screened in BALB/c mice, only a slight reduction in bacterial load (<0.5 log) in the spleens of immunized mice was obtained despite the significant induction of both humoral (higher IgG1/Ig2a ratio) and cellular (high levels of IFN-γ) responses in immunized mice (48). Sequence analysis of the B. abortus library that was used indicated that only 6.5% of the clones were actually encoding antigens instead of random insertion in six reading frames that could encode up to 17% of antigens. This decline could explain the low level of antigen representation and protection when this library was screened in mice. However, if library design included a vector that enhances in-frame cloning, the library can be more complete and much smaller. One such vector that has been designed for this purpose was described previously by Rombel et al., where a promoterless green fluorescent protein sequence was cloned downstream of multiple cloning sites (70). Moore et al. designed a different vector with a very similar purpose (61). In this vector, a His tag fusion was used to identify clones expressing recombinant protein.
(ii) dELI.
With the increases made in genomic sequencing, one can screen only the ORFs encoded in a pathogen. In a typical dELI protocol, all ORFs predicted in a genome are amplified by PCR and cloned to a mammalian expression vector such as the one used for random ELI. In launching such efforts, investigators need to be comfortable with high-throughput protocols for PCR amplification, cloning, and plasmid purifications. Once PCR products are amplified, amplicons can be cloned either by using standard molecular biology techniques or by various restriction-free cloning systems (universal cloning) (76). In universal cloning, no purification or quantification of amplified fragments is necessary before ligation to the target plasmid. To further increase the speed at which individual genes can be tested, the requirement of cloning each gene can be replaced by employing linear expression element (LEE) technology (84). This approach is based on the fact that any ORF flanked with a mammalian promoter and terminator can be expressed to elicit immune responses following genetic immunization, a feature that is exploited to facilitate vaccine design (84). In addition to speeding up the process, LEE constructs eliminates cloning biases that are invariably introduced in a standard directed library approach. Nonetheless, when ORFs are >1.5 kb, the process of constructing LEE could become tedious unless arbitrary decisions are made to restrict the size of the PCR product. Regardless of whether LEEs or full plasmids are used in dELI, the rest of library screening is very similar to the deconvolution process applied for rELI. During the deconvolution stage of dELI, pools of plasmids can be easily identified and a single antigen can be produced, which further speeds up the characterization of vaccine candidates.
So far, the only reports employing the dELI approach are those using small viral genomes such as HIV and simian immune deficiency virus. In one report, Sykes and Johnston generated an HIV-1 dELI library that contained 32 overlapping ∼550-bp fragments and that covered all of the open reading frames (83). The library, although complete and encoding all of the viral genome, did not cause infection in mice. However, diverse cellular and humoral immune responses were observed. Using this library, specific CD8+-T-cell responses were not altered in mice even in the presence of strong immunodominant antigens of HIV such as gag, nef, and pol (74). In a subsequent report, the performance of a reengineered genomic library of HIV-1 (fused with ubiquitin sequence) induced a diverse, multivalent CD8+-T-cell response superior to any comparable immunization with individual antigens such as Gag or Pol proteins (73).
An advantage of dELI over rELI is the reduction in complexity of the genomic library. Instead of screening thousands of clones containing noncoding sequences in rELI, only predicted ORFs can be screened with a smaller number of animal groups. Use of the dELI protocol decreases the number of plasmids in the library, which increases the concentration of any one gene in a pool and increases the likelihood of identifying protective antigens. Some antigens may still not function even at this higher dose, especially if they have the incompatible codon bias needed for mammalian expression. This can often be overcome by altering the codon bias; however, this can be cost prohibitive on a large scale. Finally, when a defined library is used, known toxins or previously identified vaccine candidates that can affect library screening in animals can be excluded. Nonetheless, even the dELI protocol was not able to cross the “simian barrier” that was discussed above. In baboons, a genomic library of HIV-2 virus generated CD8+-T-cell responses; however, no difference in viral loads between control and vaccinated animals was found (52). As suggested in this report, this lack of protective immunity against HIV could be attributed to the shortcomings of the genetic immunization protocol itself instead of the ELI approach.
(iii) cDELI.
Another ELI protocol that is a hybrid of both rELI and dELI, termed cDELI, takes advantage of a well-established technology of cDNA cloning. In this protocol, cDNA is used rather than genomic DNA or PCR products as the starting material for ELI (55). Such an approach is useful if the noncoding regions in the pathogen are substantial, as is the case for parasitic and fungal pathogens (2), or if a particular life cycle is being targeted for vaccine development. For example, in malaria and leishmaniasis, employing ELI is challenging because the large size of parasite genomes (≥10 Mb) (4) requires a high number of plasmid pools to obtain sufficient genome coverage. Also, the coding density of parasitic genomes is very low compared to that of bacterial pathogens, which complicates the design of genomic libraries.
So far, several groups have adopted cDELI to identify vaccine candidates against parasitic infestation. With small genomic library pools of only 30,000 clones, a reasonable protection level (up to 50%) was achieved when mice were challenged with a virulent strain of P. chabaudi adami (67, 77). Specific T-cell and humoral immune responses were demonstrated in vaccinated mice. A similar approach for genomic library immunization was also successful when applied against L. major (66), demonstrating the ability of cDELI to discover vaccines against complex parasites with multiple life cycles. In other infestations, a cDNA library was constructed from the amastigote stage of Leishmania donovani that was divided into 15 smaller sublibraries with 2,000 clones each (57). In three rounds of testing, they found a group of clones that decreased parasite burden in the spleen and liver and had an increase in IFN-γ production. In their second round, they screened two of the protective pools for protein expression to reduce the number of clones tested in round 3. Under these circumstances, much lower numbers of plasmids were screened in mice and resulted in the identification of plasmid pools that yielded a significant reduction in parasite levels (55, 57).
THE FUTURE OF ELI IN VACCINE DISCOVERY
Different protocols of ELI have been implemented in viral, bacterial, and parasitic diseases with varying degrees of success. Because of the nature of ELI and its simple design, several variations of the main concept of library screening can be easily envisioned to select vaccine candidates. A key step that needs to be addressed to ensure the application of ELI to different microbial systems is to improve antigen expression in a mammalian host. For example, a change in the codon optimization to replace rare codons in microbial transcripts to match mammalian codon usage can have a dramatic effect on the overall performance of genetic immunization (25, 64). As we explained previously, coadministration of cytokines and the use of robust promoter sequences in genetic immunization could be adopted for ELI. Although these changes are not related to the ELI protocol itself, the whole ELI screen can benefit from such modifications.
We also envision that the ELI deconvolution protocol can be combined with or adopted to different vaccine discovery systems. As an example of combining ELI with other technologies, a genomic expression library of Helicobacter pylori was first screened for immunogenic antigens using a Western blotting protocol employing sera collected from infected humans or rabbits (47). A relatively large number of clones (n = 114) were identified as immunogenic in this preliminary step of library characterization. Only pools of immunogenic antigens were further screened in primates to identify protective vaccines. In another example using an M. tuberculosis genomic library expressed in Escherichia coli, only antigens recognized by rabbit antiserum directed against M. tuberculosis were further tested in a murine challenge system as vaccine candidates (28). Another modification of the main theme of ELI is to enrich the plasmid library for antigens that trigger a desired immune response before screening in animals. Again, this strategy was applied to M. tuberculosis, where the genomic library was screened for eliciting T-cell activation, a key factor in controlling tuberculosis (75). Two novel vaccine candidates (mtb10 and mtb41) were identified in this screen. In the following section, we will discuss the potential of combining ELI screening with other technologies that could eventually lead to an improvement in the overall performance of ELI as a platform technology for vaccine discovery.
Expression microarrays and ELI.
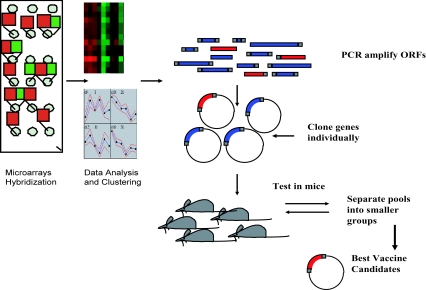
A considerable hurdle for ELI technology in vaccine discovery is the size of the genome to be screened and the number of plasmid pools that should be constructed. One way to reduce the complexity of the ELI library of plasmids is to use transcriptional profiling to screen only genes responsible for establishing an infection to discover vaccine candidates (Fig. 2). The idea behind this approach is that differentially expressed genes during infection could constitute important targets for vaccine development. Fortunately, with the advancement of transcriptional profiling techniques (such as real-time PCR and DNA microarrays), we can obtain a comprehensive profile of microbial systems on a genome-wide level. We and others have used DNA microarrays to profile pathogens of significant importance to humans and animals during natural or experimental infection (59, 65, 68, 78, 88). In all of these experiments, mixing of the host RNA with pathogen transcripts constituted a major hurdle against obtaining an accurate estimate of the pathogen transcript levels. In some cases, pathogens were directly isolated for microarray analysis using infected samples where they were present in abundance with little contamination from the host tissues (65, 68). In other cases, total RNA of the host and pathogen were extracted and only pathogen transcripts were successfully labeled using a set of genome-directed primers (87). The second logical progression for such in vivo analysis is to screen differentially expressed genes for diagnostic antigens and vaccine candidates. In particular, groups of genes of unknown function or those with a specific transcription profile can be screened using the high-throughput format of dELI.
FIG. 2.
Microarrays and vaccine development. Gene lists generated from different analyses of microarray hybridizations (such as hierarchical clustering) will be individually cloned into a mammalian expression system before further screening in an ELI protocol as outlined in Fig. 1.
Bioinformatics and ELI.
Combining the screening power of ELI with bioinformatics could identify novel vaccine candidates that could not be predicted using normal biological screening protocols (96). For example, bioinformatics analysis of the sequence of the Bacillus anthracis plasmid encoding 143 ORFs for potential virulence and pathogenesis genes was successful in predicting only 11 candidates for further analysis (3). If such an approach is used on a genome-wide level, dELI would be a realistic approach for systematic screening of vaccines against pathogens with larger genomes (e.g., parasites and fungi). Additionally, by comparing microbial sequences on a genome-wide level, we can exclude genes such as housekeeping genes or genes that are too similar to the host, but we can include genes that encode protective epitopes against multiple pathogens, especially if they are closely related (19). Alternatively, we can include genes that are predicted to encode surface-exposed proteins or putative virulence factors. This type of approach, termed “reverse vaccinology,” has been used to find vaccine candidates for a variety of different pathogens, including meningococcus, B. anthracis, Chlamydia pneumoniae, Streptococcus pyogenes, M. tuberculosis, and pneumococcus (reviewed by Mora et al. [62]). This technology relies even more on user input to determine what would make a good vaccine. One disadvantage of this type of approach is that sometimes the immunodominant region of a protein is not the one that is best for raising neutralizing antibodies. Another disadvantage is the possibility of missing unknown genes that could be protective antigens. In these circumstances, various sets of genes that are suspected to not be protective could be tested separately from the rest of the pools.
Another bioinformatics approach for vaccine discovery depends on predicting epitope binding to MHC molecules on the host antigen-presenting cells. The approach uses extensive experimental knowledge and computer prediction algorithms to find which peptides in a pathogen would have the greatest affinity and would therefore elicit the best immune response (22, 23). These methods make it possible to test possible peptides for affinity to known HLA polymorphisms in silico. Other algorithms have been developed to find “promiscuous” epitopes that can bind to multiple HLA genotypes. In the former approach, vaccine candidates were targeted to certain populations, while in the latter approach, vaccines were developed to benefit a wide group of the population. However, genetic variations in either the host or the pathogen populations can limit the effectiveness of these epitopes as vaccines. While this approach primarily helps to define the cytotoxic-T-lymphocyte epitopes, there is a component of class II-restricted T-cell epitopes that could be included by other prediction software (e.g., TEPITOPE) (81). Unfortunately, most of these predictions are done on mouse MHC and not on human MHC alleles. The type of immune response that can be predicted with epitope MHC matching programs is primarily limited to cellular immune responses. Once all of these epitopes are identified, an ELI protocol should be able to identify vaccine candidates. In these experiments, ELI will provide a platform for rapid testing of vaccine candidates.
Generally, to make safer and more-effective vaccines, we need to find an array of protective antigens for a given pathogen. In vaccinology, ELI protocols have a good chance to contribute tremendously to the discovery of novel antigens that can serve as vaccines. The ELI technology allows us the rapid discovery of vaccines against an individual pathogen or even a group of pathogens. To this end, by combining ELI with genomic technologies, we will be able to completely define the vaccinome of any pathogen.
Acknowledgments
We thank Gary Splitter for reading the manuscript.
The research in the laboratory of A.M.T. is supported by the University of Wisconsin-Madison Graduate School, the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (grants WIS04794, WIS04823, JDIP), and the Department of Defense (DARPA).
Editor: J. B. Kaper
REFERENCES
- 1.Alberti, E., A. Acosta, M. E. Sarmiento, C. Hidalgo, T. Vidal, A. Fachado, L. Fonte, L. Izquierdo, J. F. Infante, C. M. Finlay, and G. Sierra. 1998. Specific cellular and humoral immune response in Balb-c mice immunised with an expression genomic library of Trypanosoma cruzi. Vaccine 16:608-612. [DOI] [PubMed] [Google Scholar]
- 2.Almazan, C., K. M. Kocan, D. K. Bergman, J. C. Garcia-Garcia, E. F. Blouin, and J. de la Fuente. 2003. Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 21:1492-1501. [DOI] [PubMed] [Google Scholar]
- 3.Ariel, N., A. Zvi, H. Grosfeld, O. Gat, Y. Inbar, B. Velan, S. Cohen, and A. Shafferman. 2002. Search for potential vaccine candidate open reading frames in the Bacillus anthracis virulence plasmid pXO1: in silico and in vitro screening. Infect. Immun. 70:6817-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash, C. 1999. Exploring parasite genomes. Trends Microbiol. 7:10-12. [DOI] [PubMed] [Google Scholar]
- 5.Babiuk, L. A., S. L. Babiuk, B. I. Loehr, and S. van Drunnen Littel-van den Hurk. 2000. Nucleic acid vaccines: research tool or commercial reality. Vet. Immunol. Immunopathol. 76:1-23. [DOI] [PubMed] [Google Scholar]
- 6.Babiuk, L. A., P. J. Lewis, L. van Drunen, S. Tikoo, and X. Liang. 1998. Nucleic acid vaccines: veterinary applications. Curr. Top. Microbiol. Immunol. 226:90-106. [DOI] [PubMed] [Google Scholar]
- 7.Barry, M. A., D. P. Howell, H. A. Andersson, J. L. Chen, and R. A. Singh. 2004. Expression library immunization to discover and improve vaccine antigens. Immunol. Rev. 199:68-83. [DOI] [PubMed] [Google Scholar]
- 8.Barry, M. A., and S. A. Johnston. 1997. Biological features of genetic immunization. Vaccine 15:788-791. [DOI] [PubMed] [Google Scholar]
- 9.Barry, M. A., W. C. Lai, and S. A. Johnston. 1995. Protection against mycoplasma infection using expression-library immunization. Nature 377:632-635. [DOI] [PubMed] [Google Scholar]
- 10.Bennett, A. M., R. J. Phillpotts, S. D. Perkins, S. C. Jacobs, and E. D. Williamson. 1999. Gene gun mediated vaccination is superior to manual delivery for immunisation with DNA vaccines expressing protective antigens from Yersinia pestis or Venezuelan equine encephalitis virus. Vaccine 18:588-596. [DOI] [PubMed] [Google Scholar]
- 11.Bonanni, P. 1999. Demographic impact of vaccination: a review. Vaccine 17(Suppl. 3):S120-S125. [DOI] [PubMed] [Google Scholar]
- 12.Bot, A., S. Antohi, S. Bot, A. Garcia-Sastre, and C. Bona. 1997. Induction of humoral and cellular immunity against influenza virus by immunization of newborn mice with a plasmid bearing a hemagglutinin gene. Int. Immunol. 9:1641-1650. [DOI] [PubMed] [Google Scholar]
- 13.Brayton, K. A., M. van der Walt, S. W. Vogel, and B. A. Allsopp. 1998. A partially protective clone from Cowdria ruminantium identified by using a Salmonella vaccine delivery system. Ann. N. Y. Acad. Sci. 849:247-252. [DOI] [PubMed] [Google Scholar]
- 14.Brayton, K. A., S. W. Vogel, and B. A. Allsopp. 1998. Expression library immunization to identify protective antigens from Cowdria ruminantium. Ann. N. Y. Acad. Sci. 849:369-371. [DOI] [PubMed] [Google Scholar]
- 15.Buer, J., and R. Balling. 2003. Mice, microbes and models of infection. Nat. Rev. Genet. 4:195-205. [DOI] [PubMed] [Google Scholar]
- 16.Cello, J., A. V. Paul, and E. Wimmer. 2002. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science 297:1016-1018. [DOI] [PubMed] [Google Scholar]
- 17.Cohen, D., N. Orr, M. Haim, S. Ashkenazi, G. Robin, M. S. Green, M. Ephros, T. Sela, R. Slepon, I. Ashkenazi, D. N. Taylor, A.-M. Svennerholm, A. Eldad, and J. Shemer. 2000. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Israeli young adults. Infect. Immun. 68:4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn, D. L. 1997. Use of the bacille Calmette-Guerin vaccination for the prevention of tuberculosis: renewed interest in an old vaccine. Am. J. Med. Sci. 313:372-376. [DOI] [PubMed] [Google Scholar]
- 19.Cole, S. T. 2002. Comparative mycobacterial genomics as a tool for drug target and antigen discovery. Eur. Respir. J. 20:78S-86S. [DOI] [PubMed] [Google Scholar]
- 20.Comstock, G. W. 1994. Field trials of tuberculosis vaccines: how could we have done them better? Cont. Clin. Trials 94:247-276. [DOI] [PubMed] [Google Scholar]
- 21.Couillin, I., F. Letourneur, P. Lefebvre, J. G. Guillet, and F. Martinon. 2001. DNA vaccination of macaques with several different Nef sequences induces multispecific T cell responses. Virology 279:136-145. [DOI] [PubMed] [Google Scholar]
- 22.De Groot, A. S., A. Bosma, N. Chinai, J. Frost, B. M. Jesdale, M. A. Gonzalez, and W. Martin. 2001. From genome to vaccine: in silico predictions, ex vivo verification. Vaccine 19:4385-4395. [DOI] [PubMed] [Google Scholar]
- 23.De Groot, A. S., and R. Rappuoli. 2004. Genome-derived vaccines. Expert Rev. Vaccines 3:59-76. [DOI] [PubMed] [Google Scholar]
- 24.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deml, L., A. Bojak, S. Steck, M. Graf, J. Wild, R. Schirmbeck, H. Wolf, and R. Wagner. 2001. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 75:10991-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derrick, S. C., C. Repique, P. Snoy, A. L. Yang, and S. Morris. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect. Immun. 72:1685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich, W. F. 2001. Using mouse genetics to understand infectious disease pathogenesis. Genome Res. 11:325-331. [DOI] [PubMed] [Google Scholar]
- 28.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnelly, J. J., A. Friedman, J. B. Ulmer, and M. A. Liu. 1997. Further protection against antigenic drift of influenza virus in a ferret model by DNA vaccination. Vaccine 15:865-868. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly, J. J., M. A. Liu, and J. B. Ulmer. 2000. Antigen presentation and DNA vaccines. Am. J. Respir. Crit. Care Med. 162:S190-S193. [DOI] [PubMed] [Google Scholar]
- 31.Doolan, D. L., and S. L. Hoffman. 1997. Pre-erythrocytic-stage immune effector mechanisms in Plasmodium spp. infections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1361-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fachado, A., A. Rodriguez, J. Molina, J. C. Silverio, A. P. Marino, L. M. Pinto, S. O. Angel, J. F. Infante, Y. Traub-Cseko, R. R. Amendoeira, and J. Lannes-Vieira. 2003. Long-term protective immune response elicited by vaccination with an expression genomic library of Toxoplasma gondii. Infect. Immun. 71:5407-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fauci, A. S. 2001. Infectious diseases: considerations for the 21st century. Clin. Infect. Dis. 32:675-685. [DOI] [PubMed] [Google Scholar]
- 34.Fu, T. M., L. M. Guan, A. Friedman, T. L. Schofield, J. B. Ulmer, M. A. Liu, and J. J. Donnelly. 1999. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J. Immunol. 162:4163-4170. [PubMed] [Google Scholar]
- 35.Griffin, J. F. T. 2002. A strategic approach to vaccine development: animal models, monitoring vaccine efficacy, formulation and delivery. Adv. Drug Deliv. Rev. 54:851-861. [DOI] [PubMed] [Google Scholar]
- 36.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Hang, Y. J. Gan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2002. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. [DOI] [PubMed] [Google Scholar]
- 37.Hansson, M., P. A. Nygren, and S. Stahl. 2000. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 32:95-107. [DOI] [PubMed] [Google Scholar]
- 38.Hassani, M., M. C. Patel, and L. A. Pirofski. 2004. Vaccines for the prevention of diseases caused by potential bioweapons. Clin. Immunol. 111:1-15. [DOI] [PubMed] [Google Scholar]
- 39.Hassett, D. E., J. Zhang, M. Slifka, and J. L. Whitton. 2000. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 74:2620-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser, H., and S. Y. Chen. 2003. Augmentation of DNA vaccine potency through secretory heat shock protein-mediated antigen targeting. Methods 31:225-231. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman, S. L., D. L. Doolan, M. Sedegah, J. C. Aguiar, R. B. Wang, A. Malik, R. A. Gramzinski, W. R. Weiss, P. Hobart, J. A. Norman, M. Margalith, and R. C. Hedstrom. 1997. Strategy for development of a pre-erythrocytic Plasmodium falciparum DNA vaccine for human use. Vaccine 15:842-845. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh, M. J., A. P. Junqueira-Kipnis, A. Hoeffer, O. C. Turner, and I. M. Orme. 2004. Incorporation of CpG oligodeoxynucleotide fails to enhance the protective efficacy of a subunit vaccine against Mycobacterium tuberculosis. Vaccine 22:655-659. [DOI] [PubMed] [Google Scholar]
- 43.Ivey, F. D., D. M. Magee, M. D. Woitaske, S. A. Johnston, and R. A. Cox. 2003. Identification of a protective antigen of Coccidioides immitis by expression library immunization. Vaccine 21:4359-4367. [DOI] [PubMed] [Google Scholar]
- 44.Kalinna, B. H. 1997. DNA vaccines for parasitic infections. Immunol. Cell Biol. 75:370-375. [DOI] [PubMed] [Google Scholar]
- 45.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285:204-217. [DOI] [PubMed] [Google Scholar]
- 46.Kindhauser, M. K. 2003. Global defence against the infectious disease threat, p. 1-231. In World Health Organization Report Communicable Diseases 2002, vol. 15. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 47.Lazowska, I., L. Trzeciak, R. Godlewska, E. Hennig, K. Jagusztyn-Krynicka, J. Popowski, J. Regula, and J. Ostrowski. 2000. In search of immunogenic Helicobacter pylori proteins by screening of expression library. Digestion 61:14-21. [DOI] [PubMed] [Google Scholar]
- 48.Leclercq, S. Y., and S. C. Oliveira. 2003. Protective immunity induced by DNA-library immunization against an intracellular bacterial infection. J. Drug Target. 11:531-538. [DOI] [PubMed] [Google Scholar]
- 49.Levine, M. M., J. B. Kaper, D. Herrmann, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet ii:467-470. [DOI] [PubMed]
- 50.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, M. A., W. McClements, J. B. Ulmer, J. Shiver, and J. Donnelly. 1997. Immunization of non-human primates with DNA vaccines. Vaccine 15:909-912. [DOI] [PubMed] [Google Scholar]
- 52.Locher, C. P., K. F. Sykes, D. J. Blackbourn, and S. A. Johnston. 2002. Immune responses in baboons vaccinated with HIV-2 genetic expression libraries. J. Med. Primatol. 31:323-329. [DOI] [PubMed] [Google Scholar]
- 53.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, et al. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 54.Lunn, D. P., G. Soboll, B. R. Schram, J. Quass, M. W. McGregor, R. J. Drape, M. D. Macklin, D. E. McCabe, W. F. Swain, and C. W. Olsen. 1999. Antibody responses to DNA vaccination of horses using the influenza virus hemagglutinin gene. Vaccine 17:2245-2258. [DOI] [PubMed] [Google Scholar]
- 55.Manoutcharian, K., L. I. Terrazas, G. Gevorkian, and T. Govezensky. 1998. Protection against murine cysticercosis using cDNA expression library immunization. Immunol. Lett. 62:131-136. [DOI] [PubMed] [Google Scholar]
- 56.Martin, E., A. T. Kamath, H. Briscoe, and W. J. Britton. 2003. The combination of plasmid interleukin-12 with a single DNA vaccine is more effective than Mycobacterium bovis (bacille Calmette-Guerin) in protecting against systemic Mycobacterium avium infection. Immunology 109:308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melby, P. C., N. Biediger, H. Flores, C. Geldmacher, M. Melendez, G. B. Ogden, J. Uranga, and W. Zhao. 1999. Identification of vaccine candidates against visceral leishmaniasis by expression library immunization. Am. J. Trop. Med. Hyg. 61:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melby, P. C., G. B. Ogden, H. Flores, W. Zhao, C. Gelmacher, J. Uranga, N. M. Biediger, S. K. Ahuja, J. Uranga, and M. Melendez. 2000. Identification of vaccine candidates for experimental visceral leishmaniasis by immunization with sequential fractions of a cDNA expression library. Infect. Immun. 68:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Schoolnik, and A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miquel, A., I. Muller, P. Ferrer, P. D. Valenzuela, and L. O. Burzio. 2003. Immunoresponse of Coho salmon immunized with a gene expression library from Piscirickettsia salmonis. Biol. Res. 36:313-323. [DOI] [PubMed] [Google Scholar]
- 61.Moore, R. J., C. Lenghaus, S. A. Sheedy, and T. J. Doran. 2001. Improved vectors for expression library immunization—application to Mycoplasma hyopneumoniae infection in pigs. Vaccine 20:115-120. [DOI] [PubMed] [Google Scholar]
- 62.Mora, M., D. Veggi, L. Santini, M. Pizza, and R. Rappuoli. 2003. Reverse vaccinology. Drug Discov. Today 8:459-464. [DOI] [PubMed] [Google Scholar]
- 63.Morris, S., C. Kelley, A. Howard, Z. M. Li, and F. Collins. 2000. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 64.Nagata, T., M. Uchijima, A. Yoshida, M. Kawashima, and Y. Koide. 1999. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 261:445-451. [DOI] [PubMed] [Google Scholar]
- 65.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. L. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piedrafita, D., D. M. Xu, D. Hunter, R. A. Harrison, and F. Y. Liew. 1999. Protective immune responses induced by vaccination with an expression genomic library of Leishmania major. J. Immunol. 163:1467-1472. [PubMed] [Google Scholar]
- 67.Rainczuk, A., T. Scorza, P. M. Smooker, and T. W. Spithill. 2003. Induction of specific T-cell responses, opsonizing antibodies, and protection against Plasmodium chabaudi adami infection in mice vaccinated with genomic expression libraries expressed in targeted and secretory DNA vectors. Infect. Immun. 71:4506-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson, H. L., C. A. Boyle, D. M. Feltquate, M. J. Morin, J. C. Santoro, and R. G. Webster. 1997. DNA immunization for influenza virus: studies using hemagglutinin- and nucleoprotein-expressing DNAs. J. Infect. Dis. 176(Suppl. 1):S50-S55. [DOI] [PubMed] [Google Scholar]
- 70.Rombel, I. T., K. F. Sykes, S. Rayner, and S. A. Johnston. 2002. ORF-FINDER: a vector for high-throughput gene identification. Gene 282:33-41. [DOI] [PubMed] [Google Scholar]
- 71.Rosenthal, S. R., M. Merchlinsky, C. Kleppinger, and K. L. Goldenthal. 2001. Developing new smallpox vaccines. Emerg. Infect. Dis. 7:920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasaki, S., T. Tsuji, Y. Asakura, J. Fukushima, and K. Okuda. 1998. The search for a potent DNA vaccine against AIDS: the enhancement of immunogenicity by chemical and genetic adjuvants. Anticancer Res. 18:3907-3915. [PubMed] [Google Scholar]
- 73.Singh, R. A., and M. A. Barry. 2004. Repertoire and immunofocusing of CD8 T cell responses generated by HIV-1 gag-pol and expression library immunization vaccines. J. Immunol. 173:4387-4393. [DOI] [PubMed] [Google Scholar]
- 74.Singh, R. A., L. Wu, and M. A. Barry. 2002. Generation of genome-wide CD8 T cell responses in HLA-A*0201 transgenic mice by an HIV-1 ubiquitin expression library immunization vaccine. J. Immunol. 168:379-391. [DOI] [PubMed] [Google Scholar]
- 75.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 76.Smith, C., P. J. R. Day, and M. R. Walker. 1993. Generation of cohesive ends on PCR products by UDG-mediated excision of dU, and application for cloning into restriction digest-linearized vectors. PCR Methods Appl. 2:328-332. [DOI] [PubMed] [Google Scholar]
- 77.Smooker, P. M., Y. Y. Setiady, A. Rainczuk, and T. W. Spithill. 2000. Expression library immunization protects mice against a challenge with virulent rodent malaria. Vaccine 18:2533-2540. [DOI] [PubMed] [Google Scholar]
- 78.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. T. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stemke-Hale, K., B. Kaltenboeck, F. J. DeGraves, K. F. Sykes, J. Huang, C.-H. Bu, and S. A. Johnston. 2005. Screening the whole genome of a pathogen in vivo for individual protective antigens. Vaccine 23:3016-3025. [DOI] [PubMed] [Google Scholar]
- 80.Stephen, J. A., A. M. Talaat, and M. J. McGuire. 2002. Genetic immunization: what's in a name? Arch. Med. Res. 33:325-329. [DOI] [PubMed] [Google Scholar]
- 81.Sturniolo, T., E. Bono, J. Y. Ding, L. Raddrizzani, O. Tuereci, U. Sahin, M. Braxenthaler, F. Gallazzi, M. P. Protti, F. Sinigaglia, and J. Hammer. 1999. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 17:555-561. [DOI] [PubMed] [Google Scholar]
- 82.Suarez, D. L., M. L. Perdue, N. Cox, T. Rowe, C. Bender, J. Huang, and D. E. Swayne. 1998. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 72:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sykes, K. F., and S. A. Johnston. 1999. Genetic live vaccines mimic the antigenicity but not pathogenicity of live viruses. DNA Cell Biol. 18:521-531. [DOI] [PubMed] [Google Scholar]
- 84.Sykes, K. F., and S. A. Johnston. 1999. Linear expression elements: a rapid, in vivo, method to screen for gene functions. Nat. Biotechnol. 17:355-359. [DOI] [PubMed] [Google Scholar]
- 85.Sykes, K. F., M. G. Lewis, B. Squires, and S. A. Johnston. 2002. Evaluation of SIV library vaccines with genetic cytokines in a macaque challenge. Vaccine 20:2382-2395. [DOI] [PubMed] [Google Scholar]
- 86.Tacket, C. O., M. B. Sztein, G. A. Losonsky, S. S. Wasserman, J. P. Nataro, R. Edelman, D. Pickard, G. Dougan, S. N. Chatfield, and M. M. Levine. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 65:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Talaat, A. M., P. Hunter, and S. A. Johnston. 2000. Genome-directed primers for selective labeling of bacterial transcripts for DNA microarray analysis. Nat. Biotechnol. 18:679-682. [DOI] [PubMed] [Google Scholar]
- 88.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Talaat, A. M., R. Lyons, and S. A. Johnston. 2001. A combination vaccine confers full protection against co-infections with influenza, herpes simplex and respiratory syncytial viruses. Vaccine 20:538-544. [DOI] [PubMed] [Google Scholar]
- 90.Ulmer, J. B., R. R. Deck, C. M. DeWitt, T. M. Fu, J. J. Donnelly, M. J. Caulfield, and M. A. Liu. 1997. Expression of a viral protein by muscle cells in vivo induces protective cell-mediated immunity. Vaccine 15:839-841. [DOI] [PubMed] [Google Scholar]
- 91.United States National Intelligence Council. 2000. The global infectious disease threat and its implications for the United States. Environ. Change Secur. Proj. Rep. Summer:33-65. [PubMed] [Google Scholar]
- 92.van den Hurk, S. V., V. Gerdts, B. I. Loehr, R. Pontarollo, R. Rankin, R. Uwiera, and L. A. Babiuk. 2000. Recent advances in the use of DNA vaccines for the treatment of diseases of farmed animals. Adv. Drug Deliv. Rev. 43:13-28. [DOI] [PubMed] [Google Scholar]
- 93.Wang, Q. M., S. H. Sun, Z. L. Hu, M. Yin, C. J. Xiao, and J. C. Zhang. 2004. Improved immunogenicity of a tuberculosis DNA vaccine encoding ESAT6 by DNA priming and protein boosting. Vaccine 22:3622-3627. [DOI] [PubMed] [Google Scholar]
- 94.Wang, R. B., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. P. Charoenvit, T. R. Jones, P. Hobart, et al. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 95.Yero, C. D., F. R. Pajon, M. E. Caballero, A. K. Cobas, H. Y. Lopez, M. M. Farinas, B. S. Gonzales, and D. A. Acosta. 2005. Immunization of mice with Neisseria meningitidis serogroup B genomic expression libraries elicits functional antibodies and reduces the level of bacteremia in an infant rat infection model. Vaccine 23:932-939. [DOI] [PubMed] [Google Scholar]
- 96.Zagursky, R. J., and D. Russell. 2001. Bioinformatics: use in bacterial vaccine discovery. BioTechniques 31:636-640. [DOI] [PubMed] [Google Scholar]