Abstract
Toll-like receptors (TLR) are crucial for an efficient antifungal defense. We investigated the differential recognition of blastoconidia and hyphae of Candida albicans by TLRs. In contrast to Candida blastoconidia, which stimulated large amounts of gamma interferon (IFN-γ), the tissue-invasive Candida hyphae did not stimulate any IFN-γ by human peripheral blood mononuclear cells (PBMC) or murine splenic lymphocytes. After stimulation with blastoconidia, the production of IFN-γ was TLR4 dependent, as shown by the significantly decreased IFN-γ production in anti-TLR4-treated PBMC and in splenic lymphocytes from TLR4-defective ScCr mice. In addition, peritoneal macrophages from ScCr mice produced less tumor necrosis factor α (TNF-α) than macrophages of control mice did when stimulated with Candida blastoconidia, but not with hyphae, indicating that TLR4-mediated signals are lost during hyphal germination. In contrast, macrophages from TLR2 knockout mice had a decreased production of TNF-α in response to both Candida blastoconidia and hyphae. Candida hyphae stimulated production of interleukin-10 through TLR2-dependent mechanisms. In conclusion, TLR4 mediates proinflammatory cytokine induction after Candida stimulation, whereas Candida recognition by TLR2 leads mainly to anti-inflammatory cytokine release. TLR4-mediated proinflammatory signals are lost during germination of Candida blastoconidia into hyphae. Phenotypic switching during germination may be an important escape mechanism of C. albicans, resulting in counteracting host defense.
Candida albicans is a major fungal pathogen which can cause invasive infection, especially in immunocompromised hosts (11). During infection, the phenotypic switch between blastoconidia and hyphae is a virulence trait of C. albicans. Mutants that are locked in the yeast form are less virulent in experimental models of disseminated candidiasis (10, 25). Different mechanisms, such as inhibition of phagocytosis and killing or a differential induction of pro- versus anti-inflammatory cytokines, have been suggested to play a role in the increased invasiveness of hyphae. The molecular mechanism of the differential cytokine stimulation by blastoconidia and hyphae is not known.
Previous research has shown that monocytes fail to phagocytose hyphae and produce low levels of interleukin-12 (IL-12) following hyphal stimulation (6, 14); the ingestion of hyphae by dendritic cells inhibits IL-12 production and induces IL-4 production, resulting in a T helper 2 (Th2) cytokine bias (7, 20). Different signaling pathways may be induced by fungal blastoconidia and hyphae, as was recently demonstrated for Aspergillus fumigatus (19). A possible mechanism could be differential recognition by Toll-like receptors (TLR), which have been identified as the most important pattern recognition receptors for microbial ligands by host cells (1, 3). Both TLR2 and TLR4 are important for the stimulation of monocytes by A. fumigatus (19), but whereas conidia are recognized by both TLR2 and TLR4, leading to proinflammatory cytokine production, germination into hyphae leads to a loss of TLR4 recognition and an anti-inflammatory cytokine bias. We and others have demonstrated that TLR2 and TLR4 are involved in host defense against disseminated Candida infections (16, 17).
In the present study, we hypothesized that blastoconidia and hyphae of C. albicans differ in this stimulation of host cells. Using blastoconidial and hyphal forms, we investigated the kinetics of cytokines and the role of TLR2 and TLR4 in regulating host cell responses.
MATERIALS AND METHODS
C. albicans.
Heat-killed C. albicans blastoconidia (strain ATCC MYA-3573, UC 820) in a concentration of 107 CFU/ml were used throughout this study. To generate hyphae, live yeast forms of Candida were grown for 24 h at 37°C in RPMI 1640 (Gibco-BRL, Grand Island, NY), adjusted to pH 6.4 by using hydrochloric acid. After 24 h, more than 95% of blastoconidia were grown to hyphae, which were checked by microscope. Hyphae were heat killed for 45 min at 98°C and resuspended in RPMI 1640 to a hyphal inoculum size that originated from 107/ml blastoconidia (referred to as 107/ml hyphae).
PBMC preparation.
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of six healthy volunteers by density gradient centrifugation over Ficoll-Hypaque (Amersham Biosciences, Sweden), washed twice in sterile phosphate-buffered saline, and resuspended in RPMI 1640 supplemented with 10 mM l-glutamine, gentamicin at 10 μg/ml, and 10 mM pyruvate. The cells were counted in a hemacytometer, and the number of cells was adjusted to 5 × 106/ml. C. albicans blastoconidia or hyphae (107 CFU/ml) were added to 5 × 105 mononuclear cells in 100 μl in a 96-well microtiter plate (Greiner, Alphen a/d Rijn, The Netherlands) and were incubated for 24 and 48 h at 37°C in 5% CO2 atmosphere. All reagents used were endotoxin free.
In a separate set of experiments, PBMC were preincubated for 2 h at 37°C with anti-TLR4 antibodies (clone HTA125; HyCult, Uden, The Netherlands) at a final concentration of 7.5 ng/ml, diluted 1:4 in the microtiter plate. Mouse immunoglobulin G 2a isotype antibody was used as a control (Immunosource, Halle, Belgium). After incubation for 24 h and 48 h at 37°C, supernatants were collected and stored at −80°C until analysis.
PBMC were preincubated for 10 min with a Candida cell wall component, glucan phosphate, at 50 μg/ml (kindly provided by G. D. Brown, Oxford, United Kingdom) on ice, after which C. albicans blastoconidia (107 CFU/ml) were added for 30 min at 37°C. After three washes, the cells were incubated in RPMI for 24 h at 37°C in 5% CO2 atmosphere.
Animals.
TLR4-deficient C57BL/10ScCr mice were from our local colony, and control TLR4-competent C57BL/10J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). TLR2−/− mice on a C57BL/6J background were kindly provided by S. Akira (Osaka University, Japan). C57BL/6J control mice were obtained from the Jackson Laboratory. The mice were fed sterilized laboratory chow (Hope Farms, Woerden, The Netherlands) and water ad libitum. The experiments were approved by the ethics committee on animal experiments of Nijmegen University.
In vitro cytokine production by murine macrophages.
The mice were killed, and resident peritoneal macrophages were harvested by injecting 4 ml of sterile phosphate-buffered saline. After centrifugation and washing, the cells were resuspended in RPMI 1640 medium containing 1 mM pyruvate, 2 mM l-glutamine, and 100 μg/ml gentamicin. Peritoneal cell exudates consisted of 90% macrophages and 9% lymphocytes (data not shown). Cells were cultured in 96-well microtiter plates (Greiner) at 105 cells per well, in a final volume of 200 μl. The cells were stimulated with control medium, 2 × 107 CFU/ml heat-killed C. albicans blastoconidia, or 107 CFU/ml hyphae. As positive controls, we used Escherichia coli lipopolysaccharide (LPS) (O55:B5, 0.1 μg/ml; Sigma, St Louis, MO) as the TLR4 agonist and lipoteichoic acid (LTA) (0.1 μg/ml; kindly provided by T. Hartung, Konstanz University, Germany) as the TLR2 agonist (21). After incubation for 24 h at 37°C, plates were centrifuged and the supernatant was collected and stored at −80°C until cytokine assays were performed.
Stimulation of splenic lymphocytes.
Spleen cells were obtained as described previously (18). Splenocytes were washed and resuspended in RPMI-Dutch modification and counted in a hemacytometer, and the number was adjusted to 5 × 106/ml. Cells were stimulated with 2 × 107 CFU/ml heat-killed C. albicans blastoconidia or hyphae. Gamma interferon (IFN-γ) was measured in supernatants collected after 48 h of incubation at 37°C in 5% CO2 in 24-well plates (Greiner).
Cytokine assays.
Human IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-10 were measured by enzyme-linked immunosorbent assay (Pelikine; CLB, The Netherlands), according to the guidelines of the manufacturer. Murine TNF-α was determined by specific radioimmunoassay (detection limit, 20 pg/ml), as previously described (15). Murine IFN-γ and IL-10 were measured by a commercial enzyme-linked immunosorbent assay (detection limit, 16 pg/ml; BioSource International, Camarillo, CA), according to the instructions of the manufacturer.
Statistical analysis.
The differences between groups were analyzed by the Student t test, and the results were considered statistically significant at a P value of <0.05.
RESULTS
Kinetics of cytokine production.
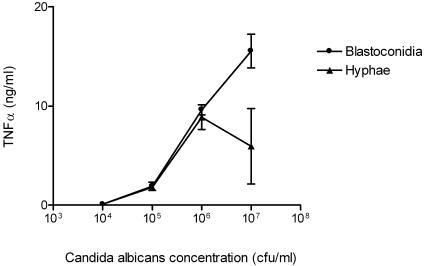
PBMC from six healthy volunteers were incubated with various concentrations of heat-killed C. albicans blastoconidia and hyphae. Candida albicans blastoconidia stimulated TNF-α release in a concentration-dependent manner (Fig. 1). On the basis of these results, we selected a concentration of 107 CFU/ml for subsequent experiments.
FIG. 1.
Production of TNF-α by PBMC from four healthy volunteers after stimulation with various concentrations of heat-killed C. albicans blastoconidia and hyphae. Data are represented as means ± standard errors of the means from two separate experiments.
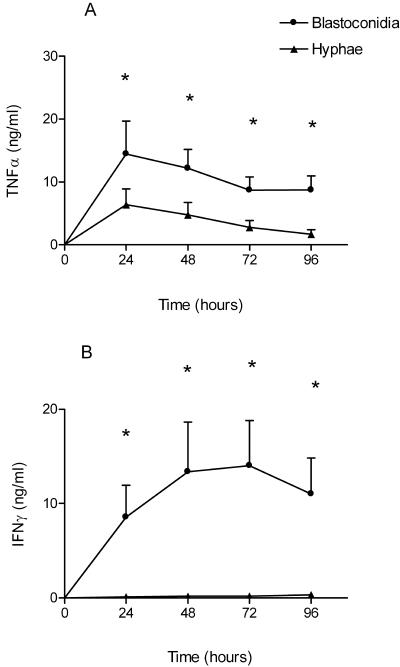
The time course of cytokine production of PBMC after stimulation with C. albicans blastoconidia and hyphae is shown in Fig. 2. Blastoconidia induced a much higher TNF-α production than hyphae did (Fig. 2A) (P < 0.05), and IFN-γ induction by hyphae was virtually absent (Fig. 2B) (P < 0.05). IL-10 production (as measured at 48 h in two separate experiments) was lower in hypha-stimulated PBMC than in blastoconidium-stimulated PBMC (274 ± 168 versus 63 ± 28 pg/ml, respectively; not significant).
FIG. 2.
Production of TNF-α (A) and IFN-γ (B) by PBMC after stimulation with heat-killed C. albicans blastoconidia (107 CFU/ml) and hyphae (107 CFU/ml). Data are represented as means ± standard errors of the means from two separate experiments with cells of six volunteers. *, P < 0.05, t test.
Role of TLR4 in blastoconidium- or hypha-induced cytokine production by PBMC.
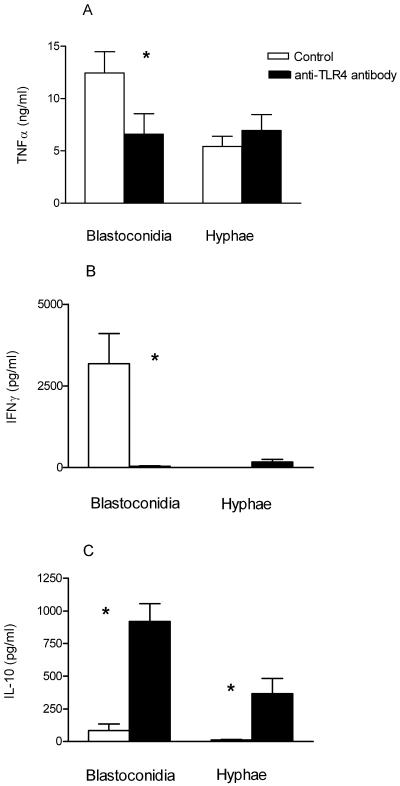
Preincubation of PBMC with anti-TLR4 antibodies almost completely blocked the LPS-induced TNF-α production (data not shown). Anti-TLR4 antibodies significantly reduced the TNF-α production by blastoconidium-stimulated cells (Fig. 3A) (P < 0.05), while a 98% reduction in IFN-γ production was found after blocking the TLR4 receptor by a neutralizing antibody (Fig. 3B) (P < 0.05). No inhibition of TNF-α production was found when using an isotype control antibody (data not shown).
FIG. 3.
Induction of TNF-α (A), IFN-γ (B), and IL-10 (C) production by PBMC from five healthy volunteers after preincubation with anti-TLR4 antibody or isotype control antibody and stimulation with heat-killed C. albicans blastoconidia (107 CFU/ml) or hyphae (107 CFU/ml). TNF-α was measured after 24 h, and IFN-γ and IL-10 were measured after 48 h. Data are represented as means ± standard errors of the means from two separate experiments. *, P < 0.05, t test.
In contrast, the IL-10 production was significantly increased by the blocking of TLR4 in blastoconidia-stimulated PBMC (Fig. 3C) (P < 0.05). After hyphal stimulation, however, we found IFN-γ production by PBMC to be almost absent, while the production of both IFN-γ and especially IL-10 significantly increased after the blocking of the TLR4 receptor (Fig. 3B and C) (P < 0.05).
The role of the TLR2/dectin-1 receptor complex in Candida-induced cytokine production.
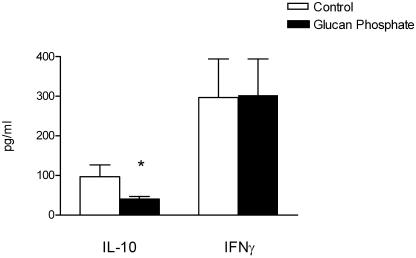
Candida-derived β-glucan has been shown to stimulate cytokine production through TLR2/dectin-1 receptor complexes. We tested the hypothesis that cytokine induction by C. albicans blastoconidia was mediated by TLR2 by the blocking of the dectin-1/TLR2 receptor complex with glucan phosphate. Glucan phosphate significantly reduced the production of IL-10, but not of IFN-γ, after stimulation with C. albicans blastoconidia at 107 CFU/ml (Fig. 4).
FIG. 4.
IFN-γ and IL-10 production by PBMC from five healthy volunteers after preincubation with RPMI as a control or glucan phosphate (50 μg/ml) and stimulation for 48 h with heat-killed C. albicans blastoconidia (107 CFU/ml). Data are represented as means ± standard errors of the means. *, P < 0.05, t test.
Role of TLR2 and TLR4 in Candida-induced cytokine production in mice.
To investigate the role of TLR2 and TLR4 in the stimulation of cytokines by blastoconidia and hyphae, we also stimulated peritoneal macrophages of TLR2−/− mice, TLR4-defective ScCr mice, and control mice in vitro with C. albicans. In all experiments, controls included stimulation with E. coli LPS, a TLR4 agonist, and LTA, a TLR2 agonist.
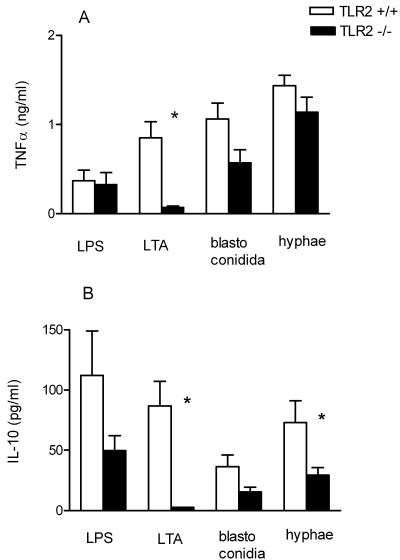
In peritoneal macrophages of TLR2−/− mice, LPS-induced cytokine production was unaffected, whereas, as expected, LTA-stimulated cytokine production was low (Fig. 5). TNF-α production in TLR2−/− cells exposed to blastoconidia was 45% lower than that in TLR2+/+ cells, although the difference did not reach statistical significance (Fig. 5A). Hypha-stimulated IL-10 production by TLR2−/− macrophages was significantly reduced by 65% (Fig. 5B) (P < 0.05).
FIG. 5.
TNF-α (A) and IL-10 (B) production by peritoneal macrophages from TLR2+/+ mice and TLR2−/− mice in response to heat-killed C. albicans blastoconidia (107 CFU/ml) or hyphae (107 CFU/ml), LTA (0.1 μg/ml), and E. coli LPS (0.1 μg/ml). Data are represented as means ± standard errors of the means from three separate experiments with three to nine mice per strain. *, P < 0.05, t test.
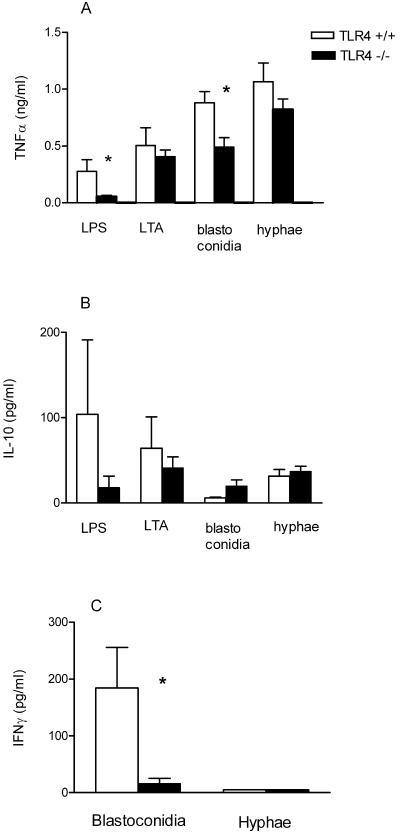
LPS-induced cytokine production was absent in TLR4−/− mice. The production of TNF-α in TLR4−/− mice upon stimulation with blastoconidia was 46% lower than that in TLR4+/+ mice and 25% lower than that in TLR4+/+ mice after stimulation with hyphae (Fig. 6A). Similar amounts of IL-10 were produced by hypha-stimulated TLR4−/− and TLR4+/+ macrophages (Fig. 6B). However, there was a trend towards a higher IL-10 release in TLR4−/− macrophages than in TLR4+/+ macrophages after stimulation with blastoconidia (Fig. 6B).
FIG. 6.
TNF-α (A) and IL-10 (B) production by peritoneal macrophages from TLR4+/+ mice and TLR4−/− ScCr mice in response to heat-killed C. albicans blastoconidia (107 CFU/ml) or hyphae (107 CFU/ml), LTA (0.1 μg/ml), and E. coli LPS (0.1 μg/ml). IFN-γ (C) production by splenic lymphocytes from TLR4+/+ mice and TLR4−/− mice in response to heat-killed C. albicans blastoconidia (107 CFU/ml) or hyphae (107 CFU/ml) during a 48-h stimulation. Data are represented as means ± standard errors of the means from two different experiments with three to nine mice per strain. *, P < 0.05, t test.
IFN-γ production by spleen cells of TLR4+/+and TLR4−/− mice.
To analyze the potential role of TLR4 in IFN-γ production after stimulation with C. albicans blastoconidia or hyphae, we harvested spleen cells from mice lacking a functional TLR4 and from wild-type mice. Splenic lymphocytes were stimulated with blastoconidia and hyphae. As shown in Fig. 6C, only splenic lymphocytes from wild-type mice stimulated with blastoconidia were capable of producing IFN-γ, which peaked after 48 h of incubation, whereas small amounts of IFN-γ were produced by TLR4−/− cells. Undetectable IFN-γ levels were found for both TLR4-defective mice and wild-type mice following stimulation with hyphae (Fig. 6C).
DISCUSSION
In this study, we compared the mechanisms of cytokine stimulation by C. albicans blastoconidia and hyphae in human and murine leukocytes. We show that C. albicans blastoconidia stimulate both TLR2 and TLR4 and that the latter receptor is responsible for IFN-γ production. In contrast, hyphae were not recognized by TLR4, and although they induced larger amounts of IL-10 through TLR2, they were not able to stimulate IFN-γ release.
Most studies on anticandidal defense mechanisms have investigated the induction of cytokines by blastoconidia, while little was known about cytokine induction by hyphae. The results of the present study demonstrate that C. albicans hyphae are unable to induce IFN-γ in either human PBMC or murine splenic lymphocytes. This is supported by other studies, demonstrating a defective IL-12 production in hypha-stimulated human monocytes (6, 7, 14). Only Levitz and North were able to observe induction of small amounts of IFN-γ after stimulation of cells with Candida hyphae, but even in their study only half of the volunteers produced small amounts of IFN-γ, whereas the others were unresponsive (13). The loss of IFN-γ production after germination of Candida blastoconidia into hyphae is very likely an important virulence mechanism induced by yeast-to-hypha transformation, as IFN-γ is a key promoter of the protective immunity against this pathogen. Recombinant IFN-γ protects against experimental candidiasis (12), and IFN-γ−/− mice have an increased susceptibility to systemic candidiasis (4). In contrast to the defective induction of IFN-γ, hyphae induced considerable amounts of IL-10, an anti-inflammatory cytokine inducing a Th2 bias. Although hyphae produced smaller amounts of IL-10 than blastoconidia did in human cells, the IFN-γ/IL-10 ratio induced by hyphae was significantly lower than that induced by blastoconidia. The preferential induction of IL-10 by Candida hyphae could have additional deleterious effects in disseminated candidiasis, as IL-10 is associated with increased susceptibility to candidiasis in mice (23).
In the second part of the study, we investigated the mechanisms responsible for the differential cell activation by blastoconidia and hyphae. Evidence that Candida stimulates through activation of TLR has been provided by us and others, using isolated Candida cell wall components, such as Candida glucan and mannan (5, 8, 16, 22). Recently, we demonstrated that Aspergillus conidia stimulate proinflammatory cytokines through TLR4-mediated signals, whereas germination into hyphae leads to a loss of TLR4 signals and an anti-inflammatory cytokine bias (19). We hypothesized that a similar mechanism could be responsible for the differential stimulation of Candida blastoconidia and hyphae.
IFN-γ production by human PBMC was TLR4 dependent, as shown by blocking PBMC with anti-TLR4 antibody. In splenic lymphocytes from TLR4-defective mice stimulated with blastoconidia, IFN-γ production was almost absent, suggesting that also in mice, the interaction of blastoconidia with TLR4 is responsible for the induction of IFN-γ. These results are sustained by several recent studies showing that TLR4 signals induce mainly a Th1 cytokine pattern, whereas TLR2 recognition leads to a release of Th2 cytokines (2). Our findings demonstrate that blastoconidium-induced IFN-γ production is TLR4 dependent, whereas germination into hyphae induces a loss of the capacity to interact with TLR4, resulting in absent IFN-γ production, while the induction of IL-10 remains intact.
A different picture emerged when cells from TLR2−/− mice were stimulated with Candida. TLR2−/− macrophages produced a 30%- to 45%-smaller amount of TNF-α than TLR2+/+ cells did when stimulated with either blastoconidia or hyphae, although the difference did not reach statistical significance. This is in line with earlier observations showing a reduced TNF-α production in TLR2−/− macrophages in response to blastoconidia and hyphae (24). More importantly, we also observed that hypha-induced IL-10 production is mainly TLR2 dependent. We have recently shown that TLR2−/− mice are more resistant to a disseminated Candida infection than are TLR2+/+ mice and that TLR2−/− mice have an impaired IL-10 production (17). Thus, Candida-induced IL-10 production is mediated through TLR2, and TLR2-mediated IL-10 release is deleterious in experimental models of acute disseminated candidiasis. Taking together the results of the human PBMC experiments and the mouse experiments, therefore, the interaction of blastoconidia with TLR4 induces a strong proinflammatory cytokine release, whereas the interaction of blastoconidia with TLR2 mainly results in an anti-inflammatory cytokine release. Hyphae induce a strong anti-inflammatory cytokine response mediated by TLR2, while they are unable to stimulate IFN-γ due to the loss of recognition by TLR4 (Fig. 7).
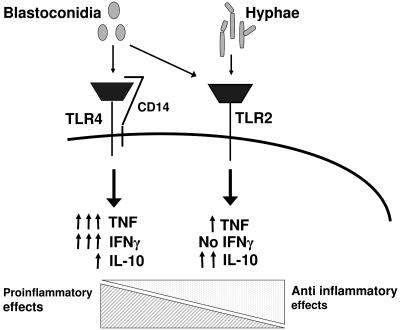
FIG. 7.
Loss of TLR4-mediated signals during yeast-to-hypha transition of C. albicans. Recognition of C. albicans blastoconidia involves both TLR2 and TLR4. The yeast-to-hypha transition results in the loss of proinflammatory TLR4-mediated signals and a TLR2-mediated anti-inflammatory cytokine profile, used by C. albicans as an escape mechanism from the host defense.
Little is known about the cell wall components of Candida that stimulate cytokine production.
Differences in cell wall composition of blastoconidia and hyphae could explain differences in signaling pathways and cytokine induction. However, further research is needed to investigate cytokine response to viable C. albicans, which only exposes external cell wall components. In our study, heat-killed C. albicans was used, potentially exposing both external cell wall and internal components.
An important finding of the present study was that blastoconidium-induced IL-10 was decreased after preincubation with glucan phosphate, which blocks the interaction of β-glucan of Candida with the TLR2/dectin-1 complex (5). In previous experiments, phospholipomannan directly initiated proinflammatory cytokine production through an interaction with TLR2 (9), whereas β-glucans have been shown to interact with dectin-1/TLR2 (5, 8). We show here that the interaction of Candida β-glucans with the TLR2/dectin-1 receptor complex also mediates the release of the anti-inflammatory cytokine IL-10.
In conclusion, we have demonstrated that TLR4 is crucial for the stimulation of proinflammatory cytokines by C. albicans. Whereas Candida blastoconidia and hyphae both stimulate cytokines through TLR2, only blastoconidia are capable of stimulating cells via TLR4. The loss of TLR4-mediated signals during germination of blastoconidia into hyphae results in a biased stimulation of IL-10 release, used as an escape mechanism of C. albicans from the host defense.
Acknowledgments
Mihai G. Netea was supported by a VIDI research grant from The Netherlands Organization of Scientific Research.
Editor: T. R. Kozel
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Balish, E., R. D. Wagner, A. Vazquez-Torres, C. Pierson, and T. Warner. 1998. Candidiasis in interferon-gamma knockout (IFN-γ−/−) mice. J. Infect. Dis. 178:478-487. [DOI] [PubMed] [Google Scholar]
- 5.Brown, G. D., J. Herre, D. L. Williams, J. A. Willment, A. S. Marshall, and S. Gordon. 2003. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 68:5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans: implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouault, T., S. Ibata-Ombetta, O. Takeuchi, P. A. Trinel, P. Sacchetti, P. Lefebvre, S. Akira, and D. Poulain. 2003. Candida albicans phospholipomannan is sensed through Toll-like receptors. J. Infect. Dis. 188:165-172. [DOI] [PubMed] [Google Scholar]
- 10.Krueger, K. E., A. K. Ghosh, B. P. Krom, and R. L. Cihlar. 2004. Deletion of the NOT4 gene impairs hyphal development and pathogenicity in Candida albicans. Microbiology 150:229-240. [DOI] [PubMed] [Google Scholar]
- 11.Kullberg, B. J., and A. M. Oude Lashof. 2002. Epidemiology of opportunistic invasive mycoses. Eur. J. Med. Res. 7:183-191. [PubMed] [Google Scholar]
- 12.Kullberg, B. J., J. W. van 't Wout, C. Hoogstraten, and R. van Furth. 1993. Recombinant interferon-gamma enhances resistance to acute disseminated Candida albicans infection in mice. J. Infect. Dis. 168:436-443. [DOI] [PubMed] [Google Scholar]
- 13.Levitz, S. M., and E. A. North. 1996. Gamma interferon gene expression and release in human lymphocytes directly activated by Cryptococcus neoformans and Candida albicans. Infect. Immun. 64:1595-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, L., K. Kang, M. Takahara, K. D. Cooper, and M. A. Ghannoum. 2001. Hyphae and yeasts of Candida albicans differentially regulate interleukin-12 production by human blood monocytes: inhibitory role of C. albicans germination. Infect. Immun. 69:4695-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea, M. G., P. N. M. Demacker, B. J. Kullberg, O. C. Boerman, C. Verschueren, A. F. H. Stalenhoef, and J. W. M. van der Meer. 1996. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe Gram-negative infections. J. Clin. Investig. 97: 1366-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea, M. G., C. A. A. van der Graaf, A. G. Vonk, I. Verschueren, J. W. M. van der Meer, and B. J. Kullberg. 2002. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185:1483-1489. [DOI] [PubMed] [Google Scholar]
- 17.Netea, M. G., R. Sutmuller, C. Hermann, C. A. A. van der Graaf, J. W. M. van der Meer, J. H. van Krieken, T. Hartung, G. Adema, and B. J. Kullberg. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172: 3712-3718. [DOI] [PubMed] [Google Scholar]
- 18.Netea, M. G., A. G. Vonk, M. van den Hoven, C. Verschueren, L. A. Joosten, J. H. van Krieken, W. B. van den Berg, J. W. M. van der Meer, and B. J. Kullberg. 2003. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur. J. Immunol. 33: 3409-3417. [DOI] [PubMed] [Google Scholar]
- 19.Netea, M. G., A. Warris, J. W. M. van der Meer, M. J. Fenton, T. J. G. Verver-Jansen, L. E. H. Jacobs, T. Andresen, P. E. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of Toll-like receptor-4 mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 20.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 21.Schröder, N. W. J., S. Morath, C. Alexander, U. B. Göbel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 22.Tada, H., E. Nemoto, H. Shimauchi, T. Watanabe, T. Mikami, T. Matsumoto, N. Ohno, H. Tamura, K. Shibata, S. Akashi, K. Miyake, S. Sugawara, and H. Takada. 2002. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46:503-512. [DOI] [PubMed] [Google Scholar]
- 23.Vasquez-Torres, A., J. Jones-Carson, R. D. Wagner, T. Warner, and E. Balish. 1999. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect. Immun. 67:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villamón, E., D. Gozalbo, P. Roig, J. E. O'Connor, D. Fradelizi, and M. L. Gil. 2004. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Warenda, A. J., S. Kauffman, T. P. Sherrill, J. M. Becker, and J. B. Konopka. 2003. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 71:4045-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]