Abstract
Chicken genetics and age affect resistance to enteric infection with Salmonella enterica serovar Typhimurium and were used to identify the immune responses that may contribute to rapid clearance. When birds were infected at 40 days of age, line 61 chickens cleared the infection more effectively than line N chickens, whereas when birds were infected at 10 days of age, both chicken lines were highly susceptible to infection. Antibody levels, T-cell responsiveness, and cytokine mRNA levels were all elevated during infection. A negative correlation between resistance and antigen-specific antibody production was observed in older chickens. However, this finding was not replicated for age-related resistance; we found that older chickens exhibited a stronger and more rapid antibody response than younger chickens. The levels of interleukin-1β (IL-1β) and gamma interferon (IFN-γ) mRNA were similar in the spleens and cecal tonsils of both line 61 and line N chickens, except for higher levels of IL-1β in the spleens of line 61 chickens at 6 days postinfection. Differences in the levels of IFN-γ and IL-1β 1β mRNA between the lines were more apparent in younger chickens, but while the increases were greater than those observed in the older chickens, the clearance of enteric S. enterica serovar Typhimurium was much slower. The level of antigen-specific proliferation of splenocytes was associated with increased resistance in both experimental systems, and the strongest responses were observed in older and genetically resistant chickens. The data presented here implicate T-cell responses in the clearance of S. enterica serovar Typhimurium from the intestine of infected chickens.
Human food poisoning associated with the consumption of Salmonella enterica-infected poultry food products (meat and eggs) remains a major public health problem. The cases are typically caused by S. enterica serovars Typhimurium and Enteritidis, which colonize the alimentary tract of chickens (age, >3 days) with little systemic infection and no obvious external signs of disease. Commercially available vaccines used by the egg-producing sector are either killed or noncharacterized live attenuated vaccines (15, 35, 48). However, the degree of protection afforded by these vaccines is less than that conferred by priming with nonattenuated Salmonella serovars (5).
The majority of immunological studies of chicken Salmonella infections have concentrated on the humoral response, and there have been well-documented increases in anti-Salmonella immunoglobulin G (IgG) and IgA antibodies (reviewed in reference 49). However, the functional importance of antibody in clearance of enteric Salmonella remains uncertain as a result of conflicting evidence from bursectomized (B-cell-deficient) chickens (1, 11, 16, 18). Cell-mediated immune responses to wild-type infections have been investigated less extensively; however, increases in the antigen-specific delayed-type hypersensitivity response and changes in the distribution of T- and B-cell subsets have been reported (9, 25). In more recent studies workers in our laboratory have monitored cellular and humoral immune responses in chickens following both primary and secondary infections with S. enterica serovar Typhimurium (7). Strong cellular and humoral immune responses correlated temporally with clearance of S. enterica serovar Typhimurium from the gut following primary infection, although these responses were less intense following rechallenge. Immediately prior to clearance of primary infection significant increases in the levels of mRNA encoding interleukin-1β (IL-1β), gamma interferon (IFN-γ), and transforming growth factor β (TGF-β) were observed. Increases in ex vivo proliferation of splenocytes following stimulation with flagella and outer membrane proteins have been demonstrated in chickens infected with heat-killed or attenuated vaccine strains (2, 3, 36).
The influence of host genetics in resistance to infectious diseases is well established, and differences in disease susceptibility are frequently associated with the effectiveness of the immune response. Hence, the difference in relative resistance between inbred host strains has been widely used as a tool to identify components of the host immune response which are important in resolution of disease in mice (17, 29, 41, 46) and in chickens (12, 22, 43). Classical genetic mapping studies have also identified a variety of genes associated with resistance in a number of different murine disease models (reviewed in reference 14), including systemic salmonellosis, in which resistance has been linked to Nramp1 (31), TLR4 (10, 38), and TLR5 (42). Genetic loci in the chicken that are associated with resistance to systemic salmonellosis have also been identified (32). The evidence for the role of the major histocompatibility complex (MHC) locus in the ability of young chicks to resist systemic infection differs according to the study (13, 28). Additional candidate loci involved in resistance of chickens to systemic salmonellosis include SAL1, Nramp1, and TLR4 (21, 26, 32). In contrast, genetic resistance of chickens to enteric colonization by S. enterica serovar Typhimurium and S. enterica serovar Enteritidis is not associated with the SAL1 or MHC loci (4). In the present study, we examined the temporal patterns of cellular and humoral immune responses in genetically resistant and susceptible lines of chickens infected at 10 or 40 days of age. These ages were selected based on the age-dependent susceptibility of chickens to enteric S. enterica serovar Typhimurium (7). The combination of these experimental systems allowed identification of genetic or age-related differences in the anti-Salmonella responses of inbred lines that correlate with resistant or susceptible phenotypes.
MATERIALS AND METHODS
Experimental animals.
Specific-pathogen-free inbred line N and line 61 (White Leghorn-derived) chickens were supplied as 1-day-old chicks by the Poultry Production Unit of the Institute for Animal Health, Compton Laboratory. Line N chickens have been characterized as susceptible to intestinal infection and line 61 chickens have been characterized as resistant based on the number and persistence of enteric Salmonella (4). The birds were reared in wire cages at 30°C from the time that they were 1 day old, and the temperature was decreased to 20°C at 3 weeks of age. They were given ad libitum access to water and a vegetable-based protein diet (Special Diet Services, Witham, United Kingdom). The birds were tagged with metal wing bands to allow identification of individuals.
Bacterial strains.
The infection studies were carried out using a spontaneous nalidixic acid-resistant mutant of S. enterica serovar Typhimurium strain F98 (phage type 14) that has been used in previous studies (4, 6, 44). Bacterial cultures were grown from stocks stored in glycerol at −70°C in Luria-Bertani broth (BD Biosciences, Oxford, United Kingdom) at 37°C in an orbital shaking incubator at 150 rpm.
T-cell proliferation assays.
The T-cell proliferation assay was performed as described previously (6). Briefly, assays were performed with 106 splenocytes/well in U-bottom microtiter plates, and preparations were cocultured in RPMI containing 5% fetal calf serum supplemented with either 8.1 μg/ml S. enterica serovar Typhimurium F98 soluble antigen preparation (STAgP), prepared as described previously (6), or 200 μg/ml phytohemagglutinin (PHA) or in RPMI containing fetal calf serum alone by using a final volume of 200 μl/well. Microtiter plates were incubated at 41°C in an atmosphere consisting of 5% CO2 in air for 24 h prior to addition of 1 μCi/well [3H]thymidine (Amersham, Little Chalfont, United Kingdom) and incubation for a further 18 h. Cells were harvested with a Tomtec Mach IIIM cell harvester (Receptor Technologies, Banbury, United Kingdom), and incorporation of [3H]thymidine was determined with a 1450 Microbeta Trilux scintillation counter (Perkin-Elmer, Beaconsfield, United Kingdom).
ELISA.
Levels of serum immunoglobulins (IgM, IgG, and IgA) specific for STAgP were measured by an enzyme-linked immunosorbent assay (ELISA) as described previously (7). As the levels of IgA in the serum have been shown to be correlated with the levels secreted in the gut and bile duct (40), the IgA in the serum was measured. Briefly, flat-bottom 96-well ELISA plates (BD Biosciences, Oxford, United Kingdom) were coated with STAgP diluted to a concentration of 16.2 μg/ml in carbonate/bicarbonate buffer (pH 9.6) overnight at 4°C and then washed with phosphate-buffered saline containing Tween 20 (0.05%). Following blocking with phosphate-buffered saline containing 0.05% Tween 20 supplemented with 3% skim milk powder for 1 h, serum samples were diluted 1:400 in blocking buffer (for detection of IgM and IgG) and 1:12.5 (for detection of IgA), added to the plates, and incubated at 37°C for 1 h. The plates were washed, and bound immunoglobulins were detected by incubation at 37°C for 1 h with horseradish peroxidase conjugated to either goat anti-chicken IgM (1:1,000; Serotec, Oxford, United Kingdom), rabbit anti-chicken IgG (1:2,000; Sigma), or goat anti-chicken IgA (1:20,000; Serotec) diluted in blocking buffer. The plates were washed, and a solution containing 2,2-azino-di(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and a hydrogen peroxide solution (50 μl/well) was added as the chromogen. The plates were incubated at room temperature in the dark for 30 to 60 min, and the reaction was stopped by addition of 1% sodium dodecyl sulfate. Absorbance at 405 nm was determined with a Benchmark microplate reader (Bio-Rad, Hemel Hempstead, United Kingdom).
Quantitative analysis of cytokine mRNA.
Cytokine levels were quantified by real-time reverse transcription (RT)-PCR using the ABI Prism 7700 sequence detection system (TaqMan; PE Applied Biosystems, Warrington, United Kingdom) as described by Kaiser et al. (23). Total RNA was extracted from samples stored in RNA Later at −20°C (QIAGEN, Crawley, United Kingdom) using an RNeasy mini kit by following the manufacturer's instructions (QIAGEN). Primers and probes for IFN-γ, IL-1β, and 28S RNA-specific amplification have been described previously (23). RT-PCR was performed using an RT qPCR Mastermix kit (Oswel Research Products Ltd., Southampton, United Kingdom) as described previously (7). Differences in cytokine mRNA levels between infected and uninfected groups were calculated relative to reference 28S rRNA as described previously (37).
Statistics.
Statistical comparisons of groups were carried out using Student's t test in Microsoft Excel or using a regression analysis (F test) with Genstat. Differences between experimental groups were considered significant if the P value was <0.05.
Experimental protocol. (i) Infections in 40-day-old chickens.
Line N and line 61 chickens were given 0.1 ml of an adult gut flora preparation when they were 1 day old to minimize variation in the composition of the gut flora (4). Both lines of chicken were randomly assigned to four groups (line N, groups 1 and 2; line 61, groups 3 and 4). At 40 days of age groups 2 and 4 were infected orally with 1.8 × 108 CFU of S. enterica serovar Typhimurium F98 Nalr, while groups 1 and 3 were not infected and were used as controls.
(ii) Infections in 10-day-old chickens.
Line N and line 61 chickens were given 0.1 ml of an adult gut flora preparation when they were 1 day old. Both line N and line 61 chickens were randomly assigned to four groups (line N, groups 1 and 2; line 61, groups 3 and 4). At 10 days of age groups 2 and 4 were infected orally with 1.1 × 108 CFU of S. enterica serovar Typhimurium F98 Nalr, and groups 1 and 3 were not infected and were used as controls.
Postmortem analysis.
Five birds from each group were killed at 6, 13, 20, and 27 days postinfection (p.i.) for analysis. Samples of spleen were used for assessment of lymphocyte proliferation, and samples of spleen and cecal tonsils were transferred into RNA Later (QIAGEN, Crawley, United Kingdom) for determination of cytokine mRNA expression. Samples of liver, spleen, and cecal contents were taken and processed for bacteriological analysis as described previously (8), before they were plated onto brilliant green agar supplemented with 20 μg/ml sodium nalidixate and 1 μg/ml novobiocin and incubated at 37°C for 24 h prior to enumeration.
RESULTS
Clearance of Salmonella in chickens infected at 10 and 40 days of age.
In chickens infected at 40 days of age, significantly higher numbers of viable Salmonella cells (P < 0.05) were detected in the cecal contents of line N chickens at 13, 20, and 27 days p.i. than in the cecal contents of line 61 chickens at the same times (Table 1). Clearance of Salmonella from the gut in line 61 chickens was more rapid than clearance from the gut in line N chickens, and no Salmonella was detected in the cecal contents at 13 days p.i.. By comparison, Salmonella was detected in the cecal contents of four of five line N birds at 27 days p.i. The Salmonella counts for the systemic organs (spleen and liver) were low in both lines throughout the study and did not differ significantly at any time following infection, although generally more of the line N birds than of the line 61 birds were positive for Salmonella in the spleen and liver at 13 days p.i. (Table 1). Salmonella was not detected in the cecal contents, livers, or spleens of uninfected birds (data not shown).
TABLE 1.
Viable counts of S. enterica serovar Typhimurium in the cecal contents, spleens, and livers of line N and line 61 chickens infected 40 days after hatching
| Day p.i. | Line N
|
Line 61
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cecal contents
|
Spleen
|
Liver
|
Cecal contents
|
Spleen
|
Liver
|
|||||||
| Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | |
| 6 | 4.38 (0.21) | 5/5a | 2.61 (0.27) | 5/5 | 2.66 (1.44) | 5/5 | 4.00 (0.45) | 5/5 | 3.01 (0.269) | 5/5 | 2.58 (0.25) | 5/5 |
| 13 | 3.03 (0.48)b | 5/5 | 1.4 (0.6) | 3/5 | 0.9 (0.55) | 2/5 | 0.4 (0.4)b | 1/5 | 1.29 (0.79) | 2/5 | 1.2 (0.49) | 3/5 |
| 20 | 2.63 (0.75)b | 4/5 | 0.89 (0.55) | 2/5 | 0.9 (0.55) | 2/5 | 0 (0)b | 0/5 | 0 (0) | 0/5 | 0 (0) | 0/5 |
| 27 | 1.6 (0.4)b | 4/5 | 0 (0) | 0/5 | 0.87 (0.87) | 1/5 | 0 (0)b | 0/5 | 0 (0) | 0/5 | 0.4 (0.4) | 1/5 |
Number positive/total number.
There was a significant difference (P < 0.05) between line N and line 61 chickens at this time postinfection (as determined by Student's t test).
The number of Salmonella organisms in the cecal contents of chickens infected at 10 days of age did not differ significantly for the two lines at any of the times examined (Table 2). For both lines the cecal contents of four of five birds remained Salmonella positive at 27 days p.i., and the counts were approximately 3 logs higher than those in chickens infected at 40 days of age. The numbers of Salmonella organisms in the spleen and liver were also higher in the chickens infected at 10 days of age than in the chickens infected at 40 days of age (Tables 1 and 2), although the rates of systemic clearance were similar regardless of the age.
TABLE 2.
Viable counts of S. enterica serovar Typhimurium in the cecal contents, spleens, and livers of line N and line 61 chickens infected 10 days after hatching
| Day p.i. | Line N
|
Line 61
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cecal contents
|
Spleen
|
Liver
|
Cecal contents
|
Spleen
|
Liver
|
|||||||
| Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | Mean log10 CFU/g (SEM) | No. positive | |
| 6 | 7.57 (0.14) | 5/5a | 3.98 (0.07) | 5/5 | 3.46 (0.19) | 5/5 | 7.43 (0.17) | 5/5 | 4.29 (0.15) | 5/5 | 3.35 (0.19) | 5/5 |
| 13 | 6.75 (0.27) | 5/5 | 2.42 (0.20) | 3/5 | 0 (0) | 0/5 | 7.19 (0.31) | 5/5 | 2.83 (0.14) | 5/5 | 2.06 (0.06) | 1/5 |
| 20 | 5.83 (0.42) | 5/5 | 0 (0) | 0/5 | 0 (0) | 0/5 | 5.36 (0.73) | 5/5 | 0 (0) | 0/0 | 0 (0) | 0/5 |
| 27 | 5.13 (0.33) | 4/5 | 0 (0) | 0/5 | 0 (0) | 0/5 | 4.43 (0.99) | 4/5 | 0 (0) | 0/0 | 0 (0) | 0/5 |
Number positive/total number.
Immune responses in chickens infected at 40 days of age.
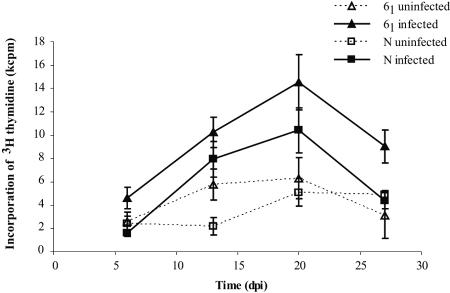
Antigen-specific proliferation of splenocytes in response to STAgP was minimal with uninfected chickens, indicating that the antigen preparation caused little nonspecific proliferation. After infection, STAgP-specific proliferation was detected with splenocytes of both chicken lines, and the levels peaked at 20 days p.i. (Fig. 1). Of note, the proliferative response of line N splenocytes at 27 days p.i. was comparable to the response of uninfected birds despite the continued presence of Salmonella in the guts of four of five chickens and the continued presence of Salmonella in the liver of one of five chickens. Although the magnitude of STAgP-specific splenocyte proliferation in line 61 chickens was reduced at 27 days p.i. compared with the magnitude at 20 days p.i., the proliferative response remained higher than that in uninfected controls at the same time (P < 0.05). When proliferation data for infected line N and 61 splenocytes were compared over the course of infection by regression analysis (F test), a significant difference was observed (P = 0.026). Chickens in all groups responded similarly to stimulation with PHA (data not shown).
FIG. 1.
Antigen-specific proliferation of splenocytes from line N and line 61 chickens infected with S. enterica serovar Typhimurium 40 days after hatching as measured by incorporation of [3H]thymidine. The error bars indicate standard errors (n = 5). Regression analysis using the F test between the infected line N and line 61 chickens resulted in a P value of 0.026. dpi, days postinfection.
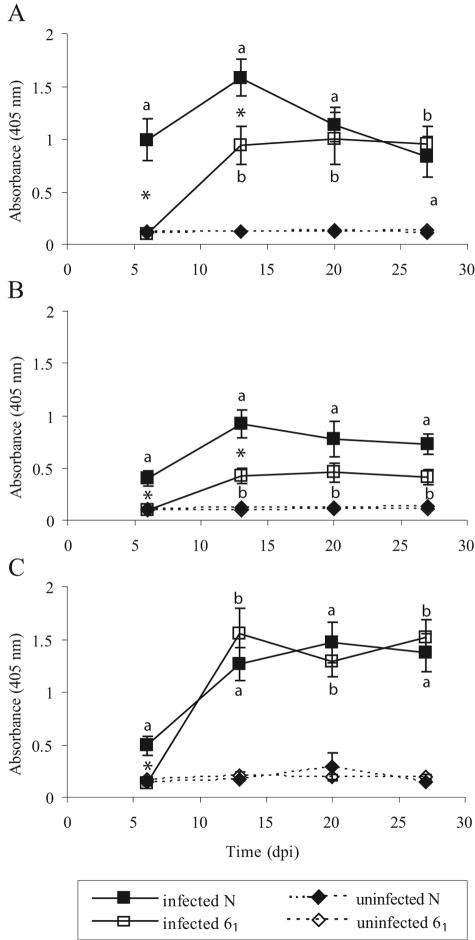
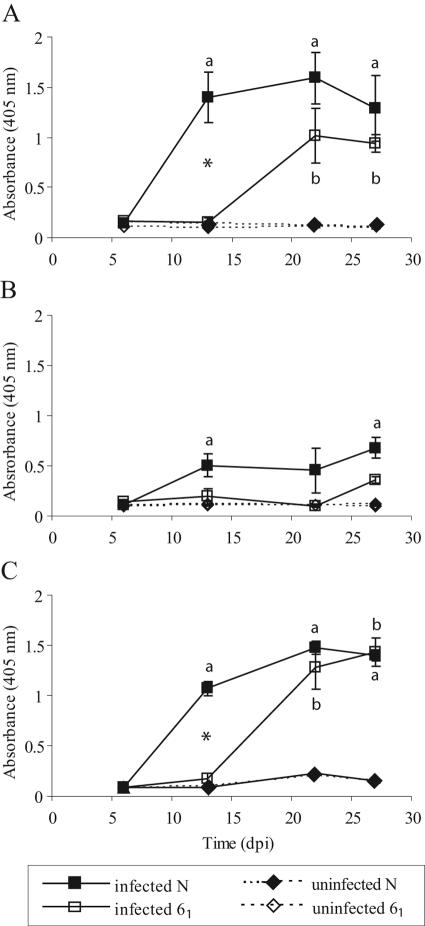
Significantly increased levels of STAgP-specific serum antibodies (IgM, IgG, and IgA) were associated with infection of both chicken lines (Fig. 2). For both IgM and IgG, elevated levels were detected more rapidly following infection (and peaked at higher levels) in line N chickens. The level of antigen-specific serum IgG remained higher in line N chickens than in line 61 chickens throughout the experimental period (27 days p.i.). The levels of STAgP-specific serum IgA were elevated at 13 days p.i. in both chicken lines and remained significantly higher than the levels in uninfected chickens for the duration of the experiment (Fig. 2C).
FIG. 2.
Antigen-specific serum IgM (A), IgG (B), and IgA (C) in line N and 61 chickens infected with S. enterica serovar Typhimurium 40 days after hatching. The error bars indicate standard errors (n = 5). a and b indicate that there were significant differences between infected and uninfected line N and 61 chickens, respectively (as determined by a t test). An asterisk indicates that there was a significant difference between infected line N and line 61 chickens (P < 0.05). dpi, days postinfection.
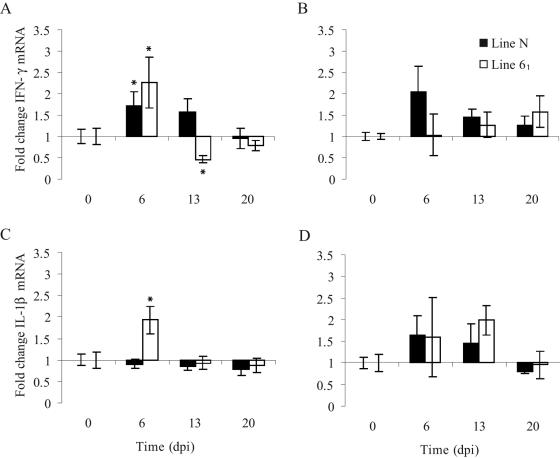
Changes in the expression of IFN-γ and IL-1β mRNA were measured by real-time quantitative RT-PCR by using splenic or cecal tonsil RNA during the course of infection. The levels of splenic IFN-γ mRNA were significantly increased at 6 days p.i. in both chicken lines (Fig. 3A). Following the initial increase, the IFN-γ mRNA levels returned to the preinfection levels at 20 days p.i. in line N chickens and at 13 days p.i. in line 61 chickens. Increased IFN-γ mRNA levels were detected in the cecal tonsils, but the changes compared to uninfected chickens from the same line were not statistically significant (Fig. 3B). The splenic levels of IL-1β mRNA were elevated transiently in line 61 chickens at 6 days p.i., whereas they were not elevated in line N chickens at any time (Fig. 3C). As observed with IFN-γ mRNA, there was great variability in the levels of IL-1β mRNA between individuals; no statistically significant differences between groups were detected. However, for both lines of chickens some individuals expressed elevated levels of IL-1β mRNA (spleen and cecal tonsils) at 6 and 13 days p.i. (Fig. 3C and D).
FIG. 3.
Changes in expression of IFN-γ (A and B) and Il-1β (C and D) mRNA as determined by quantitative RT-PCR in the spleens (A and C) and cecal tonsils (B and D) of chickens infected with S. enterica serovar Typhimurium 40 days after hatching. The data are expressed as fold increases compared to uninfected control chickens. The error bars indicate standard errors (n = 5). An asterisk indicates that there was a significant difference in mRNA expression between infected chickens and uninfected controls (as determined by a t test). dpi, days postinfection.
Immune responses in chickens infected at 10 days of age.
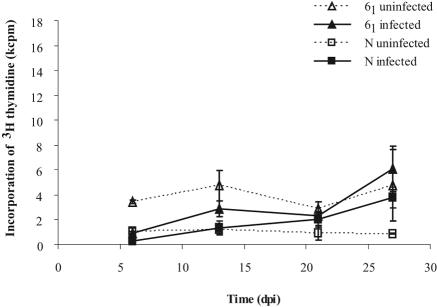
The proliferation of splenocytes in response to STAgP was low in both chicken lines following infection at 10 days of age; no significant differences between the two lines were detected (Fig. 4). Similarly, the level of responsiveness to the T-cell mitogen PHA was low in young birds (10 to 27 days old). In comparison, the response in older chickens (>40 days) was much greater (data not shown). The response of splenocytes to PHA was not affected by infection with Salmonella.
FIG. 4.
Antigen-specific proliferation of splenocytes from line N and line 61 chickens infected with S. enterica serovar Typhimurium 10 days after hatching as measured by incorporation of [3H]thymidine. The error bars indicate standard errors (n = 5). No statistical differences were observed between any groups, as determined by regression analysis. dpi, days postinfection.
Increased levels of STAgP-specific IgM were detected from 13 days p.i. in line N chickens and from 22 days p.i. in line 61 chickens, although there were no significant differences in the magnitudes of the responses after 22 days p.i. (Fig. 5a). Similarly, when STAgP-specific IgG (Fig. 5B) and IgA (Fig. 5C) were studied, line N chickens responded more rapidly than line 61 chickens.
FIG. 5.
Antigen-specific serum IgM (A), IgG (B), and IgA (C) in line N and 61 chickens infected with S. enterica serovar Typhimurium 10 days after hatching. The error bars indicate standard errors (n = 5). a and b indicate that there were significant differences between infected and uninfected line N and 61 chickens, respectively (as determined by a t test). An asterisk indicates that there was a significant difference between infected line N chickens and infected line 61 chickens (P < 0.05). dpi, days postinfection.
Increased levels of antibodies specific for STAgP were observed at later times following infection in birds infected at 10 days of age (Fig. 5) than in birds infected at 40 days of age (Fig. 2). Compared to chickens infected at 40 days of age, chickens infected at 10 days of age produced relatively low levels of IgG at all of the times tested, although the levels continued to rise until termination of the experiment (27 days p.i.), at which point S. enterica serovar Typhimurium was still present in the cecal contents of four of five birds sampled in both infected groups. Induction of STAgP-specific IgA in line 61 chickens following infection was slower in chickens infected at 10 days of age than in chickens infected at 40 days of age (22 and 13 days p.i., respectively). In contrast induction of antigen-specific IgA production in line N chickens occurred at 13 days p.i. regardless of the age at infection.
Significant increases in the amount of IFN-γ mRNA were detected in the spleens of line N chickens at 6 days p.i., and the levels remained significantly higher than those in uninfected controls up to 21 days p.i. (Fig. 6A). Similarly, the levels of splenic IFN-γ mRNA were also elevated in line 61 chickens, although to a lesser degree than that observed in line N chickens at 6 days p.i. In contrast, the magnitude of the increase in the IFN-γ mRNA level detected in the cecal tonsils was greater in line 61 chickens than in line N chickens following infection at 6 days p.i. (Fig. 6B). The increased level of IFN-γ mRNA was transient in both chicken lines.
FIG. 6.
Changes in expression of IFN-γ (A and B) and Il-1β (C and D) mRNA as determined by quantitative RT-PCR in the spleens (A and C) and cecal tonsils (C and D) of chickens infected with S. enterica serovar Typhimurium 10 days after hatching. The data are expressed as fold increases compared to uninfected control chickens. The error bars indicate standard errors (n = 5). An asterisk indicates that there was a significant difference in mRNA expression between infected chickens and uninfected controls (as determined by a t test). dpi, days postinfection.
The levels of IL-1β mRNA increased in the spleens and cecal tonsils in both chicken lines following infection (Fig. 6C and D). In the spleen, increases in IL-1β mRNA levels were observed in lines N and 61 at 6 days p.i. A large increase in the IL-1β mRNA level was detected at 6 days p.i. in the cecal tonsils of line 61 chickens, and there was a more modest increase in line N chickens, following which the levels dropped back to those observed in uninfected controls.
DISCUSSION
In this study we focused on B-cell (antibody), T-cell, and cytokine (IL-1β and IFN-γ) responses induced by enteric infection in resistant and susceptible chicken lines at different ages.
Previous studies of genetic resistance either have examined immune correlates with disease outcome or have employed genetic mapping approaches. Resistance to systemic salmonellosis in chickens is associated with the MHC (28, 50), Nramp1 (21), and TLR4 loci (26). Using a candidate gene approach, Kramer et al. (24) found that resistance to enteric S. enterica serovar Enteritidis was associated with polymorphisms in 9 of 12 candidate genes examined. These genes included genes encoding inducible nitric oxide synthase, IFN-γ, IL-2, NRAMP-1, caspase 1, TGF-β2, and TGF-β4 and genes involved in antibody kinetics (24). In the present study we examined the association of immune responses with resistance to enteric S. enterica serovar Typhimurium infection according to host genetics (two different chicken lines) and the age at the time of infection (10 and 40 days). To date, the study of immune correlates with enteric salmonellosis in the chicken has been limited to ex vivo studies of heterophils, which showed an increased capacity to respond to S. enterica serovar Enteritidis when they were derived from resistant chickens (45). A wide range of immune responses are induced by exposure of chickens to the human food-borne serovars of Salmonella (S. enterica serovars Typhimurium and Enteritidis), including antibody, T-cell, and cytokine responses measured in this study and reported previously (6, 7, 9). The combination of genetic and age-related resistance as test phenotypes provided a much more stringent test of association with disease outcome than the use of a single phenotype.
Since S. enterica serovar Typhimurium resides primarily in the lumen of the gut of 10- and 40-day-old chickens, an obvious potential clearance mechanism is the activity of specific secreted IgA. There was no association between levels of specific antibody and outcome of infection, although there were genetic differences in the levels of all antibody classes.
The capacity of T cells to respond to challenge is dependent upon age, as determined by mitogen- and antigen-driven proliferative assays (30; this study), which are associated strongly with the age-related changes in susceptibility to enteric salmonellosis. The mechanisms that underpin age-related T-cell responsiveness in young birds are unknown, but they may involve structural and compositional changes in the enteric lymphoid system (20, 27, 47). In murine systems dendritic cell immaturity has been proposed to be a major mechanism of neonatal hyporesponsiveness (39); unfortunately, dendritic cell biology in the chicken is poorly understood, and at present it is not possible to examine the role of these cells.
Substantial antigen-specific class-switched antibody was detected (IgG and IgA) in the young chickens (albeit it was slower than that with older birds), which is indicative of gut-associated TCRαβ+ CD4+ T-cell involvement (although classical antigen-specific proliferation was not detected in the spleen). Genetic differences in susceptibility to infection became apparent in older birds, and this was associated with more robust antigen-induced proliferation of splenocytes in resistant birds. Interestingly, single nucleotide polymorphism analysis identified the autocrine growth factor IL-2 as a candidate locus associated with resistance to S. enterica serovar Enteritidis in 3-week-old chickens (along with 8 of 12 other chickens tested) (24).
In murine salmonellosis, control of bacterial numbers at systemic sites can be attributed to the coordinated activity of innate and T-cell populations (33), with B cells playing a role during secondary challenge (34). IFN-γ is well established as a central mediator of immunity to S. enterica serovar Typhimurium in mice and is induced by challenge of chickens with S. enterica serovar Typhimurium despite the largely enteric nature of the infection (6). However, the level of IFN-γ mRNA and the timing of induction were not associated with the outcome of infection in young chickens (10 days) or older chickens (40 days). Indeed, the highest levels of IFN-γ mRNA were observed in the spleens of young line N chickens and in the cecal tonsils of young line 61 chickens, which did not display differential susceptibility to infection with S. enterica serovar Typhimurium. Similarly, the levels of IL-1β mRNA were not associated with susceptibility or resistance to infection of either young or older line N or line 61 chickens. The highest levels of splenic IL-1β mRNA were present in young chickens, which probably reflected the larger numbers of bacteria in the guts of these chickens than in the guts of older birds having either genetic background. Nonetheless, our data for IL-1β mRNA do not preclude a role in resistance since substantial posttranslational modification is required for activity (19) and polymorphisms in caspase-1 (also known as IL-1 converting enzyme) are associated with resistance to enteric S. enterica serovar Enteritidis (24).
Stringent assessment of immune responses that are associated with resistance to infection, using host genetics and age, revealed that only T-cell responsiveness was associated with disease outcome. Some immunological changes, such as increased antibody production, were associated with host genetics but not with the resistance phenotype. Similarly, other features of the immune response, such as increased IFN-γ and IL-1β production in the cecal tonsils, differed according to age, but they were not associated with genetic susceptibility in older birds. These studies support the hypothesis that S. enterica serovar Typhimurium clearance from the chicken gut is T cell dependent and indicate the usefulness of multiple-phenotype strategies for identifying features that are strongly associated with resistance.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (grant 8/BFP11365).
We thank Lisa Rothwell for technical advice and the staff of the production and experimental units at the Institute for Animal Health.
Editor: F. C. Fang
REFERENCES
- 1.Arnold, J. W., and P. S. Holt. 1995. Response to Salmonella Enteritidis infection by the immunocompromised avian host. Poult. Sci. 74:656-665. [DOI] [PubMed] [Google Scholar]
- 2.Babu, U., R. A. Dalloul, M. Okamura, H. S. Lillehoj, H. Xie, R. B. Raybourne, D. Gaines, and R. A. Heckert. 2004. Salmonella enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet. Immunol. Immunopathol. 101:251-257. [DOI] [PubMed] [Google Scholar]
- 3.Babu, U., M. Scott, M. J. Myers, M. Okamura, D. Gaines, H. F. Yancy, H. Lillehoj, R. A. Heckert, and R. B. Raybourne. 2003. Effects of live attenuated and killed Salmonella vaccine on T-lymphocyte mediated immunity in laying hens. Vet. Immunol. Immunopathol. 91:39-44. [DOI] [PubMed] [Google Scholar]
- 4.Barrow, P. A., N. Bumstead, K. Marston, M. A. Lovell, and P. Wigley. 2004. Faecal shedding and intestinal colonization of Salmonella enterica in in-bred chickens: the effect of host-genetic background. Epidemiol. Infect. 132: 117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow, P. A., J. O. Hassan, and A. Berchieri, Jr. 1990. Reduction in faecal excretion of Salmonella Typhimurium strain F98 in chickens vaccinated with live and killed S. Typhimurium organisms. Epidemiol. Infect 104:413-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beal, R. K., C. Powers, P. Wigley, P. A. Barrow, and A. L. Smith. 2004. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 33:25-33. [DOI] [PubMed] [Google Scholar]
- 7.Beal, R. K., P. Wigley, C. Powers, S. D. Hulme, P. A. Barrow, and A. L. Smith. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 100:151-164. [DOI] [PubMed] [Google Scholar]
- 8.Berchieri, A., Jr., P. Wigley, K. Page, C. K. Murphy, and P. A. Barrow. 2001. Further studies on vertical transmission and persistence of Salmonella enterica serovar Enteritidis phage type 4 in chickens. Avian Pathol. 30:297-310. [DOI] [PubMed] [Google Scholar]
- 9.Berndt, A., and U. Methner. 2001. Gamma/delta T cell response of chickens after oral administration of attenuated and non-attenuated Salmonella Typhimurium strains. Vet. Immunol. Immunopathol. 78:143-161. [DOI] [PubMed] [Google Scholar]
- 10.Bihl, F., L. Salez, M. Beaubier, D. Torres, L. Lariviere, L. Laroche, A. Benedetto, D. Martel, J. M. Lapointe, B. Ryffel, and D. Malo. 2003. Overexpression of Toll-like receptor 4 amplifies the host response to lipopolysaccharide and provides a survival advantage in transgenic mice. J. Immunol. 170:6141-6150. [DOI] [PubMed] [Google Scholar]
- 11.Brownwell, J. R., W. W. Sadler, and M. J. Fanelli. 1970. Role of Bursa of Fabricius in chicken resistance to Salmonella Typhimurium. Avian Dis. 14:142-152. [PubMed] [Google Scholar]
- 12.Bumstead, J. M., N. Bumstead, L. Rothwell, and F. M. Tomley. 1995. Comparison of immune responses in inbred lines of chickens to Eimeria maxima and Eimeria tenella. Parasitology 111:143-151. [DOI] [PubMed] [Google Scholar]
- 13.Bumstead, N., and P. A. Barrow. 1988. Genetics of resistance to Salmonella Typhimurium in newly hatched chicks. Br. Poult. Sci. 29:521-529. [DOI] [PubMed] [Google Scholar]
- 14.Casanova, J. L., E. Schurr, L. Abel, and E. Skamene. 2002. Forward genetics of infectious diseases: immunological impact. Trends Immunol. 23:469-472. [DOI] [PubMed] [Google Scholar]
- 15.Clifton-Hadley, F. A., M. Breslin, L. M. Venables, K. A. Sprigings, S. W. Cooles, S. Houghton, and M. J. Woodward. 2002. A laboratory study of an inactivated bivalent iron restricted Salmonella enterica serovars Enteritidis and Typhimurium dual vaccine against Typhimurium challenge in chickens. Vet. Microbiol. 89:167-179. [DOI] [PubMed] [Google Scholar]
- 16.Corrier, D. E., M. H. Elissalde, R. L. Ziprin, and J. R. DeLoach. 1991. Effect of immunosuppression with cyclophosphamide, cyclosporin, or dexamethasone on Salmonella colonization of broiler chicks. Avian Dis. 35:40-45. [PubMed] [Google Scholar]
- 17.de Gee, A. L., G. Sonnenfeld, and J. M. Mansfield. 1985. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J. Immunol. 134:2723-2726. [PubMed] [Google Scholar]
- 18.Desmidt, M., R. Ducatelle, J. Mast, B. M. Goddeeris, B. Kaspers, and F. Haesebrouck. 1998. Role of the humoral immune system in Salmonella Enteritidis phage type four infection in chickens. Vet. Immunol. Immunopathol. 63:355-367. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello, C. A. 1997. Interleukin-1. Cytokine Growth Factor Rev. 8: 253-265. [DOI] [PubMed] [Google Scholar]
- 20.Gomez Del Moral, M., J. Fonfria, A. Varas, E. Jimenez, J. Moreno, and A. G. Zapata. 1998. Appearance and development of lymphoid cells in the chicken (Gallus gallus) caecal tonsil. Anat. Rec. 250:182-189. [DOI] [PubMed] [Google Scholar]
- 21.Hu, J., N. Bumstead, P. Barrow, G. Sebastiani, L. Olien, K. Morgan, and D. Malo. 1997. Resistance to salmonellosis in the chicken is linked to NRAMP1 and TNC. Genome Res. 7:693-704. [DOI] [PubMed] [Google Scholar]
- 22.Jarosinski, K. W., R. Yunis, P. H. O'Connell, C. J. Markowski-Grimsrud, and K. A. Schat. 2002. Influence of genetic resistance of the chicken and virulence of Marek's disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis. 46:636-649. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser, P., L. Rothwell, E. E. Galyov, P. A. Barrow, J. Burnside, and P. Wigley. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella Typhimurium, Salmonella Enteritidis and Salmonella Gallinarum. Microbiology 146:3217-3226. [DOI] [PubMed] [Google Scholar]
- 24.Kramer, J., M. Malek, and S. J. Lamont. 2003. Association of twelve candidate gene polymorphisms and response to challenge with Salmonella Enteritidis in poultry. Anim. Genet. 34:339-348. [DOI] [PubMed] [Google Scholar]
- 25.Lee, G. M., G. D. Jackson, and G. N. Cooper. 1983. Infection and immune responses in chickens exposed to Salmonella Typhimurium. Avian Dis. 27:577-583. [PubMed] [Google Scholar]
- 26.Leveque, G., V. Forgetta, S. Morroll, A. L. Smith, N. Bumstead, P. Barrow, J. C. Loredo-Osti, K. Morgan, and D. Malo. 2003. Allelic variation in TLR4 is linked to susceptibility to Salmonella enterica serovar Typhimurium infection in chickens. Infect. Immun. 71:1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lillehoj, H. S., and K. S. Chung. 1992. Postnatal development of T-lymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet. Immunol. Immunopathol. 31:347-360. [DOI] [PubMed] [Google Scholar]
- 28.Liu, W., M. M. Miller, and S. J. Lamont. 2002. Association of MHC class I and class II gene polymorphisms with vaccine or challenge response to Salmonella Enteritidis in young chicks. Immunogenetics 54:582-590. [DOI] [PubMed] [Google Scholar]
- 29.Locksley, R. M., F. P. Heinzel, M. D. Sadick, B. J. Holaday, and K. D. Gardner, Jr. 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744-749. [DOI] [PubMed] [Google Scholar]
- 30.Lowenthal, J. W., T. E. Connick, P. G. McWaters, and J. J. York. 1994. Development of T cell immune responsiveness in the chicken. Immunol. Cell Biol. 72:115-122. [DOI] [PubMed] [Google Scholar]
- 31.Malo, D., K. Vogan, S. Vidal, J. Hu, M. Cellier, E. Schurr, A. Fuks, N. Bumstead, K. Morgan, and P. Gros. 1994. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics 23:51-61. [DOI] [PubMed] [Google Scholar]
- 32.Mariani, P., P. A. Barrow, H. H. Cheng, M. M. Groenen, R. Negrini, and N. Bumstead. 2001. Localization to chicken chromosome 5 of a novel locus determining salmonellosis resistance. Immunogenetics 53:786-791. [DOI] [PubMed] [Google Scholar]
- 33.Mastroeni, P., J. A. Chabalgoity, S. J. Dunstan, D. J. Maskell, and G. Dougan. 2001. Salmonella: immune responses and vaccines. Vet. J. 161: 132-164. [DOI] [PubMed] [Google Scholar]
- 34.Mastroeni, P., C. Simmons, R. Fowler, C. E. Hormaeche, and G. Dougan. 2000. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 68:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Methner, U., A. Berndt, and G. Steinbach. 2001. Combination of competitive exclusion and immunization with an attenuated live Salmonella vaccine strain in chickens. Avian Dis. 45:631-638. [PubMed] [Google Scholar]
- 36.Okamura, M., H. S. Lillehoj, R. B. Raybourne, U. S. Babu, and R. A. Heckert. 2004. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-gamma production. Comp. Immunol. Microbiol. Infect. Dis. 27:255-272. [DOI] [PubMed] [Google Scholar]
- 37.Philbin, V. J., M. Iqbal, Y. Boyd, M. J. Goodchild, R. K. Beal, N. Bumstead, J. Young, and A. L. Smith. 2005. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 114:507-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 39.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 40.Rose, M. E., E. Orlans, A. W. Payne, and P. Hesketh. 1981. The origin of IgA in chicken bile: its rapid active transport from blood. Eur. J. Immunol. 11:561-564. [DOI] [PubMed] [Google Scholar]
- 41.Rose, M. E., D. Wakelin, and P. Hesketh. 1990. Eimeria vermiformis: differences in the course of primary infection can be correlated with lymphocyte responsiveness in the BALB/c and C57BL/6 mouse, Mus musculus. Exp. Parasitol. 71:276-283. [DOI] [PubMed] [Google Scholar]
- 42.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine Toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 43.Sharma, J. M. 1981. Natural killer cell activity in chickens exposed to Marek's disease virus: inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 25:882-893. [PubMed] [Google Scholar]
- 44.Smith, H. W., and J. F. Tucker. 1975. The effect of antibiotic therapy on the faecal excretion of Salmonella Typhimurium by experimentally infected chickens. J Hyg. 75:275-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swaggerty, C. L., M. H. Kogut, P. J. Ferro, L. Rothwell, I. Y. Pevzner, and P. Kaiser. 2004. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 113: 139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagliabue, A., L. Nencioni, L. Villa, and D. Boraschi. 1984. Genetic control of in vitro natural cell-mediated activity against Salmonella Typhimurium by intestinal and splenic lymphoid cells in mice. Clin. Exp. Immunol. 56: 531-536. [PMC free article] [PubMed] [Google Scholar]
- 47.Vervelde, L., and S. H. Jeurissen. 1993. Postnatal development of intra-epithelial leukocytes in the chicken digestive tract: phenotypical characterization in situ. Cell Tissue Res. 274:295-301. [DOI] [PubMed] [Google Scholar]
- 48.Woodward, M. J., G. Gettinby, M. F. Breslin, J. D. Corkish, and S. Houghton. 2002. The efficacy of Salenvac, a Salmonella enterica subsp. Enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens. Avian Pathol. 31:383-392. [DOI] [PubMed] [Google Scholar]
- 49.Zhang-Barber, L., A. K. Turner, and P. A. Barrow. 1999. Vaccination for control of Salmonella in poultry. Vaccine 17:2538-2545. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, H., and S. J. Lamont. 2003. Chicken MHC class I and II gene effects on antibody response kinetics in adult chickens. Immunogenetics 55: 133-140. [DOI] [PubMed] [Google Scholar]