Abstract
In order to develop CD8+-T-cell-mediated immunotherapy against intracellular infectious agents, vaccination using recombinant virus vectors has become a promising strategy. In this study, we generated recombinant adenoviral and vaccinia virus vectors expressing a single CD8+-T-cell epitope, ANYNFTLV, which is derived from a Trypanosoma cruzi antigen. Immunogenicity of these two recombinant virus vectors was confirmed by the detection of ANYNFTLV-specific CD8+ T cells in the spleens of immunized mice. Priming/boosting immunization using combinations of these two recombinant virus vectors revealed that the adenovirus vector was efficient for priming and the vaccinia virus vector was effective for boosting the CD8+-T-cell responses. Moreover, we also demonstrated that the ANYNFTLV-specific CD8+-T-cell responses were further augmented by coadministration of recombinant vaccinia virus vector expressing the receptor activator of NFκB (RANK) ligand as an adjuvant. By priming with the adenovirus vector expressing ANYNFTLV and boosting with the vaccinia virus vectors expressing ANYNFTLV and RANK ligand, the immunized mice were efficiently protected from subsequent challenge with lethal doses of T. cruzi. These results indicated, for the first time, that the induction of immune responses against a single CD8+-T-cell epitope derived from an intrinsic T. cruzi antigen was sufficient to control lethal T. cruzi infection.
Trypanosoma cruzi is the etiological agent of Chagas' disease in Central and South America (8, 20, 21, 35). As it invades and replicates in essentially all types of cells of mammalian hosts, T-cell-mediated immunity is critical for resolving the infection (3, 9). In accordance with this notion, the depletion of CD8+ or CD4+ T cells results in unrelenting parasitemia and a fatal outcome in mice (38, 42, 43, 44). Considering the paucity of therapeutic drugs against T. cruzi (46), the development of a vaccine to induce effective T-cell-mediated immunity has been eagerly expected (50, 51). We previously identified a major epitope of trans-sialidase surface antigen (TSSA) recognized by CD8+ T cells in T. cruzi-infected C57BL/6 mice and have demonstrated that vaccination with plasmid DNA encoding TSSA can induce CD8+-T-cell-mediated protective immunity against lethal T. cruzi infection (19, 27, 28, 29).
Vaccination using recombinant virus vectors has become a promising strategy to induce T-cell immunity against intracellular infectious agents (37, 40). Adenovirus and vaccinia virus have been shown to be the most efficient vectors for inducing protective immune responses against human immunodeficiency virus (12, 15, 16, 39) and malaria (22, 30, 31, 36, 54). The generation of replication-deficient virus mutants makes this strategy safer and more effective for containing the threat and spread of infections. The recombinant virus vector vaccination was also demonstrated effective for conferring protective immunity against T. cruzi, which was artificially engineered to express a well-characterized, immunogenic foreign antigen (26). However, it remains to be determined if the recombinant virus vectors expressing an intrinsic T. cruzi antigen are really effective for conferring immunological protection. In addition, it also remains to be determined whether the vaccine-induced CD8+-T-cell responses are sufficient for controlling the T. cruzi infection.
In the present study, we demonstrated that vaccination with recombinant adenoviral and vaccinia virus vectors expressing a single CD8+-T-cell epitope, ANYNFTLV, which is derived from a T. cruzi TSSA antigen, was effective for protecting mice from lethal T. cruzi infection. We also found that recombinant vaccinia virus expressing RANK ligand exhibited an adjuvant effect for enhancing the induction of ANYNFTLV-specific CD8+ T cells. These findings demonstrate that the immune response directed against a single CD8+-T-cell epitope is sufficient for controlling the lethal T. cruzi infection, providing a new basis for improving vaccine strategies against Chagas' disease.
MATERIALS AND METHODS
Animals and parasite.
Female C57BL/6 (H-2b) mice, 5 to 8 weeks of age, were purchased from SEAC Yoshitomi (Yoshitomi, Fukuoka, Japan). Blood-form trypomastigotes of T. cruzi Tulahuen strain (24) were maintained in outbred CD1 or inbred BALB/c mice by intramuscular or intraperitoneal inoculation of 5,000 trypomastigotes into naïve mice every 2 week. An institutional review committee at Juntendo University has approved the animal studies described here.
Cells and culture.
The C57BL/6-derived thymoma cell line EL-4 was used as antigen-presenting cells for CD8+-T-cell cultures and assays. The cell line has been widely used for CD8+-T-cell assays, since it was well reported that it expresses only class I antigens but not class II (23, 49), which was confirmed by our own hands (data not shown). In that respect, the EL4 cells are suitable antigen-presenting cells for the detection of antigen-specific CD8+ T cells during the gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. BHK-21 cell (American Type Culture Collection, Manassas, VA) was used for growing highly attenuated vaccinia virus strain called modified vaccinia virus Ankara (MVA) (American Type Culture Collection) (41) or MVA-derived recombinant viruses. The transformed human embryonic kidney cell line 293 (American Type Culture Collection) was used for growing replication-deficient adenovirus or recombinant adenoviruses. These cells were cultured in high-glucose Dulbecco's modified Eagle's medium (Life Technologies/BRL, Rockville, MD) supplemented with 10% fetal calf serum, 2 g/liter sodium bicarbonate (Sigma-Aldrich Co., St. Louis, MO), 200 mg/liter l-arginine hydrochloride (Life Technologies/BRL), 36 mg/liter l-asparagine (Life Technologies/BRL), 2.6 g/liter HEPES (Sigma-Aldrich), 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich), and antibiotics (complete Dulbecco's modified Eagle's medium). The medium used for ELISPOT assays and the culture of lymphocytes was supplemented with phorbol myristate acetate-stimulated EL-4 cell culture supernatant as a source of 30 U/ml interleukin (IL)-2 (complete Dulbecco's modified Eagle's medium-IL-2) (25).
Peptide.
An H-2Kb-restricted CD8+-T-cell epitope peptide, ANYNFTLV, derived from TSSA (19, 27, 28, 29) (Fig. 1A) was synthesized and used for immunological assays. The gene encoding for TSSA was first identified to be present among the clusters of genes encoding for enzymes involved in de novo pyrimidine biosynthesis in T. cruzi (11). One of the figures in the report (11) showed a schematic representation of the 25-kb segment containing five genes that encode all six enzymes of de novo pyrimidine biosynthesis. In that scheme, however, there was an additional gene, orf, which was described only as a surface protein of T. cruzi (DNA accession number AB010287). We designated the “surface protein” TSSA, since its amino acid sequence was highly homologous to the T. cruzi trans-sialidase superfamily protein. Although we have not determined yet whether the TSSA actually has neuraminidase and trans-sialidase activities, we demonstrated that the DNA encoding TSSA was highly immunogenic, conferring protective immunity in C57BL/6 mice against T. cruzi infection (19, 27, 28, 29).
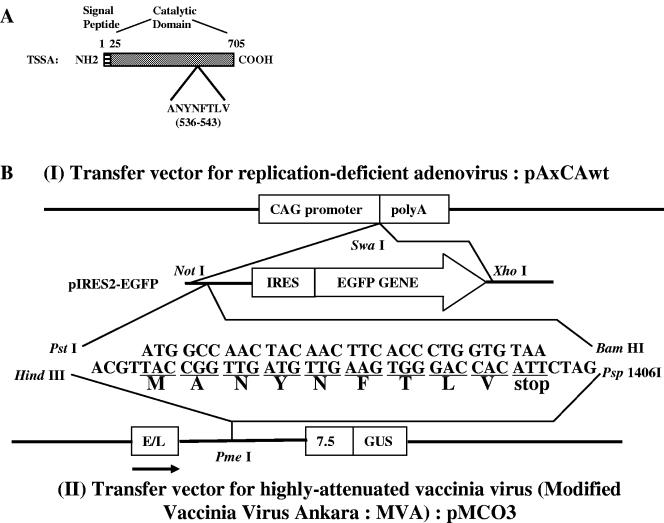
FIG. 1.
Generation of recombinant virus vectors. A. Primary structure of T. cruzi trans-sialidase surface antigen (TSSA) and an H-2Kb-restricted CD8+-T-cell epitope, ANYNFTLV. The gene encoding for TSSA was first identified to be present among the clusters of genes encoding for enzymes involved in de novo pyrimidine biosynthesis in the genome of T. cruzi Tulahuen strain (11). The report (11) showed a schematic representation of the 25 kb segment containing not only five genes that encode all six enzymes of de novo pyrimidine biosynthesis but also an additional gene, orf, which was described only as surface protein of T. cruzi (DNA accession number: AB010287). We designated the surface protein as TSSA, since its amino acid sequence was highly homologous to the T. cruzi trans-sialidase superfamily protein. The T. cruzi trans-sialidase usually consists of four parts; i.e., signal peptide, catalytic domain, C-terminal repeats, and hydrophobic region for GPI anchor. TSSA, however, consists of only two parts, signal peptide and catalytic domain. An H-2Kb-restricted CD8+-T-cell epitope, ANYNFTLV (536-543), was identified on TSSA (19). B. A minigene encoding the MANYNFTLV peptide was inserted either into pAxCAwt, a transfer vector for replication-deficient adenovirus, or into pMCO3, a transfer vector for highly attenuated vaccinia virus (MVA). Detailed procedures for generating recombinant viruses are described in the Materials and Methods. CAG, modified chicken β-actin promoter with the cytomegalovirus immediate-early enhancer (32); poly A, poly(A) addition signal; IRES, internal ribosome entry site; EGFP, enhanced green fluorescent protein; E/L, synthetic early/late MVA promoter; 7.5, MVA P7.5 promoter; GUS, gene encoding Escherichia coli β-glucuronidase.
Generation of recombinant adenoviruses.
To construct a minigene encoding the peptide MANYNFTLV, synthesized oligonucleotides 5′-ATGGCCAACTACAACTTCACCCTGGTGTAA-3′ and 5′-GATCTTACACCAGGGTGAAGTTGTAGTTGGCCATTGCA-3′ were annealed and inserted into pIRES2-EGFP (Becton Dickinson and Company, Franklin Lakes, NJ), which was predigested with PstI and BamHI (Fig. 1B). The sequences of oligonucleotides were modified to optimize the expression of peptides in mammalian cells. The resulting plasmid DNA was designated as pIRES-MANY.
To generate recombinant adenovirus vectors, the NotI-XhoI fragments of pIRES-MANY or pIRES2-EGFP were treated with Klenow fragment (Takara Bio Inc., Shiga, Japan) and inserted into SwaI site of the cosmid vector pAxCAwt (Takara). The resulting cosmid vectors were designated pAdex/MANY and pAdex/GFP, respectively. Using the adenovirus expression vector kit (Takara), recombinant adenoviruses, designated Ad-MANY and Ad-GFP, were generated by homologous recombination between the cosmid vector pAdex/MANY or pAdex/GFP and adenovirus genomic DNA, respectively. After picking up green fluorescence-emitting, virus-infected cells and six rounds of purification, recombinant viruses were amplified in 293 cells, and then purified by centrifugation through a cushion of cesium chloride (18). The titer of the virus stocks was determined by detecting green fluorescence-emitting cells in 293 cell cultures.
Generation of recombinant vaccinia viruses.
For the generation of recombinant MVAs, the HindIII-Psp1406I fragment of the pIRES-MANY was treated with KOD DNA polymerase (Toyobo Co. Ltd., Osaka, Japan) and inserted into the PmeI site of the vaccinia virus insertion vector, pMCO3 (6), downstream of a strong synthetic early/late virus promoter (Fig. 1B). The resulting plasmid or the unmodified pMCO3 was used to transfect BHK-21 cells, which were coinfected with the MVA. Cells that stained blue upon addition of X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) (Clontech, Palo Alto, CA) were selected. After six rounds of plaque purification, recombinant viruses were amplified in BHK-21 cells and purified by centrifugation through a cushion of 36% sucrose (10). They were designated MVA-MANY and MVA-p3, respectively. The titer of purified viruses was determined by staining virus-infected BHK-21 cells with X-Gluc.
In order to generate the recombinant MVA expressing RANK ligand (RANKL), murine RANKL cDNA (29) was inserted into the PmeI site of the pMCO3. The isolation and purification of the recombinant MVA expressing RANKL, designated as MVA-RANKL, was performed as described above. The expression of RANKL by the MVA-RANKL was confirmed by staining the virus-infected BHK-21 cells with biotin-conjugated anti-RANKL monoclonal antibody (IK22-5) (29), or rat IgG isotype control (BD PharMingen, San Diego, CA). After washing with PBS twice, the cells were incubated with PE-labeled streptavidin (BD PharMingen), washed with PBS twice, and then analyzed on a FACSCalibur (BD Biosciences, San Jose, CA). The data were processed using the CellQuest program (BD Biosciences).
Quantification of antigen-specific T cells by ELISPOT assay.
The frequency of antigen (ANYNFTLV)-specific T cells was determined by ELISPOT assay for IFN-γ-secreting cells essentially as described previously (25). Briefly, serial dilutions of freshly isolated splenocytes or cultured T cells (1 × 104 to 100 × 104) were cocultured with irradiated EL-4 cells that had been pulsed with 1 μM ANYNFTLV peptide in anti-IFN-γ monoclonal antibody-coated plates for 24 to 28 h. The spots formed by IFN-γ-secreting cells were detected with biotinylated anti-IFN-γ monoclonal antibody followed by peroxidase-labeled streptavidin and diaminobenzidine. The developed spots were counted under a microscope and expressed as the number of spots per 106 cells.
Vaccination schedule, dosages, and challenge infection.
All the vaccination schedules and dosages are described in detail in each figure legend. The number of ANYNFTLV-specific CD8+ T cells was quantified by ELISPOT assay. The immunized mice were challenged with lethal or sublethal dose of Tulahuen strain of T. cruzi blood-form trypomastigotes 10 to 14 days after the last immunization. Blood from all infected mice was collected periodically from the tail vein, and the number of parasites in 5 μl blood (parasitemia) was counted microscopically. Survival of host mice was monitored daily.
Statistical analyses.
Statistical analyses were performed by the unpaired Student's t test or Dunnett's two-tailed t test for the ELISPOT assays and for the parasitemia. The unpaired Mann-Whitney U test was used to determine significant differences in survival data. P values less than 0.05 were considered significant.
RESULTS
Immunogenicity of recombinant adenovirus expressing ANYNFTLV.
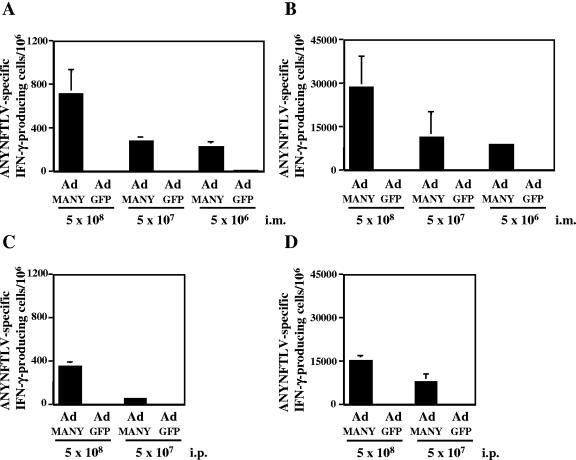
In order to test the immunogenicity of recombinant adenovirus expressing the H-2Kb-restricted CD8+-T-cell epitope of TSSA (ANYNFTLV), we immunized C57BL/6 mice intramuscularly or intraperitoneally with different doses of Ad-MANY or Ad-GFP. The induction of ANYNFTLV-specific CD8+ T cells in the spleen of Ad-MANY-immunized mice was demonstrated by ELISPOT assay using freshly isolated or in vitro expanded splenocytes as effector cells in a dose-dependent manner (Fig. 2A to D). In contrast, Ad-GFP did not induce the ANYNFTLV-specific CD8+ T cells at any doses. When the immunization was done via intraperitoneal route, the numbers of induced antigen-specific CD8+ T cells were consistently lower than those induced by intramuscular immunization (Fig. 2A and C) (354 ± 34 versus 712 ± 224 after inoculating 5 × 108 PFU viral load and 64 ± 15 versus 284 ± 30 after inoculating 5 × 107 PFU viral load, determined by ELISPOT assay using freshly isolated splenocytes as effector cells). It has been demonstrated that the induction of antigen-specific CD8+ T cells was significantly affected by the route of immunization (14, 34). We therefore decided that the immunization with Ad-MANY should be done via the intramuscular route in the following experiments.
FIG. 2.
Immunogenicity of recombinant adenovirus expressing ANYNFTLV. C57BL/6 mice were administered with either Ad-MANY or Ad-GFP intramuscularly at three different doses (A, B) or intraperitoneally at two different doses (C, D). The mice were sacrificed 14 days after the immunization, and their spleens were removed. A half of splenocytes from individual mice were cultured with irradiated EL-4 cells pulsed with ANYNFTLV peptide for one week. The freshly isolated splenocytes (A, C) or the 1-week cultured splenocytes (B, D) were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells/106 cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± standard deviation of three mice in each group. The data are representative one of three independent experiments.
Immunogenicity of recombinant MVA expressing ANYNFTLV.
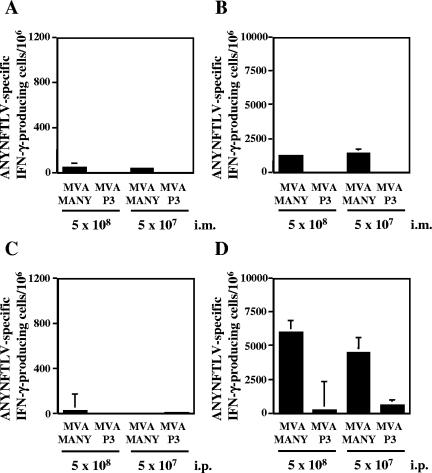
We next tested the immunogenicity of recombinant MVA expressing the ANYNFTLV epitope. When C57BL/6 mice were immunized either intramuscularly or intraperitoneally with two different doses of MVA-MANY, few ANYNFTLV-specific CD8+ T cells were detected in freshly isolated splenocytes by ELISPOT assay (Fig. 3A and C). However, a significant expansion of the specific CD8+ T cells was observed when the immune splenocytes were stimulated with the ANYNFTLV peptide in vitro for one week (Fig. 3B and D). In contrast, MVA-p3 did not significantly induce the specific CD8+ T cells. Since a larger number of antigen-specific CD8+ T cells were induced by intraperitoneal immunization compared to intramuscular immunization, the intraperitoneal route was used for MVA-MANY in the following experiments.
FIG. 3.
Immunogenicity of recombinant MVA expressing ANYNFTLV. C57BL/6 mice were administered intramuscularly (A, B) or intraperitoneally (C, D) with two different doses of either MVA-MANY or MVA-p3. The mice were sacrificed 11 days after the immunization, and their spleens were removed. A half of splenocytes from individual mice were cultured with irradiated EL-4 cells pulsed with ANYNFTLV peptide for 1 week. The freshly isolated splenocytes (A, C) or the one-week cultured splenocytes (B, D) were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells/106 cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± standard deviation of three mice in each group. The data are representative one of three independent experiments.
Ad-MANY is efficient for priming and MVA-MANY is efficient for boosting the ANYNFTLV-specific CD8+-T-cell responses.
The above results indicated that Ad-MANY was more efficient than MVA-MANY for priming the ANYNFTLV-specific CD8+-T-cell response (Fig. 2 and Fig. 3). To further expand the ANYNFTLV-specific CD8+ T cells, we next examined the effect of a boost immunization with Ad-MANY or MVA-MANY after the Ad-MANY priming. As shown in Fig. 4A, the boost immunization with Ad-MANY augmented the expansion of ANYNFTLV-specific CD8+ T cells only modestly compared to that with Ad-GFP. In contrast, the boost immunization with MVA-MANY markedly increased the frequency of ANYNFTLV-specific CD8+ T cells, while that with MVA-p3 did not (Fig. 4A). These results indicated that MVA-MANY was much more efficient than Ad-MANY for boosting the ANYNFTLV-specific CD8+-T-cell response after Ad-MANY priming. In addition, the induction of ANYNFTLV-specific CD8+ T cells by the Ad-MANY priming/MVA-MANY boost was superior to the one induced either by the MVA-MANY priming/Ad-MANY boost or by the MVA-MANY priming/MVA-MANY boost described as follows.
FIG. 4.
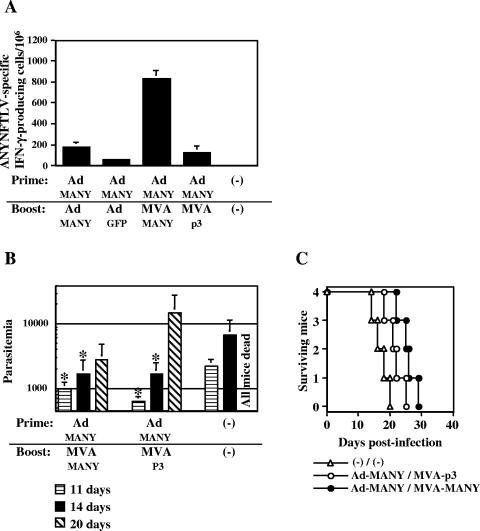
Immune responses induced by prime/boost immunization using Ad-MANY and MVA-MANY. A. C57BL/6 mice were primed intramuscularly with 5 × 107 PFU of Ad-MANY. Twelve days later, the mice were boosted intramuscularly with 5 × 107 PFU of Ad-MANY or Ad-GFP, or intraperitoneally with 5 × 107 PFU of MVA-MANY or MVA-p3. The mice were sacrificed 14 days after the boost immunization, and their spleens were removed. The freshly isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells× 106 cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± standard deviation of three mice in each group. B. Some groups of the prime/boosted mice (n = 4) were infected intramuscular with 10,000 T. cruzi blood-form trypomastigotes at 10 days after the boost immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted at 11, 14, and 20 days postinfection. *, P < 0.05 compared to unimmunized mice by the unpaired Student's t test. Statistical analysis at 20 days postinfection was not achievable due to the death of all unimmunized mice. C. Survival was monitored daily. The survival of Ad-MANY/MVA-MANY-immunized mice was significantly different (P < 0.05 by the unpaired Mann-Whitney U test) from that of unimmunized mice. The data are representative one of two independent experiments.
When mice were first immunized with one of the recombinant viruses followed by the booster injection of Ad-MANY or Ad-GFP, we didn't detect significant differences in the induced numbers of ANYNFTLV-specific CD8+ T cells between a group of mice immunized with MVA-MANY priming/Ad-MANY boost and the one immunized with MVA-p3 priming/Ad-MANY boost (472 ± 28 versus 512 ± 147 per 106 splenocytes). Repeated immunization of Ad-MANY was also not effective for enhancing the induction of antigen-specific CD8+ T cells, and on the contrary, it was even significantly lower than that induced by MVA-p3 priming/Ad-MANY boost immunization (184 ± 41 versus 512 ± 147 per 106 splenocytes, P < 0.05). When the mice were first immunized with one of the recombinant viruses followed by the booster injection of MVA-MANY or MVA-p3, we detected the enhanced induction of antigen-specific CD8+ T cells by the combined immunization of Ad-MANY priming/MVA-MANY boost (832 ± 79 per 106 splenocytes) compared to either Ad-MANY priming/MVA-p3 boost (125 ± 65 per 106 splenocytes) or Ad-GFP priming/MVA-MANY boost (11 ± 2 per 106 splenocytes). Repeated immunization with MVA-MANY was also effective, against our expectation, for enhancing the induction of antigen-specific CD8+ T cells compared to that induced by MVA-p3 priming/MVA-MANY boost immunization (350 ± 105 versus 5 ± 8 per 106 splenocytes, P < 0.05), however, its immunogenicity is still inferior to the Ad-MANY priming/MVA-MANY boost immunization.
To determine the induction of protective immunity mediated by the ANYNFTLV-specific CD8+ T cells in vivo, we challenged the mice with a lethal dose of T. cruzi blood-form trypomastigotes after the prime/boost immunization with Ad-MANY/MVA-MANY. As shown in Fig. 4B and C, the parasitemia at 11, 14, and 20 days postinfection was significantly suppressed and the survival was significantly prolonged by the prime/boost immunization with Ad-MANY/MVA-MANY. The priming with Ad-MANY and boosting with MVA-p3 also significantly suppressed the parasitemia, but survival was not significantly prolonged.
Prime/boost immunization could be enhanced by increased viral loads.
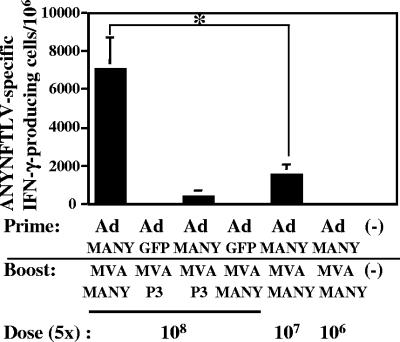
In the above experiments, the parasitemia was significantly suppressed and the survival was significantly prolonged by the prime/boost immunization with 5 × 107 PFU of Ad-MANY and 5 × 107 PFU of MVA-MANY, but all of the immunized mice eventually succumbed to T. cruzi infection. We then examined whether a prime/boost immunization with 10-fold higher doses (5 × 108 PFU) of Ad-MANY and MVA-MANY could induce a higher ANYNFTLV-specific CD8+-T-cell response. The priming with 5 × 108 PFU of Ad-MANY and the subsequent boosting with 5 × 108 PFU of MVA-MANY was not toxic, since the immunized mice did not show an apparent pathological symptom such as ruffled fur or hunched posture. As shown in Fig. 5, the prime/boost immunization with 5 × 108 PFU of Ad-MANY/MVA-MANY markedly increased the frequency of ANYNFTLV-specific CD8+ T cells compared to that with 5 × 107 PFU of Ad-MANY/MVA-MANY. In contrast, priming with 5 × 108 PFU of Ad-GFP or boosting with 5 × 108 PFU of MVA-p3 did not significantly induce the ANYNFTLV-specific CD8+ T cells.
FIG. 5.
Induction of ANYNFTLV-specific CD8+ T cells by prime/boost immunization with high doses of Ad-MANY and MVA-MANY. C57BL/6 mice (n = 3) were first primed with different doses (5 × 108, 5 × 107, or 5 × 106 PFU) of Ad-MANY or 5 × 108 PFU of Ad-GFP, and boosted 14 days later with different doses (5 × 108, 5 × 107, or 5 × 106 PFU) of MVA-MANY or 5 × 108 PFU of MVA-p3. The mice were sacrificed 10 days after the boost immunization, and their spleens were removed. The freshly isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells× 106 cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± standard deviation of three mice in each group. *, P < 0.05 determined by the Dunnett's two-tailed t test. The data are representative one of three independent experiments.
Adjuvant effect of recombinant MVA expressing murine RANKL.
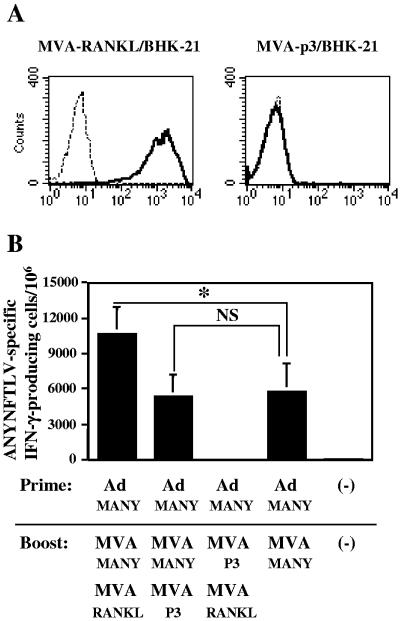
To further increase the frequency of ANYNFTLV-specific CD8+ T cells, we next tried to use recombinant MVA expressing RANKL as an adjuvant, since we previously demonstrated a potent adjuvant effect of RANKL-expressing plasmid for the induction of ANYNFTLV-specific CD8+-T-cell response by DNA vaccination (29). As shown in Fig. 6A, recombinant MVA inserted with RANKL cDNA was very efficient for expressing murine RANKL on the surface of BHK-21 cells as well as various mouse cells (data not shown).
FIG. 6.
Adjuvant effect of recombinant MVA expressing murine RANKL. (A) BHK-21 cells were infected with either MVA-RANKL or MVA-p3 and then were stained with biotinylated anti-RANKL monoclonal antibody followed by PE-labeled streptavidin. The bold histograms indicate the staining with anti-RANKL monoclonal antibody and the thin histograms indicate the staining with isotype-matched control rat immunoglobulin G. (B) C57BL/6 mice (n = 3) were first primed with 5 × 108 PFU of Ad-MANY and then boosted 11 days later with 5 × 108 PFU of MVA-MANY or MVA-p3 and 5 × 107 PFU of MVA-RANKL or MVA-p3. The mice were sacrificed 10 days after the boost immunization, and their spleens were removed. The freshly isolated splenocytes were subjected to the ELISPOT assay for IFN-γ-producing cells in response to ANYNFTLV peptide-pulsed EL-4 cells. The number of IFN-γ-secreting cells/106 cells was counted 24 h later. The number of IFN-γ-secreting cells that appeared against peptide-unpulsed EL-4 was subtracted from the number of IFN-γ-secreting cells that appeared against peptide-pulsed EL-4. Data represent the mean ± standard deviation of three mice in each group. *, P < 0.05 by the Dunnett's two-tailed t test. NS, not significantly different. The data are representative one of two independent experiments.
We then included 5 × 107 PFU of MVA-RANKL as an adjuvant in the boosting with 5 × 108 PFU of MVA-MANY after the priming with 5 × 108 PFU of Ad-MANY. As shown in Fig. 6B, the inclusion of MVA-RANKL significantly increased the frequency of ANYNFTLV-specific CD8+ T cells induced by prime/boost immunization with Ad-MANY/MVA-MANY compared to that of MVA-p3.
Protection from lethal T. cruzi infection.
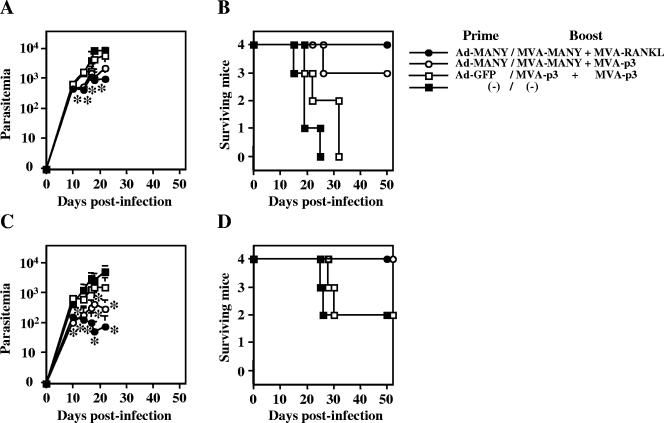
We finally challenged the mice with a lethal (10,000) or sublethal (2,000) dose of T. cruzi blood-form trypomastigotes after the prime/boost immunization with high doses (5 × 108 PFU) of Ad-MANY/MVA-MANY or Ad-GFP/MVA-p3 and the inclusion of 5 × 107 PFU of MVA-RANKL or MVA-p3 (control) as an adjuvant. As shown in Fig. 7A and C, the parasitemia was significantly suppressed by the high doses of Ad-MANY/MVA-MANY with or without MVA-RANKL, but protection of all mice from lethal T. cruzi infection was only achieved by the high doses of Ad-MANY/MVA-MANY with MVA-RANKL. These results indicated that the CD8+-T-cell response to a single epitope (ANYNFTLV) of an intrinsic T. cruzi antigen (TSSA) could control lethal T. cruzi infection, if it could be efficiently induced by recombinant virus vector vaccination and adjuvant.
FIG. 7.
Prime/boost immunization with Ad-MANY/MVA-MANY + MVA-RANKL can control lethal T. cruzi infection. C57BL/6 mice (n = 4) were first primed with 5 × 108 PFU of Ad-MANY or Ad-GFP, and then boosted with 5 × 108 PFU of MVA-MANY or MVA-p3 and 5 × 107 PFU of MVA-RANKL or MVA-p3 11 days later. The mice were infected intraperitoneally with 10,000 (A, B) or 2,000 (C, D) Tulahuen strain of T. cruzi blood-form trypomastigotes 14 days after the boost immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted periodically, and the data represent the mean ± standard deviation of four mice in each group (A, C). Survival was monitored daily (B, D). *, P < 0.05 compared to the unimmunized mice by the Dunnett's two-tailed t test (A, C). The longer survival of Ad-MANY/MVA-MANY + MVA-RANKL group was significantly different (P < 0.05 by the unpaired Mann-Whitney U test) from that of Ad-GFP/MVA-p3 plus MVA-p3 group of mice (B). The survival of other groups was not significantly different from that of the Ad-GFP/MVA-p3 plus MVA-p3 group of mice (B, D).
DISCUSSION
In the present study, we generated two recombinant virus vectors (Ad-MANY and MVA-MANY) expressing a single CD8+-T-cell epitope (ANYNFTLV) derived from an intrinsic T. cruzi antigen (TSSA). In order to explore CD8+-T-cell-mediated immunotherapeutic strategies against T. cruzi infection, we evaluated the immunogenicity of these recombinant viruses with regard to the induction of protective immune responses.
The expression of ANYNFTLV peptide by Ad-MANY and MVA-MANY was verified by detecting the ANYNFTLV-specific CD8+ T cells in immunized mice (Fig. 2 and Fig. 3). However, the immunogenicities of Ad-MANY and MVA-MANY were different, especially when we evaluated the induction of ANYNFTLV-specific CD8+ T cells in freshly isolated splenocytes. The induction of ANYNFTLV-specific CD8+ T cells by Ad-MANY was robust, reaching up to approximately 800 cells (intramuscularly) (Fig. 2A) or 400 cells (intraperitoneally) (Fig. 2C) per 106 splenocytes. In contrast, few ANYNFTLV-specific CD8+ T cells were detected in freshly isolated splenocytes when mice were immunized with MVA-MANY (Fig. 3A and C). However, modest but substantial induction of ANYNFTLV-specific CD8+ T cells by MVA-MANY was detectable after the stimulation of immune splenocytes with the ANYNFTLV peptide in vitro for a week. This inferior ability of MVA-MANY to prime ANYNFTLV-specific CD8+-T-cell response in naïve mice seems most likely due to the immunological phenomenon called “immunodominance” which is potentially a serious pitfall in using virus vectors to deliver foreign antigens for the induction of immune responses (53). Thus, in the MVA-MANY-immunized mice, T-cell responses against MVA antigens might predominate over and hinder the ANYNFTLV-specific CD8+-T-cell response.
To augment the induction of ANYNFTLV-specific CD8+ T cells, we next evaluated the effects of boost immunization with Ad-MANY or MVA-MANY after Ad-MANY priming. In a sharp contrast with the priming, MVA-MANY was far more effective than Ad-MANY for the boosting (Fig. 4A). This superior ability of MVA-MANY at boosting might be due to circumvention of the “immunodominance” by the Ad-MANY priming so that the expanded ANYNFTLV-specific CD8+ T cells by the Ad-MANY priming predominated over MVA-specific T cells. In contrast, the boosting effect of Ad-MANY was only modest (Fig. 4A). This seems at least partly due to inhibition of adenoviral reinfection by neutralizing antibodies against adenovirus induced by the Ad-MANY priming. Therefore, the use of two different virus vectors expressing the same target antigen for prime/boost immunization is an efficient strategy to circumvent this problem.
The vaccination strategy using recombinant adenovirus for priming followed by the booster injection of recombinant vaccinia virus was also effective against other infections such as malaria (4, 13) and human immunodeficiency virus (7). To our experiences, the use of two recombinant virus vectors for adenovirus priming/MVA boosting was more effective for the induction of ANYNFTLV-specific CD8+ T cells than the use of TSSA gene-encoding DNA vaccine for priming followed by the booster injection of MVA-MANY. Even though the induction of ANYNFTLV-specific CD8+ T cells of DNA vaccine priming/MVA-MANY boosting was significantly enhanced (145 ± 50 per 106 splenocytes) when it was compared to those induced by either DNA vaccine alone (−12 ± 11 per 106 splenocytes) or MVA-MANY alone (24 ± 23 per 106 splenocytes), the number of ANYNFTLV-specific CD8+ T cells induced by that regimen was constantly and significantly lower than that induced by the combined immunizations of two recombinant virus vectors.
When the immunized mice were challenged with a lethal dose of T. cruzi after the prime/boost immunization with 5 × 107 PFU of Ad-MANY/MVA-MANY, the parasitemia was significantly suppressed (Fig. 4B) and the survival was significantly prolonged but all mice eventually died (Fig. 4C). To achieve survival of all immunized mice from lethal T. cruzi infection, we further enhanced the induction of ANYNFTLV-specific CD8+ T cells by the prime/boost immunization with 10-fold higher doses (5 × 108 PFU) of Ad-MANY/MVA-MANY (Fig. 5) and the inclusion of MVA-RANKL as an adjuvant (Fig. 6B).
Since the Ad and MVA vectors used here were highly attenuated, no apparent adverse effect manifested by pathological symptoms was observed after the priming with 5 × 108 PFU of Ad-MANY and the boosting with 5 × 108 PFU of MVA-MANY, while the frequency of ANYNFTLV-specific CD8+ T cells was dramatically increased (approximately 7,000 per 106 splenocytes). Moreover, the inclusion of MVA-RANKL at the boosting further increased the frequency of ANYNFTLV-specific CD8+ T cells (approximately 10,000 per 106 splenocytes). RANKL is a member of the tumor necrosis factor family, which has been implicated in immune regulation and bone homeostasis (2, 45). A major target for RANKL in the immune system is dendritic cells (DCs) that are potent antigen-presenting cells and express a high level of RANK (1, 52). It has been reported that RANKL not only enhances the survival of DCs but also up-regulates the production of cytokines, such as IL-12 (2, 17), and the expression of costimulatory molecules, such as B7, by dendritic cells thereby augments T-cell responses (47, 48). It would be interesting to determine the profiles of cytokine production in vivo with regard to the protective immunity against T. cruzi infection, since cytokines could modify the course of infection in combination with cellular components including CD8+ T cells. We previously demonstrated that plasmid expressing RANKL was a potent genetic adjuvant for enhancing the induction of TSSA-specific CD8+ T cells by DNA vaccination (29). We have now shown that MVA expressing RANKL is also useful as an adjuvant for recombinant MVA vaccination.
By the prime/boost immunization with 5 × 108 PFU of Ad-MANY/MVA-MANY and the inclusion of MVA-RANKL as adjuvant, all the immunized mice now survived against lethal T. cruzi infection (Fig. 7B). Although we previously demonstrated that recombinant virus vector vaccination against a highly immunogenic murine malaria antigen could protect mice from genetically modified T. cruzi expressing the same malaria antigen (26), this is the first indication that the CD8+-T-cell response against a single CD8+-T-cell epitope of intrinsic T. cruzi antigen was sufficient for controlling lethal T. cruzi infection, if it could be efficiently induced by recombinant virus vector vaccination and adjuvant. It is obvious that we should further define the optimal immunization protocols such as the immunization intervals of both recombinant viruses (4) and should determine how long the induced protective immune responses would last after the booster immunization. It would be also interesting to analyze if the enhanced T-cell-mediated immune responses could have detrimental effects both in the acute and in the chronic phase of T. cruzi infections, since there are few reports regarding this issue in the context of developing T-cell-mediated vaccination strategies. We are particularly interested in the immunopathological outcome caused by T cells during the chronic stage of Chagas' disease. This important issue will be analyzed thoroughly in closely combined with the development of effective T-cell-mediated vaccination strategies.
Our vaccination strategy targeting the induction of immune responses against a single CD8+-T-cell epitope may be applicable for the induction of protective immunity against intracellular pathogens such as human immunodeficiency virus, which tends to be resistant to neutralizing antibodies. Moreover, since antigen-specific CD8+-T-cell responses play a pivotal role not only in protective immunity against infections but also in antitumor immunity, a similar strategy using recombinant viral vaccines and adjuvant may be also useful for efficient induction of tumor-specific CD8+-T-cell responses, for which many CD8+-T-cell epitopes have been identified (5, 33). Further studies are now under way to address these possibilities.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (no. 16590346) from the Ministry of Education, Science, Sports, and Culture of Japan to Y.M. The work was performed in part at the Department of Parasitology, Juntendo University School of Medicine Tokyo, Japan.
We thank Bernard Moss at the National Institutes of Health in Bethesda, Maryland, for providing the pMCO3 transfer vector. We also thank Haruki Uemura at the Institute of Tropical Medicine, Nagasaki University, for helping us draw a view of T. cruzi TSSA. Y.M. expresses sincere appreciation to the warm research environment and useful and constructive discussions at the Atopy Research Center, Juntendo University.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anderson, D. M., E. Maraskovsky, W. L. Billingsley, W. C. Dougall, M. E. Tometsko, E. R. Roux, M. C. Teepe, R. F. DuBose, D. Cosman, and L. Galibert. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175-179. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., B. R. Wong, R. Josien, R. M. Steinman, A. Oxenius, and Y. Choi. 1999. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J. Exp. Med. 189:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. [DOI] [PubMed] [Google Scholar]
- 4.Bruna-Romero, O., G. Gonzalez-Aseguinolaza, J. C. Hafalla, M. Tsuji, and R. S. Nussenzweig. 2001. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct virus vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98:11491-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buus, S., and M. H. Claesson. 2004. Identifying multiple tumor-specific epitopes from large-scale screening for overexpressed mRNA. Curr. Opin. Immunol. 16:137-142. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, M. W., and B. Moss. 1995. E. coli β-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19:352-. 354:356. [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. J. Bett, T. M. Fu, M. E. Davies, A. Tang, K. A. Wilson, M. Chen, R. Long, T. McKelvey, M. Chastain, S. Gurunathan, J. Tartaglia, E. A. Emini, and J. Shiver. 2004. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 78:11434-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chagas, C. 1909. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., agente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1:159-218. [Google Scholar]
- 9.DosReis, G. A. 1997. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol. Today 13:335-342. [DOI] [PubMed] [Google Scholar]
- 10.Earl, P. L., and B. Moss. 1991. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene and Wiley, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 11.Gao, G., T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Novel organization and sequences of five genes encoding all six enzymes for de novo pyrimidine biosynthesis in Trypanosoma cruzi. J. Mol. Biol. 285:149-161. [DOI] [PubMed] [Google Scholar]
- 12.Gherardi, M. M., E. Perez-Jimenez, J. L. Najera, and M. Esteban. 2004. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J. Immunol. 172:6209-6220. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T-cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Aseguinolaza, G., Y. Nakaya, A. Molano, E. Dy, M. Esteban, D. Rodriguez, J. R. Rodriguez, P. Palese, A. Garcia-Sastre, and R. S. Nussenzweig. 2003. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expressing a CD8+-T-cell epitope derived from the circumsporozoite protein of Plasmodium yoelii. J. Virol. 77:11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanke, T., T. J. Blanchard, J. Schneider, C. M. Hannan, M. Becker, S. C. Gilbert, A. V. Hill, G. L. Smith, and A. McMichael. 1998. Enhancement of MHC class I-restricted peptide-specific T-cell induction by a DNA prime/MVA boost vaccination regime. Vaccine 16:439-445. [DOI] [PubMed] [Google Scholar]
- 16.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic t lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josien, R., B. R. Wong, H. L. Li, R. M. Steinman, and Y. Choi. 1999. TRANCE, a TNF family member, is differentially expressed on T-cell subsets and induces cytokine production in dendritic cells. J. Immunol. 162:2562-2568. [PubMed] [Google Scholar]
- 18.Kanegae, Y., M. Makimura, and I. Saito. 1994. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn. J. Med. Sci. Biol. 47:157-166. [DOI] [PubMed] [Google Scholar]
- 19.Katae, M., Y. Miyahira, K. Takeda, H. Matsuda, H. Yagita, K. Okumura, T. Takeuchi, T. Kamiyama, A. Ohwada, Y. Fukuchi, and T. Aoki. 2002. Coadministration of interleukin-12 gene with a Trypanosoma cruzi gene improves vaccine efficacy. Infect. Immun. 70:4833-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff, L. V. 1990. Trypanosoma species (American trypanosomiasis, Chagas disease): biology of trypanosomes. p. 2077-2084. In G. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 21.Kirchhoff, L. V. 1993. American trypanosomiasis (Chagas' disease)-a tropical disease now in the United States. N. Engl. J. Med. 329:639-644. [DOI] [PubMed] [Google Scholar]
- 22.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+-T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minami, M., H. Nariuchi, H. Kawasaki, and M. E. Dorf. 1986. The role of class II MHC molecules in the activation of class I-reactive T-cell hybridomas. J. Immunol. 136:3341-3345. [PubMed] [Google Scholar]
- 24.Miyahira, Y., and J. A. Dvorak. 1994. Kinetoplastidae display naturally occurring ancillary DNA-containing structures. Mol. Biochem. Parasitol. 65:339-349. [DOI] [PubMed] [Google Scholar]
- 25.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 26.Miyahira, Y., S. Kobayashi, T. Takeuchi, T. Kamiyama, T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Induction of CD8+-T-cell-mediated protective immunity against Trypanosoma cruzi. Int. Immunol. 11:133-141. [DOI] [PubMed] [Google Scholar]
- 27.Miyahira, Y., M. Katae, K. Takeda, H. Yagita, K. Okumura, S. Kobayashi, T. Takeuchi, T. Kamiyama, Y. Fukuchi, and T. Aoki. 2003. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect. Immun. 71:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahira, Y., M. Katae, S. Kobayashi, T. Takeuchi, Y. Fukuchi, R. Abe, K. Okumura, H. Yagita, and T. Aoki. 2003. Critical contribution of CD28-CD80/CD86 costimulatory pathway to protection from Trypanosoma cruzi infection. Infect. Immun. 71:3131-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyahira, Y., H. Akiba, M. Katae, K. Kubota, S. Kobayashi, T. Takeuchi, A. Garcia-Sastre, Y. Fukuchi, K. Okumura, H. Yagita, and T. Aoki. 2003. Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-κB gene for inducing antigen-specific CD8+-T-cell response by DNA and virus vector vaccination. J. Immunol. 171:6344-6348. [DOI] [PubMed] [Google Scholar]
- 30.Moore, A. C., and A. V. Hill. 2004. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol. Rev. 199:126-143. [DOI] [PubMed] [Google Scholar]
- 31.Murata, K., Garcia-Sastre, A., M. Tsuji, M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, P. Palese, M. Esteban, and F. Zavala. 1996. Characterization of in vivo primary and secondary CD8+-T-cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 173:96-107. [DOI] [PubMed] [Google Scholar]
- 32.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 33.Oka, Y., A. Tsuboi, T. Taguchi, T. Osaki, T. Kyo, H. Nakajima, O. A. Elisseeva, Y. Oji, M. Kawakami, K. Ikegame, N. Hosen, S. Yoshihara, F. Wu, F. Fujiki, M. Murakami, T. Masuda, S. Nishida, T. Shirakata, S. Nakatsuka, A. Sasaki, K. Udaka, H. Dohy, K. Aozasa, S. Noguchi, I. Kawase, and H. Sugiyama. 2004. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA 101:13885-13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira-Ferreira, J., Y. Miyahira, G. T. Layton, N. Savage, M. Esteban, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and F. Zavala. 2000. Immunogenicity of Ty-VLP bearing a CD8+-T-cell epitope of the CS protein of P. yoelii: enhanced memory response by boosting with recombinant vaccinia virus. Vaccine 17:1863-1869. [DOI] [PubMed] [Google Scholar]
- 35.Prata, A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues, E. G., F. Zavala, D. Eichinger, J. M. Wilson, and M. Tsuji. 1997. Single immunizing dose of recombinant adenovirus efficiently induces CD8+-T-cell-mediated protective immunity against malaria. J. Immunol. 158:1268-1274. [PubMed] [Google Scholar]
- 37.Rolph, M. S., and I. A. Ramshaw. 1997. Recombinant viruses as vaccines and immunological tools. Curr. Opin. Immunol. 9:517-524. [DOI] [PubMed] [Google Scholar]
- 38.Rottenberg, M. E., A. Riarte, L. Sorrong, J. Altcheh, P. Petray, A. M. Ruiz, and H. Wigzell. 1995. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 45:53-60. [DOI] [PubMed] [Google Scholar]
- 39.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 40.Stephenson, J. R. 2001. Genetically modified viruses: vaccines by design. Curr. Pharm. Biotechnol. 2:47-76. [DOI] [PubMed] [Google Scholar]
- 41.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 43.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 44.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 45.Theill, L. E., W. J. Boyle, and J. M. Penninger. 2002. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20:795-823. [DOI] [PubMed] [Google Scholar]
- 46.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas' disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 47.Wiethe, C., K. Dittmar, T. Doan, W. Lindenmaier, and R. Tindle. 2003. Enhanced effector and memory CTL responses generated by incorporation of receptor activator of NF-κB (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J. Immunol. 171:4121-4130. [DOI] [PubMed] [Google Scholar]
- 48.Williamson, E., J. M. Bilsborough, and J. L. Viney. 2002. Regulation of mucosal dendritic cell function by receptor activator of NF-κB (RANK)/RANK ligand interactions: impact on tolerance induction. J. Immunol. 169:3606-3612. [DOI] [PubMed] [Google Scholar]
- 49.Wizel, B., Rogers, W. O., Houghten, R. A., Lanar, D. E., Tine, J. A., and S. L. Hoffman. 1994. Induction of murine cytotoxic T lymphocytes against Plasmodium falciparum sporozoite surface protein 2. Eur. J. Immunol. 24:1487-1495. [DOI] [PubMed] [Google Scholar]
- 50.Wizel, B., M. Nunes, and R. L. Tarleton. 1997. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J. Immunol. 159:6120-6130. [PubMed] [Google Scholar]
- 51.Wizel, B., N. Garg, and R. L. Tarleton. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong, B. R., R. Josien, S. Y. Lee, B. Sauter, H. L. Li, R. M. Steinman, and Y. Choi. 1997. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J. Exp. Med. 186:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 54.Zavala, F., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and M. Esteban. 2001. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280:155-159. [DOI] [PubMed] [Google Scholar]