Abstract
Clostridium perfringens type D enterotoxemias have significant economic impact by causing rapid death of several domestic animal species. Consequently, domestic animals are commonly vaccinated, at varying efficacy, with inactivated type D vegetative supernatants. Improved type D vaccines might become possible if the lethal toxins produced by type D isolates were characterized and the contributions of those toxins to supernatant-induced lethality were established. Therefore, the current study evaluated the presence of lethal toxins in supernatants prepared from late-log-phase vegetative cultures of a large collection of genotype D isolates. Under this growth condition, most genotype D isolates produced variable levels of at least three different lethal toxins, including epsilon-toxin (ETX). To model the rapid lethality of type D enterotoxemias, studies were conducted involving intravenous (i.v.) injection of genotype D vegetative supernatants into mice, which were then observed for neurotoxic distress. Those experiments demonstrated a correlation between ETX (but not alpha-toxin or perfringolysin O) levels in late-log-phase genotype D supernatants and lethality. Consistent with the known proteolytic activation requirement for ETX toxicity, trypsin pretreatment was required for, or substantially increased, the lethality of nearly all of the tested genotype D vegetative supernatants. Finally, the lethality of these trypsin-pretreated genotype D supernatants could be completely neutralized by an ETX-specific monoclonal antibody but not by an alpha-toxin-specific monoclonal antibody. Collectively, these results indicate that, under the experimental conditions used in the present study, ETX is necessary for the lethal properties of most genotype D vegetative supernatants in the mouse i.v. injection model.
Clostridium perfringens is an important cause (19) of both histotoxic infections (e.g., human gas gangrene) and enteric diseases (e.g., C. perfringens type A human food poisoning and severe enterotoxemias in domestic animals). The virulence of C. perfringens is largely attributable to its ability to produce >15 different toxins, several of which have lethal properties (15, 20). However, individual isolates of this bacterium do not express this entire toxin repertoire, providing the basis for a classification scheme (15, 20) that assigns C. perfringens isolates to one of five different toxinotypes (type A to E) depending upon their production of four (α, β, ɛ, and ι) lethal toxins. With the exception of alpha-toxin (CPA), the typing toxins are encoded by genes present on large plasmids (28).
In sheep, goats, and probably other domestic animals, C. perfringens type D isolates cause enterotoxemias that initiate with production of toxins in the intestines. Those toxins (including epsilon-toxin [ETX], a CDC/USDA overlap select toxin) can be absorbed through the intestinal mucosa (18) and then spread via the circulation to internal organs, where they cause blood pressure elevation and fluid accumulation in body cavities, as well as edema in several organs, notably brain, heart, lungs, liver, and kidney (24, 29). Type D enterotoxemias can result in peracute, acute, or chronic disease (18). In sheep, these infections primarily produce neurologic signs, which may or may not include classical brain edema-induced focal symmetrical encephalomalacia, often resulting in sudden death (18). Similar peracute and acute neurologic disease, including sudden death, is also observed in type D enterotoxemias of kids and some adult goats, whereas other adult goats develop a chronic gastrointestinal form of type D enterotoxemia that is characterized by a fibrinonecrotic colitis (18).
Understanding the rapid lethality associated with many cases of type D enterotoxemia could lead to improved vaccine design. In the absence of a well-characterized, small animal oral-challenge model, intravenous (i.v.) injection of vegetative culture supernatants into mice is commonly used to study the systemic lethality associated with type D enterotoxemias. However, the potential presence of several lethal toxins in those type D supernatants could complicate interpretation of mouse i.v. injection results. For example, vegetative cultures of type D isolates (by definition) produce at least two potent lethal toxins, i.e., ETX and CPA. Although not yet systematically evaluated with a large isolate collection, some or all type D isolates could produce additional lethal toxins, such as perfringolysin O (PFO), enterotoxin (CPE), or beta2 toxin (CPB2). Variations in lethal toxin levels among type D vegetative culture supernatants could impact their lethal activity. For example, some of those supernatants might possess sublethal ETX concentrations but lethal CPA concentrations. However, to date, variations in supernatant lethal toxin levels have not been assessed with a sizeable collection of type D isolates. Finally, although the effects of i.v. injection of some pure C. perfringens toxins into animals have been well studied, the relative contribution of different toxins to the lethal properties of type D vegetative culture supernatants has not yet been rigorously determined.
In response, the present study genotypically and phenotypically characterized lethal toxin production by a large collection of type D isolates. Collectively, several results from the present study support the importance of ETX in causing the mouse lethality induced by i.v. injection of late log-phase vegetative supernatants prepared from most type D isolates.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
Of the 47 putative type D strains examined in the present study, 30 originated from the Burroughs-Wellcome (BW) collection; those BW isolates were primarily gathered from diseased animals during the 1940s to 1960s and had been stored in a lyophilized form. They were kindly provided by Russell Wilkinson (University of Melbourne). Ten other isolates were recent North American animal disease isolates that were kindly provided by J. Glenn Songer (University of Arizona). The remaining seven isolates examined in the present study came from our laboratory collections and had diverse origins, mostly including veterinary infections.
All C. perfringens isolates were initially grown overnight at 37°C under anaerobic conditions on TSC agar media (SFP agar [Difco Laboratories], 0.04% d-cycloserine [Sigma Aldrich]) to ensure culture purity. Unless otherwise specified, FTG (fluid thioglycolate medium; Difco Laboratories) or TGY (3% tryptic soy broth [Becton-Dickinson]; 2% glucose [Sigma Aldrich], 1% yeast extract [Becton-Dickinson], 0.1% l-cysteine [Sigma Aldrich]) were used for growing broth cultures.
Multiplex PCR.
Brain heart infusion agar (Becton-Dickinson) plates were inoculated with a putative type D isolate and then grown anaerobically overnight at 37°C. Three or four colonies were picked from each plate and used to prepare template DNA as described previously (37). These DNA preparations were then subjected to a multiplex PCR assay (10) capable of detecting six genes encoding C. perfringens lethal toxins or lethal toxin components, i.e., the CPA gene (plc), the beta-toxin gene (cpb), the CPB2 gene (cpb2), the CPE gene (cpe), the ETX gene (etx), and the iap gene encoding the A component of iota toxin. Products from each multiplex PCR were electrophoresed on 2% agarose gels; after electrophoresis, these gels were stained with ethidium bromide for visualization. Isolates carrying both plc and etx genes are genotypically type D and henceforth are referred to as genotype D isolates (10).
Optimization of vegetative culture conditions for ETX production.
A single isolated colony from a TSC plate streaked with a type D isolate was inoculated into 10 ml of FTG medium, which was then incubated overnight at 37°C. A 0.1-ml aliquot of each overnight culture was then inoculated into 10 ml of FTG, TGY, brain heart infusion broth (Difco), or differential reinforced clostridial broth (EM Science). Those cultures were grown at 37°C, with aliquots of each culture removed at specific times. For each removed culture aliquot, optical density at 600 nm values were determined prior to centrifugation. Each resultant supernatant was then mixed with an equal volume of protein sample buffer before boiling for 10 min and loading on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gel for ETX Western blotting (see below).
Preparation of vegetative culture supernatants from genotype D isolates.
Single isolated colonies of genotype D isolates were inoculated into 10 ml of FTG medium, which was incubated overnight at 37°C. Based upon the pilot ETX production studies described above (see Results), a 0.1-ml aliquot of each overnight culture was inoculated into 10 ml of TGY, and these cultures were grown at 37°C to late log phase. Bacteria were removed from each TGY culture by centrifugation, and the resultant supernatants were filter sterilized with a 0.45-μm-pore-size filter.
Quantification of toxin levels in supernatants from genotype D isolates. (i) ETX.
For stronger (>400 ng of ETX/ml) ETX producers, sterile late-log-phase supernatants (prepared as described above) were diluted, as needed, to bring their ETX levels within the standard curve range of purified ETX used for Western blot quantification (see Results). For weaker (<400 ng of ETX/ml) ETX producers, the sterile supernatants were concentrated 10-fold using Amicon Ultra-15 centrifugal ultrafiltration devices (10,000 molecular weight cutoff). All sterile supernatants were mixed with SDS sample buffer, boiled for ∼10 min, and electrophoresed on 10% acrylamide gels containing SDS. After electrophoresis, separated supernatant proteins (and pure ETX standards) were transferred onto a nitrocellulose membrane (Bio-Rad Laboratories). For ETX Western immunoblotting (31), an ETX-specific monoclonal antibody (5B7; kindly provided by Paul Hauer, Center for Veterinary Biologics, Ames, Iowa) was used as primary antibody, followed by rabbit anti-mouse immunoglobulin G (IgG)-peroxidase conjugate (Sigma) as a secondary antibody. ETX Western blots run with normal (nonconcentrated) or concentrated supernatants were then developed with SuperSignal West Pico or West Femto chemiluminescent substrate (Pierce), respectively. For each genotype D isolate, supernatant ETX levels were quantified for three independent cultures. All Western blot results were analyzed and preserved using a Bio-Rad ChemiDoc imaging system.
(ii) CPB2.
To quantify CPB2 toxin levels, an aliquot of sterile late-log-phase supernatant from a culture of cpb2-positive genotype D isolate was concentrated 10- to 50-fold using an Amicon Ultra-15 device prior to mixing with protein sample buffer. Each concentrated vegetative supernatant was then boiled and electrophoresed on a 12% acrylamide gel containing SDS. A dilution series of CPB2, purified to homogeneity as described previously (9), was also run on each gel to construct a standard curve. After electrophoresis and sample transfer onto nitrocellulose, Western immunoblotting was performed using a rabbit polyclonal CPB2 antiserum (kindly provided by Michel Popoff, Institut Pasteur, Paris, France) as primary antibody, followed by goat anti-rabbit IgG-peroxidase conjugate (Sigma) as a secondary antibody. Blots were developed with SuperSignal West Pico chemiluminescent substrate. For each cpb2-positive genotype D isolate, supernatant CPB2 levels were quantified for three independent cultures.
(iii) CPE.
CPE production by sporulating cultures of cpe-positive genotype D isolates was assessed by inoculating a 0.1-ml aliquot of an overnight FTG culture into 10 ml of Duncan-Strong (DS) sporulation medium (17, 30). Those cultures were then incubated for ∼14 h, at which time sporulation was assessed by phase-contrast microscopy. Sporulating cells in the cultures were sonicated to release their internal CPE. After centrifugation, supernatants from the sonicated sporulating culture lysates were used for CPE Western blotting (17).
For CPE Western blotting, a 100-μl aliquot of supernatant from each sporulating (prepared as described above) or vegetative (prepared as described for ETX Western blotting) culture was mixed with 100 μl of SDS sample buffer, and 30 μl of that mixture was then electrophoresed on 10% acrylamide gels containing SDS (no sample boiling). To quantify CPE levels present in each supernatant, a dilution series of purified CPE (21) was run on the gel to establish a standard curve. After electrophoresis and transfer onto nitrocellulose, CPE Western blotting was performed with a rabbit polyclonal CPE antiserum as primary antibody, followed by a goat anti-rabbit IgG-peroxidase conjugate (Sigma) as a secondary antibody. Blots were developed with SuperSignal West Pico chemiluminescent substrate. For each genotype D isolate, supernatant CPE levels were quantified for three independent sporulating and vegetative cultures.
(iv) CPA.
A 10-ml aliquot of sterile late-log-phase supernatant from genotype D vegetative culture supernatant, prepared as described above for the ETX Western blots, was freeze dried. That lyophilized material was then resuspended in 1 ml of phosphate buffered saline (pH 7.4) and subjected to a phospholipase C (PLC) activity assay using nutrient agar supplemented with 4% egg yolk (vol/vol) as described previously (32). C. perfringens PLC (Sigma) was used as a standard for quantifying CPA activity. Total protein present in each 10-fold-concentrated vegetative supernatant sample was determined by using the BCA kit (Pierce), and the specific activity was expressed as PLC units/mg of total protein.
(v) PFO.
A 1-ml aliquot of sterile late log-phase supernatant from a vegetative genotype D culture (prepared as described above) was subjected to a series of twofold dilutions with 5 mM dithiothreitol (Roche) in DPBS. These diluted samples were then subjected to a quantitative PFO assay using horse red blood cells (Biolab, Melbourne, Australia) as described previously (34). The PFO titer was defined as the reciprocal of the last dilution showing complete hemolysis, as indicated by a significant decrease in absorbance at 570 nm recorded with a Multiskan spectrophotometer (Labsystems).
Mouse lethality assay of genotype D vegetative culture supernatants.
For initial toxicity testing, each sterile late log-phase genotype D supernatant (prepared as described above) was divided into two aliquots. Because trypsin activation is necessary for ETX activation (23, 35), one of the paired supernatant aliquots was treated with 0.05% trypsin for 30 min at 37°C, whereas the other aliquot was similarly incubated without trypsin. Two BALB/c mice (male or female, ca. 17 to 20 g; Charles River Laboratories) each received an i.v. injection (tail vein) of 0.5 ml of the trypsinized (i.e., trypsin-treated) supernatant, while two other mice each received a similar 0.5-ml i.v. injection of the nontrypsinized supernatant. All mice were observed for up to 48 h to monitor the development of significant neurological distress, at which point those mice were immediately euthanized with CO2. Our IACUC permit did not allow death as a routine expected experimental endpoint, so supernatants that produced significant neurologic distress (defined by the development of one or more of the following signs: incoordination, ataxia, paralysis, blindness, or convulsions) within 48 h were considered to possess lethal activity. However, pilot experiments indicated that death typically follows the onset of neurologic distress in mice receiving i.v. injections of genotype D late-log-phase supernatants.
For vegetative culture supernatants inducing neurologic distress, a 50% lethal dose (LD50)/ml was determined with additional pairs of mice, who received i.v. injections containing twofold dilutions (between 1/50 to 1/800) in 1% peptone water of late-log-phase supernatant aliquots that were or were not trypsinized (as described above). Negative and positive control mice were also included in each assay, with negative control mice receiving an i.v. injection of 1% peptone water that did or did not contain trypsin (two mice each). Positive control mice received i.v. injections containing twofold dilutions of a filtered C. perfringens type D (CPE-negative and CPB2-negative) late-log-phase supernatant of known toxicity, given at the same dilutions used for the test samples. The toxin titration was calculated as double the reciprocal of the highest dilution inducing lethality, within 48 h, in at least one of the two paired mice; this result was then expressed as the 50% mouse lethal dose/ml.
For these lethality assays, at least two batches of each vegetative culture supernatant were prepared and tested in mice as described above. Lethality results were first averaged for each supernatant batch, followed by averaging the means for the two or more different supernatant preparations tested for each genotype D isolate. If results for different preparations of late-log-phase supernatant for a particular isolate showed >3-fold dilution difference, the samples were retested. All experimental procedures were approved by the Animal Care and Use Committee of the California Animal Health and Food Safety Laboratory, University of California, Davis (permit 34).
Neutralization of genotype D vegetative culture supernatant lethality.
To help identify which toxins are responsible for the lethal activity of supernatants prepared from late-log-phase genotype D cultures, monoclonal antibody (MAb) neutralization experiments were performed as follows.
(i) Preincubation of ETX MAb with genotype D vegetative culture supernatants.
A 0.6-ml volume of an undiluted genotype D late-log-phase supernatant, prepared as described above, was divided into two aliquots that either were or were not trypsinized as described above. The paired aliquots were then filter sterilized prior to the addition of 0.1 ml of a solution containing 2 mg of ETX-neutralizing MAb 5B7/ml. Those mixtures were then brought to 1.2 ml with 1% peptone water and incubated for 30 min at room temperature. A 0.5-ml aliquot of each mixture was injected i.v. into two mice, while an additional pair of mice received a similar i.v. injection of the same sterile trypsinized or nontrypsinized supernatants that had been identically prepared, except for the omission of ETX MAb.
(ii) Preincubation of CPA MAb with genotype D vegetative culture supernatants.
Filter-sterilized supernatants from late-log-phase cultures of six representative C. perfringens genotype D strains were incubated with 2 mg of an anti-CPA MAb (kindly provided by P. Hauer)/ml. Mouse inoculations using those mixtures were performed as described above for ETX neutralization studies.
(iii) Preincubation of CPE MAb with genotype D vegetative culture supernatants.
Sterile supernatants were prepared from late log-phase cultures of four selected C. perfringens genotype D strains as described above. The sterile supernatants were then preincubated at room temperature for 30 min with 0.1 ml of a solution containing 2 mg of the CPE-neutralizing MAb 3C9 (38)/ml before being i.v. injected into mice, as described for ETX supernatant neutralization experiments.
MAb neutralization of semipurified CPA.
A 0.5-ml aliquot (containing a 30 LD50 dose) of semipurified, ultrafiltered CPA was obtained from a vaccine production batch (CSL, Ltd., Melbourne, Australia) prepared from an ovine isolate of C. perfringens type A. That toxin preparation was preincubated at room temperature for 30 min with 0.1 ml of a solution containing 2 mg of either the same CPA- or ETX-neutralizing MAb/ml used in the genotype D supernatant neutralization experiments described above. A 0.5-ml aliquot of each CPA-antibody solution was then injected i.v. into mice.
MAb neutralization of purified ETX.
ETX was purified to homogeneity using a modification of the classical method of Habeeb (14). Briefly, late-log-phase supernatant from type D isolate NCTC8346 was precipitated with 45% ammonium sulfate, followed by successive ion-exchange chromatography on Macro-Prep DEAE support (Bio-Rad) and Macro-Prep CM cation-exchange support (Bio-Rad). A 0.5-ml aliquot of pure ETX was then activated with trypsin (as described above) and preincubated at room temperature for 30 min with 0.1 ml of a solution containing 2 mg of either the same CPA- or ETX-neutralizing MAb/ml used in the genotype D supernatant neutralization experiments described above. A 0.5-ml aliquot of each ETX-antibody solution was then injected i.v. into mice.
RESULTS
Lethal toxin gene carriage by putative C. perfringens type D isolates.
Multiplex PCR analysis was performed to survey the presence of genes encoding six major C. perfringens lethal toxins among a collection of 47 putative type D isolates. The 30 surveyed isolates from the BW collection had been previously classified as type D many years ago using the classical toxin neutralization typing method (15, 33). Multiplex PCR analyses confirmed (data not shown) that 22 of the 30 BW isolates are genotype D, since they carry plc and etx genes but not cpb or iap genes (10). Of the remaining eight BW isolates formerly classified as type D by classical toxin neutralization approaches, seven were identified as genotype A (i.e., these isolates carry the plc gene, but not the etx gene required of genotype D isolates), while the other putative type D isolate from the BW collection was found to classify as genotype B (i.e., it carries the plc, etx, and cpb genes). Similar multiplex PCR analysis was also performed on 10 recent animal disease isolates, which confirmed their previous identification by J. G. Songer (unpublished data) as genotype D. Finally, multiplex PCR analysis of seven other putative type D isolates collected from various other sources confirmed their identity as genotype D.
The 39 isolates identified as genotype D by multiplex PCR analysis were heterogeneous with respect to their carriage of genes encoding two other lethal toxins, i.e., CPE and CPB2. Nearly 70% of these genotype D isolates were found to lack both the cpe and cpb2 genes, while the remaining 12 genotype D isolates carried cpe, cpb2, or both the cpe and the cpb2 genes (Table 1).
TABLE 1.
Multiplex PCR analysis of genotype D isolates
| No. of isolates (%) | Genotype |
|---|---|
| 27 (69) | plc etx |
| 5 (13) | plc etx cpe |
| 5 (13) | plc etx cpe cpb2 |
| 2 (5) | plc etx cpb2 |
Lethal toxin levels in genotype D vegetative culture supernatants.
Phenotypic studies were then conducted to evaluate whether the 39 surveyed genotype D isolates express their lethal toxin genes during vegetative growth and to establish whether these isolates produce PFO, a lethal toxin whose gene (pfoA) is not detected by the conventional multiplex PCR assay. PLC activity on egg yolk agar plates and hemolytic activity against horse erythrocytes were measured to determine CPA or PFO activity levels, respectively, in vegetative culture supernatants of genotype D isolates. Because specific activity assays are not available for ETX, CPB2, or CPE, the presence of these three lethal toxins in genotype D supernatants was assessed by Western blotting.
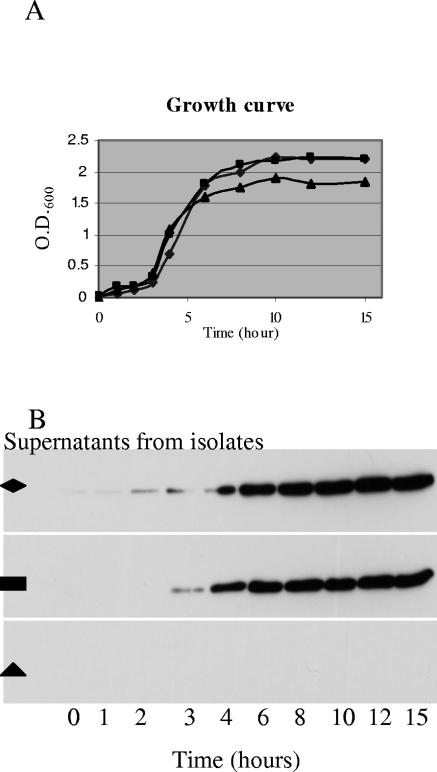
Since our genotype D supernatant characterization studies would eventually also include extensive lethality determinations, which would use large numbers of mice, we could only choose a single growth condition for preparing vegetative supernatants from genotype D isolates. Before picking that growth condition for vegetative supernatant characterization studies, pilot studies were conducted to optimize conditions for ETX production by type D vegetative cultures. Those studies revealed (data not shown) that, of four tested media (TGY, FTG, brain heart infusion, and differential reinforced clostridial broth), TGY most consistently supported ETX production by several randomly selected D isolates. Additional pilot studies with three genotype D isolates growing in TGY then determined (Fig. 1) that one genotype D isolate failed to produce detectable levels of ETX at any point in the growth curve. However, the other two pilot isolates produced large amounts of ETX by 8 to 10 h (i.e., by late log phase), with their ETX levels then remaining relatively constant for several hours thereafter. Since, (i) late log phase is also optimum for CPB2 and CPA production (5, 9) and (ii) late-log-phase cultures also express PFO (25), supernatants from late-log-phase culture were used for all further studies characterizing the toxin content and lethality of type D vegetative culture supernatants.
FIG. 1.
Pilot studies to optimize ETX expression in genotype D vegetative cultures. Three randomly selected genotype D isolates (▪, ▴, and ⧫) were inoculated into TGY medium and incubated at 37°C. (A) At the specified times, aliquots were removed from each culture and the optical density at 600 nm of the aliquot was determined with a spectrophotometer. (B) After centrifugation, supernatant ETX levels for each isolate were evaluated by Western blotting, as described in the Materials and Methods. The results shown are representative of two repetitions. Note the absence of any detectable ETX in the supernatant of isolate (▴).
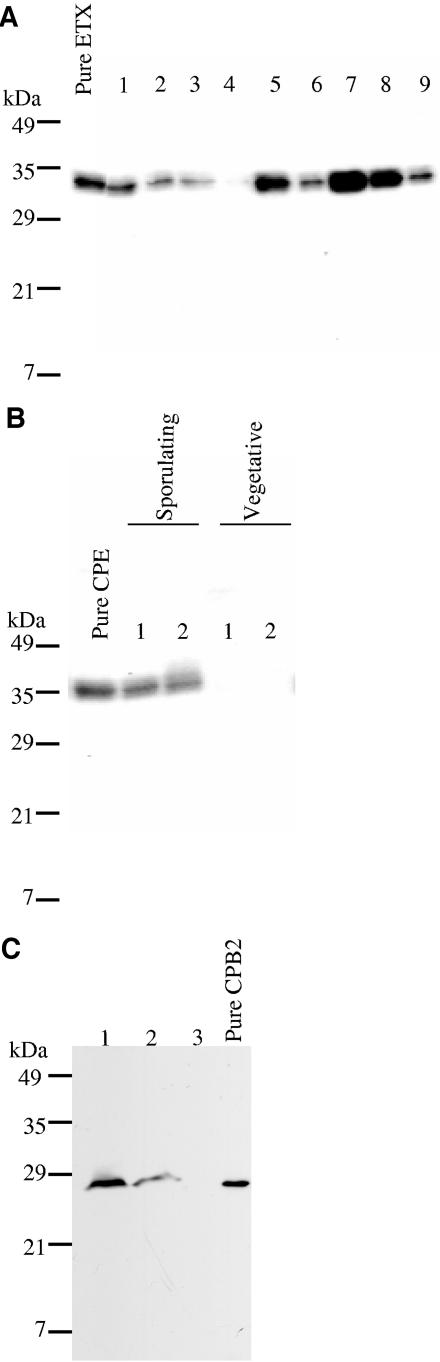
Using those supernatants, Western blot analyses revealed (see Fig. 2A, for representative results) significant variations in supernatant ETX levels among the surveyed genotype D isolates. Late-log-phase culture supernatants from two genotype type D isolates contained no detectable ETX, even using 10-fold-concentrated supernatants and a highly sensitive Western blot detection substrate, while similar supernatants from 13 other genotype D isolates had only low ETX levels (i.e., <1.7 μg of ETX/ml of supernatant). However, medium to high ETX levels (i.e., >1.7 to 53 μg of ETX/ml) were detected in late-log-phase supernatants prepared from the 24 remaining genotype D isolates.
FIG.2.
Western blot analysis of ETX, CPE, and CPB2 toxin production by genotype D isolates. (A) Variations in ETX production by representative genotype D isolates probed with a monoclonal antibody against ETX. Pure epsilon-toxin (far left lane) was also electrophoresed as a control. (B) Variations in CPE production by sporulating and vegetative culture of representative cpe-positive genotype D isolates probed with CPE polyclonal antiserum. The pure CPE lane shows sporulating culture; sporulating culture lanes 1 and 2 show supernatants of two cpe-positive genotype D isolates grown in DS sporulation medium. Vegetative culture lanes 1 and 2 show late-log-phase culture supernatant of these same two isolates grown in TGY medium. (C) Variations in CPB2 toxin production by three representative cbp2-positive type D isolates probed with a polyclonal antibody against CPB2 toxin. Pure CPB2 toxin (far right lane) was also electrophoresed as a control.
As previously established for cpe-positive type A isolates (7), CPE expression by cpe-positive genotype D isolates was strongly sporulation associated. None of the 10 surveyed cpe-positive isolates produced any CPE during vegetative growth (see Fig. 2B for representative Western blot results). However, the eight cpe-positive genotype D isolates that were able to sporulate in DS sporulation medium also could produce CPE in that medium (representative results shown in Fig. 2B). The remaining two cpe-positive type D isolates neither sporulated nor produced CPE in DS medium.
Of the seven cpb2-positive genotype D isolates surveyed, six were able to express CPB2 during late-log-phase growth (Fig. 2C). However, CPB2 production by those isolates was modest (ranging up to 0.4 μg/ml), becoming detectable only when 50-fold-concentrated vegetative supernatants were used for Western blotting.
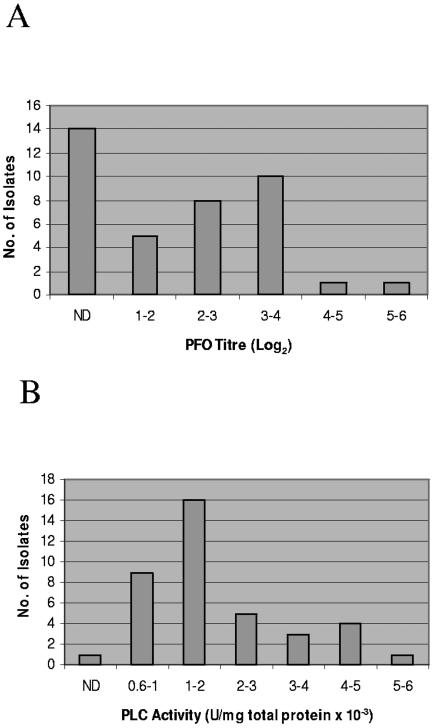
Nearly two-thirds of these 39 genotype D isolates were found to produce both CPA and PFO activity during late log phase (Fig. 3). However, similar vegetative supernatants from one third of the surveyed isolates contained no detectable PFO activity (Fig. 3A), while late-log-phase supernatant from one PFO-positive genotype D isolate contained no detectable CPA activity (Fig. 3B). When present, PFO or CPA activity levels in these vegetative supernatants showed considerable isolate-to-isolate variation. PFO activity levels (Fig. 3A) in late-log-phase supernatants ranged from 1 to 5.5 (log2 titer), while their CPA activity levels (Fig. 3B) ranged from 0.6 to 5.1 PLC units/mg × 10−3. No consistent correlations (i.e., R2 values were always <0.4) were observed between the production of ETX, CPA, or PFO by individual genotype D isolates (data not shown).
FIG. 3.
PFO (A) or alpha-toxin (B) activities in vegetative supernatants of surveyed genotype D isolates. Isolates are grouped based upon the mean of three independent toxin activity determinations. ND, no significant activity detected.
Lethal properties of genotype D vegetative culture supernatants.
Collectively, the results shown in Fig. 2 and 3 indicated that late-log-phase cultures of nearly all genotype D isolates produce multiple lethal toxins, although with considerable variation in amounts. Therefore, 21 of these genotype D isolates were selected for further study to evaluate the contribution of various toxins to the lethal properties of their vegetative supernatants in the mouse i.v. injection model. These 21 isolates included a range of CPA, PFO, and ETX producers (Table 2), including one non-ETX producer that expresses medium amounts of CPA. In addition, those selected genotype D isolates included one expressing CPB2 and 3 others that are cpe-positive (although they do not express CPE during vegetative growth).
TABLE 2.
Supernatant neutralization with different antibodies
| Supernatant | Vegetative culture (phenotype 1)a | Epsilon antibody | Alpha antibody |
|---|---|---|---|
| 1 | PLC(4.2), PFO(5.5), ETX(53) | Yes | NDb |
| 2 | PLC(1.6), PFO(<1), ETX(17) | Yes | ND |
| 3 | PLC(2.5), PFO(<1), ETX(6) | Yes | No |
| 4 | PLC(<1), PFO(1.8), ETX(6) | Yes | ND |
| 5 | PLC(1.9), PFO(<1), ETX(25) | Yes | ND |
| 6 | PLC(2.1), PFO(<1), ETX(13) | Yes | ND |
| 7 | PLC(4.6), PFO(3.5), ETX(5) | Yes | ND |
| 8 | PLC(2.1), PFO(<1), ETX(8) | Yes | No |
| 9 | PLC(1.5), PFO(<1), ETX(10) | Yes | No |
| 10 | PLC(1.2), PFO(1.6), ETX(5) | Yes | ND |
| 11 | PLC(<1), PFO(2.7), ETX(3) | ND | ND |
| 12 | PLC(5.1), PFO(2.9), ETX(2) | Yes | ND |
| 13 | PLC(2.1), PFO(<1), ETX(8) | ND | ND |
| 14 | PLC(3.8), PFO(2.9), ETX(4) | Yes | No |
| 15 | PLC(1.1), PFO(<1), ETX(4) | Yes | No |
| 16 | PLC(<1), PFO(2.7), ETX(3) | ND | ND |
| 17 | PLC(1.4), PFO(<1), ETX(<1) | ND | ND |
| 18 | PLC(2.0), PFO(3.7), ETX(52), CPE(0) | Yes | ND |
| 19 | PLC(4.6), PFO(3.5), ETX(2), CPE(0) | ND | ND |
| 20 | PLC(2.1), PFO(<1), ETX(13) | ND | ND |
| 21 | PLC(<1), PFO(1.6), ETX(5), CPB2(0.03) | ND | ND |
| Alpha-toxin | PLC | No | Yes |
Values shown in subscript parentheses for ETX, CPB2, and CPE are micrograms of protein/ml and for PFO and PLC are activity levels (log2 titer or U/ml [10−3], respectively).
ND, not determined.
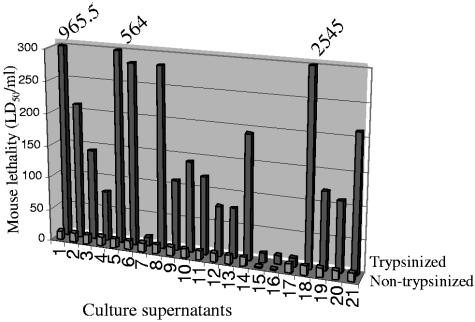
Several of the lethal toxins produced by late-log-phase cultures of genotype D isolates in Fig. 2 and 3 can be trypsin sensitive (11, 13), as confirmed by our pilot study demonstrating that a 30-min pretreatment with 0.05% trypsin completely abrogates the lethality of a 30 LD50/ml dose of semipurified CPA. In contrast, the ETX prototoxin must be proteolytically activated to acquire lethal properties (23, 35), which we confirmed by demonstrating that a 30-min preincubation with 0.05% trypsin decreases the LD50 of pure ETX prototoxin from 1 to 10 μg/ml to ∼12.5 ng/ml (∼312 ng/kg). Because of these trypsin sensitivity differences, initial experiments were performed to determine whether similar trypsin pretreatment affects the lethal properties of late-log-phase supernatants prepared from the 21 selected genotype D isolates (Fig. 4). These studies revealed that, without trypsin pretreatment, these supernatants cause little or no lethality when injected i.v. into mice. Nor was lethality observed in mice receiving injections of trypsin-containing buffer alone. However, trypsin pretreatment initiated, or substantially increased, the lethality of late-log-phase supernatants from 19 of the 21 tested genotype D isolates. A non-ETX producer was one of the two genotype D isolates whose vegetative supernatant did not show trypsin enhancement of lethality; even undiluted, late-log-phase supernatant from that ETX-negative isolate was not consistently lethal, with or without trypsin pretreatment.
FIG. 4.
Mouse lethality assay results with trypsinized or nontrypsinized genotype D supernatants. The front row shows mouse lethality results with a nontrypsinized aliquot of vegetative culture supernatants prepared from 21 different genotype D isolates (see Table 2 for their phenotype). The back row depicts mouse lethality results for the same genotype D vegetative supernatants after trypsinization. Numbers at top of supernatants 1, 5, and 18 had lethality levels too large to depict on this graph. The results shown are the mean of two independent determinations. Note that culture supernatants were arbitrarily assigned numbers 1 to 21 to avoid possible misuse by identifying genotype D isolates producing highly lethal supernatants. Genotype D isolates producing supernatants 1 to 4, 7 to 12, 14, and 18 to 20 are animal disease isolates from the BW collection; isolates producing supernatants 13, 17, and 21 are recent North American animal disease genotype D isolates provided by Glenn Songer; and the remaining supernatants (5, 6, and 16) were from animal disease genotype D isolates obtained from miscellaneous sources.
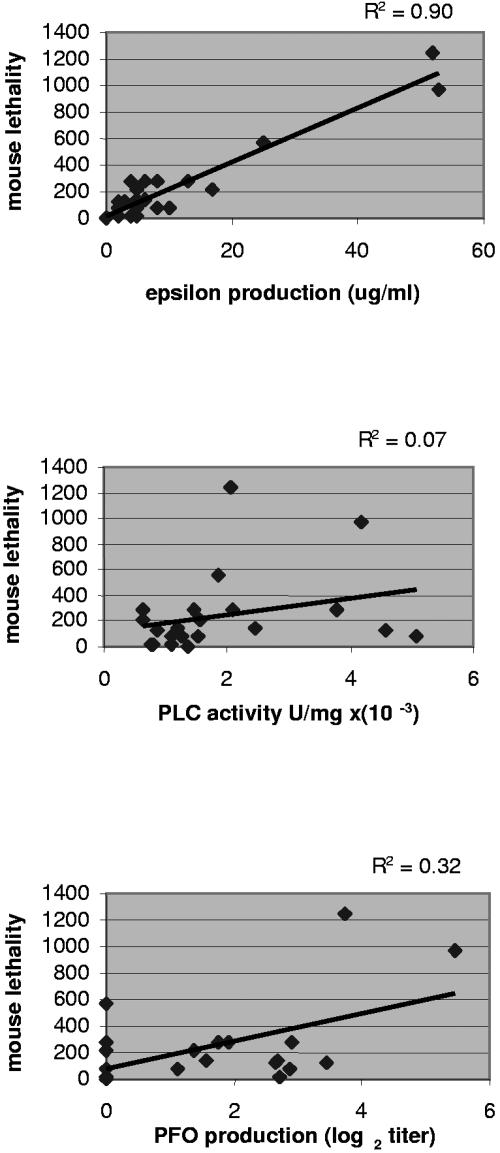
Since the Fig. 4 results were consistent with ETX playing an important role in genotype D vegetative supernatant-induced lethality, the lethal toxin content of late-log-phase supernatants from these 21 isolates were compared against their trypsin-activated lethality, if any. Those analyses revealed a strong correlation between the ETX levels of these trypsin-activated supernatants and their lethality (Fig. 5, top panel). Similar comparisons indicated that neither CPA (Fig. 5, middle panel) nor PFO (Fig. 5, bottom panel) activity levels significantly correlate with trypsin-activated lethality of late-log-phase supernatants. No attempt was made to correlate CPE or CPB2 expression with lethality because Fig. 2 (middle and bottom panels) results demonstrated that little or none of those two toxins are present in vegetative culture supernatants of genotype D isolates.
FIG. 5.
Correlations between mouse lethality and lethal toxin levels in the vegetative supernatants of genotype D isolates. (Top panel) Regression analysis of late-log-phase supernatant ETX levels (μg/ml) versus mouse lethality. Regression analyses of late-log-phase supernatant alpha-toxin (middle panel) or PFO (bottom panel) levels versus mouse lethality are also shown. For the bottom panel, isolates graphed at 0 on the x axis had an undetectable PFO activity level. Mouse lethality results shown represent the mean of two independent determinations and toxin level, or activity determinations represent the mean of three independent determinations. The R2 shown for each panel is the correlation coefficient.
MAb neutralization of genotype D vegetative supernatant lethality.
To definitively evaluate whether ETX is required for the lethal properties of most trypsin-activated genotype D vegetative culture supernatants, MAb neutralization experiments were performed. Preincubation of neutralizing anti-ETX MAb 5B7 with each of 14 different genotype D late-log-phase supernatants, all containing ETX and exhibiting trypsin-activated lethality in Fig. 4 and 5 experiments, completely neutralized their lethality for mice (Table 2). Furthermore, this anti-ETX MAb also neutralized the lethal activity of late-log-phase supernatant from the one ETX-producing genotype D isolate that did not exhibit enhanced lethality after trypsin pretreatment in Fig. 4 studies. In contrast, preincubation of an anti-CPA MAb with trypsin-activated late-log-phase supernatants from 5 of these 14 genotype D isolates failed to neutralize their lethality, even though these 5 isolates included the strongest CPA and PFO producers analyzed in this survey.
Several controls support the specificity of these neutralization results. First, under preincubation conditions identical to those used above with the genotype D supernatants, the anti-ETX MAb neutralized the lethality of 5 μg of pure trypsin-activated ETX but not a 30 LD50 dose of semipurified CPA. However, that CPA dose (but not activated ETX) was neutralized by similar preincubation with an anti-CPA MAb. A final control for antibody specificity included the failure of an anti-CPE MAb to neutralize the lethality of trypsin-pretreated late-log-phase supernatants from five selected genotype D isolates.
DISCUSSION
In domestic ruminants, Clostridium perfringens type D isolates are a major cause of fatal enterotoxemias (18, 33). To reduce the economic impact of these enterotoxemias, vaccination is widely practiced using vaccines that typically consist of inactivated supernatant filtrate prepared from a vegetative culture of a type D isolate (18, 33). Whether those type D supernatants commonly contain lethal amounts of ETX and other toxins has been unclear but could be important for improving vaccine efficacy. For example, if vegetative cultures of type D isolates typically produce lethal levels of several toxins, optimally protective vaccines should probably be produced using type D supernatants that contain high levels of all of those lethal toxins and not just ETX.
Classical C. perfringens toxin typing studies (15, 20) have demonstrated that antiserum raised against type A vegetative culture supernatants cannot neutralize the lethal properties of type D vegetative culture supernatants in the mouse i.v. injection model, which is consistent with ETX (produced by type D, but not by type A, isolates) playing a role in type D supernatant-induced lethality in mice. However, results from the present study imply that the polyclonal antisera raised against type D supernatants and then used in classical toxin typing studies would typically contain a mix of antibodies against several lethal toxins. Therefore, previous toxin typing results did not preclude the possibility of other lethal toxins (besides ETX) contributing to, or even being sufficient for, the mouse lethality induced by some or all type D vegetative culture supernatants.
Consequently, the major goals of the present study were to comprehensively characterize lethal toxin production by genotype D isolates and to start identifying which of these exotoxins are important for the lethality of genotype D vegetative supernatants in the mouse i.v. injection model. By determining the carriage and expression of lethal toxin genes among our large collection of genotype D isolates, our analyses now establish that genotype D isolates typically produce widely varying levels of at least three lethal toxins during vegetative growth. The basis for these variations in toxin expression among type D isolates is not clear but could involve such factors as promoter sequence variations or isolate-to-isolate variations in the activity of two-component regulatory systems (e.g., VirR/VirS), which are known to positively regulate CPB2, CPA, and PFO expression levels in type A isolates (2, 6, 26). Currently, little or no information is available regarding the regulation of ETX production.
With respect to identifying which specific toxins are important for the lethality of genotype D vegetative supernatants, three results from the present study indicate that, under the experimental conditions used, ETX is required for the lethal properties of most late-log-phase type D supernatants in the mouse i.v. injection model. First, our studies identified a strong correlation between genotype D vegetative supernatant ETX levels and mouse lethality, whereas CPA or PFO activity levels in those supernatants showed no correlation with mouse lethality. The current results also revealed that ∼5% of the surveyed genotype D isolates produce no detectable ETX, at least under the current experimental conditions. Further study of these non-ETX-producing isolates is under way, but the apparent discovery of silent etx genes in some genotype D isolates has potential diagnostic significance, i.e., genotype D isolates with silent etx genes would classify differently using classical toxin typing neutralization techniques compared to multiplex PCR genotyping approaches. To our knowledge, silent etx genes have not been identified previously, but a precedent exists for silent C. perfringens lethal toxin genes, e.g., type E isolates usually carry silent cpe genes (3) and some type A isolates (and possibly some type D isolates, see Fig. 2) apparently carry silent cpb2 genes (4, 9, 16, 36). Similarly, several isolates previously classified as type D by toxin neutralization typing approaches were shown in our study to belong to genotype B; this classification discrepancy might be attributable to isolates carrying silent cpb genes.
A second line of evidence in support of ETX as the major toxin contributing to the lethality of most genotype D vegetative supernatants is provided by our trypsin pretreatment results. These studies indicate that trypsin pretreatment was either required for, or substantially increased, the mouse lethality of vegetative supernatants prepared from 19 of the 21 tested genotype D isolates. That determination is consistent with ETX playing the major role in type D supernatant lethality given (i) the known requirement for proteolytic activation of ETX toxicity (23) and (ii) observations that CPB2 (11) and CPA (13) can be trypsin sensitive. ETX activation explaining the trypsin pretreatment-induced increase in mouse lethality observed for most surveyed genotype D vegetative supernatants is also consistent with our observation that an ETX-negative genotype D isolate produced a late-log-phase supernatant that was nonlethal, with or without trypsin pretreatment.
Our antibody neutralization results provide the final piece of evidence that ETX plays a major role in genotype D vegetative supernatant-induced mouse lethality. An anti-ETX MAb was shown to specifically block the lethality of trypsin-activated late-log-phase supernatants from most genotype D isolates, which indicates ETX is necessary for the lethal properties of these supernatants in the mouse i.v. injection model. Notably, this anti-ETX MAb (but not an anti-CPA MAb) even neutralized the one ETX-containing supernatant that exhibited the same lethality in the presence or absence of trypsin-pretreatment. Besides confirming ETX is responsible for the lethal properties of that supernatant, this result also suggests that genotype D isolate produces extracellular proteases to self-activate ETX, a phenomenon noted previously for some type D isolates (22). For other type D isolates, ETX is activated by intestinal proteases prior to its absorption (14).
The present study's conclusion that, under the experimental conditions used, ETX is necessary for the lethality of most genotype D vegetative culture supernatants in the mouse i.v. injection model is consistent with the potency of this toxin, which has the lowest LD50 (0.1 to 0.3 μg/kg) of all C. perfringens toxins when administered via the i.v. route (12). By comparison, the other lethal toxins consistently present in type D supernatants, i.e., CPA and PFO, have LD50 values of 3 or 13 μg/kg, respectively, by the i.v. route (12). Establishing that ETX is required for the lethality of most genotype D vegetative supernatants is also consistent with results from a previous study by el Idrissi and Ward (8), who noted a correlation between mouse i.v. lethality and ETX levels (measured by enzyme-linked immunosorbent assay) in late-log-phase supernatants prepared from six type D isolates. These authors noted that their putative correlation between ETX levels and lethality was preliminary and required confirmation by analysis of additional C. perfringens type D isolates; in that regard, only a single genotype D isolate was common to both studies. Although el Idrissi and Ward did not attempt to neutralize type D supernatant lethality with an ETX-specific antibody, our results demonstrating that ETX is required for genotype D vegetative supernatant lethality are consistent with, and offer support for continuing, the common practice of evaluating type D veterinary vaccination efficacy by measuring serum anti-ETX titers in immunized animals (18). These new findings are also consistent with a previous study showing that immunization with an anti-idiotypic antibody raising antibodies against an ETX neutralizing epitope can protect mice against challenge with a virulent type D isolate (27).
There are also several obvious limitations to our study. First, although this survey included a large number of genotype D isolates with diverse geographic origins and dates of isolation, rare genotype D isolates could exist that cause ETX-independent mouse lethality by producing exceptionally large amounts of another (non-ETX) lethal toxin. Supporting that possibility, a few type A isolates have been identified that express exceptionally large amounts of CPA activity, e.g., late-log-phase supernatants of type A strain ATCC 13124 produce CPA activity levels of 18.8 U/mg of total protein × 10−3 (determined in the present study). Second, variations in culture conditions could affect the relative contributions to mouse lethality by different lethal toxins in genotype D vegetative supernatants. That possibility should be evaluated by future studies, but the culture conditions used in our study for preparing vegetative supernatants were chosen for maximal toxin content (see Results) and closely resemble the culture conditions used by el Idrissi and Ward, thus allowing direct comparisons between these two studies. Third, our studies do not necessarily rule out the possibility that additive or synergistic interactions between ETX, CPA, and PFO could affect lethality. For example, such toxin interactions might hasten time to death of mice, a factor not investigated in the current study. There is precedent for interactions between C. perfringens toxins, since CPA and PFO have been shown to act synergistically in a mouse model of C. perfringens type A myonecrosis (1). Fourth, species differences could affect the relative sensitivity of animals to type D lethal toxins (see Introduction). Finally, although injecting supernatants i.v. into mice is useful for modeling the rapid lethality of type D enterotoxemias, this approach obviously does not completely mimic the natural infection. Conceivably, other lethal toxins could play a role in, or even be required, for ETX absorption across the intestinal mucosa. This important question cannot currently be addressed for large numbers of genotype D isolates in the absence of a well-characterized small animal oral-challenge model amenable to antibody neutralization approaches. Such oral challenge models would also be useful for dissecting the lethal properties of type D isolates using toxin knockout mutants. Therefore, efforts are currently under way to develop improved animal models for studies aimed at elucidating the virulence of type D isolates.
Acknowledgments
This research was supported by grants AI056177-03 and T32 AI49820 (Molecular Microbial Persistence and Pathogenesis Graduate Training Program) from the National Institute of Allergy and Infectious Diseases. Research in the Monash Laboratory was also supported by a grant from the Australian Research Council to the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
We thank J. Glenn Songer for supplying some of the type D isolates used in this study, Michel Popoff for supplying antiserum to CPB2, P. Hauer for supplying monoclonal antibodies to ETX and CPA, and Jon Brazier for providing information on the BW strains.
Editor: D. L. Burns
REFERENCES
- 1.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ba-Thein, W., M. Lyristis, K. Ohtani, I. T. Nisbet, H. Hayashi, J. I. Rood, and T. Shimizu. 1996. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J. Bacteriol. 178:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billington, S. J., E. U. Wieckowski, M. R. Sarker, D. Bueschel, J. G. Songer, and B. A. McClane. 1998. Clostridium perfringens type E animal enteritis isolates with highly conserved, silent enterotoxin sequences. Infect. Immun. 66:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Bueschel, D. M., B. H. Jost, S. J. Billington, H. T. Trinh, and J. G. Songer. 2003. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121-129. [DOI] [PubMed] [Google Scholar]
- 5.Bullifent, H. L., A. Moir, and R. W. Titball. 1995. The construction of a reporter system and use for the investigation of Clostridium perfringens gene expression. FEMS Microbiology Lett. 131:99-105. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, J. K., and J. I. Rood. 2000. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J. Bacteriol. 182:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collie, R. E., J. F. Kokai-Kun, and B. A. McClane. 1998. Phenotypic characterization of enterotoxigenic Clostridium perfringens isolates from non-foodborne human gastrointestinal diseases. Anaerobe 4:69-79. [DOI] [PubMed] [Google Scholar]
- 8.el Idrissi, A. H., and G. E. Ward. 1992. Evaluation of enzyme-linked immunosorbent assay for diagnosis of Clostridium perfringens enterotoxemias. Vet. Microbiol. 31:389-396. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, D. J., K. Miyamoto, B. Harrision, S. Akimoto, M. R. Sarker, and B. A. McClane. 2005. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747-762. [DOI] [PubMed] [Google Scholar]
- 10.Garmory, H. S., N. Chanter, N. P. French, D. Busechel, J. G. Songer, and R. W. Titball. 2000. Occurrence of Clostridium perfringens β2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol. Infect. 124:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibert, M., C. Jolivet-Reynaud, and M. R. Popoff. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65-73. [DOI] [PubMed] [Google Scholar]
- 12.Gill, D. M. 1982. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginter, A., E. D. Williamson, F. Dessy, P. Coppe, H. Bullifent, A. Howells, and R. Titball. 1996. Molecular variation between the alpha-toxins from the type strain (NCTC 8237) and clinical isolates of Clostridium perfringens associated with disease in man and animals. Microbiology 142:191-198. [DOI] [PubMed] [Google Scholar]
- 14.Habeeb, A. F. 1969. Studies on epsilon-prototoxin of Clostridium perfringens type D. I. purification methods: evidence for multiple forms of epsilon-prototoxin. Arch. Biochem. Biophys. 130:430-440. [DOI] [PubMed] [Google Scholar]
- 15.Hatheway, C. 1990. Toxigenic clostridia. Clin. Microb. Rev. 3:66-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jost, B. H., S. J. Billington, H. T. Trinh, D. M. Bueschel, and J. G. Songer. 2005. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect. Immun. 73:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokai-Kun, J. F., J. G. Songer, J. R. Czeczulin, F. Chen, and B. A. McClane. 1994. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J. Clin. Microbiol. 32:2533-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClane, B. A., F. A. Uzal, M. F. Miyakawa, D. Lyerly, and T. Wilkins. 2004. The enterotoxic clostridia. In The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., vol. 2004. [Online.] Springer-Verlag, New York, N.Y. http://141.150.157.117:8080/prokPUB/chaprender/jsp/showchap.jsp?chapnum=443.
- 19.McClane, B. A., and J. I. Rood. 2001. Clostridial toxins involved in human enteric and histotoxic infections, p. 169-209. In H. Bahl and P. Duerre (ed.), Clostridia: biotechnology and medical applications. Wiley-VCH, Weinheim, Germany.
- 20.McDonel, J. L. 1986. Toxins of Clostridium perfringens types A, B, C, D, and E, p. 477-517. In F. Dorner and H. Drews (ed.), Pharmacology of bacterial toxins. Pergamon Press, Oxford, England.
- 21.McDonel, J. L., and B. A. McClane. 1988. Production, purification and assay of Clostridium perfringens enterotoxin. Method Enzymol. 165:94-103. [DOI] [PubMed] [Google Scholar]
- 22.Minami, J., S. Katayama, O. Matsushita, C. Matsushita, and A. Okabe. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527-535. [DOI] [PubMed] [Google Scholar]
- 23.Miyata, S., O. Matsushita, J. Minami, S. Katayama, S. Shimamoto, and A. Okabe. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens ɛ-toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778-13783. [DOI] [PubMed] [Google Scholar]
- 24.Nagahama, M., H. Iida, and J. Sakurai. 1993. Effect of Clostridium perfringens epsilon toxin on rat isolated aorta. Microbiol. Immunol. 37:447-450. [DOI] [PubMed] [Google Scholar]
- 25.Ohtani, K., S. K. Bhowmik, H. Hayashi, and T. Shimizu. 2002. Identification of a novel locus that regulates expression of toxin genes in Clostridium perfringens. FEMS Microbiol. Lett. 209:109-114. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani, K., H. I. Kawsar, K. Okumura, H. Hayashi, and T. Shimizu. 2003. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens. FEMS Microbiol. Lett. 222:137-141. [DOI] [PubMed] [Google Scholar]
- 27.Percival, D., A. D. Shuttleworth, E. D. Williamson, and D. C. Kelly. 1990. Anti-idiotypic antibody-induced protection against Clostridium perfringens type D. Infect. Immun. 58:2487-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit, L., M. Gilbert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai, J., M. Nagahama, and Y. Fujii. 1983. Effect of Clostridium perfringens epsilon toxin on the cardiovascular system of rat. Infect. Immun. 42:1183-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarker, M. R., R. J. Carman, and B. A. McClane. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946-958. [DOI] [PubMed] [Google Scholar]
- 31.Singh, U., C. M. Van Itallie, L. L. Mitic, J. M. Anderson, and B. A. McClane. 2000. CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J. Biol. Chem. 275:18407-18417. [DOI] [PubMed] [Google Scholar]
- 32.Sloan, J., T. A. Warner, P. T. Scott, T. L. Bannam, D. I. Berryman, and J. I. Rood. 1992. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27:207-219. [DOI] [PubMed] [Google Scholar]
- 33.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens, D. L., J. Mitten, and C. Henry. 1987. Effects of alpha and theta toxins from Clostridium perfringens on human polymorphonuclear leukocytes. J. Infect. Dis. 156:324-333. [DOI] [PubMed] [Google Scholar]
- 35.Uzal, F. A., M. Ghoddusi, W. R. Kelly, and M. Rozmanec. 1999. Comparison of the effects of Clostridium perfringens type D culture supernatants in ligated intestinal loops of goats and sheep. J. Comp. Pathol. 121:127-138. [DOI] [PubMed] [Google Scholar]
- 36.Waters, M., A. Savoie, H. S. Garmory, D. Bueschel, M. R. Popoff, J. G. Songer, R. W. Titball, B. A. McClane, and M. R. Sarker. 2003. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J. Clin. Microbiol. 41:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wen, Q., and B. A. McClane. 2004. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 70:2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wnek, A. P., R. J. Strouse, and B. A. McClane. 1985. Production and characterization of monoclonal antibodies against Clostridium perfringens type A enterotoxin. Infect. Immun. 50:442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]