Abstract
Individuals with blood group O are more susceptible than other individuals to severe cholera, although the mechanism underlying this association is unknown. To assess the respective roles of both intrinsic host factors and adaptive immune responses that might influence susceptibility to infection with Vibrio cholerae, we prospectively followed a cohort of household contacts of patients with cholera in Bangladesh. In this study, we made the novel observation that persons with blood group O were less likely than those with other blood groups to become infected with V. cholerae O1 (odds ratio [OR], 0.67; 95% confidence interval [CI], 0.53 to 0.85; P = 0.008). Consistent with prior studies, however, household contacts with blood group O were more likely to develop severe illness if infected with V. cholerae O1 (OR, 2.3; 95% CI, 0.98 to 5.59; P = 0.05). While blood group O protected significantly against infection with V. cholerae O1, there was no evidence of protection against V. cholerae O139. A multivariate analysis demonstrated that the association between blood group O and protection from infection with V. cholerae O1 was independent of age, gender, and baseline anti-cholera toxin and vibriocidal antibody titers. Based on this epidemiologic evidence, we propose a hypothesis for understanding the association between blood group O and the risk of infection with V. cholerae O1 and O139 as well as the risk of developing severe symptoms once infected.
Vibrio cholerae is a gram-negative bacterium that causes a spectrum of infection ranging from asymptomatic colonization to rapidly fatal secretory diarrhea known as cholera gravis. V. cholerae is differentiated serologically by the O side chain of its lipopolysaccharide; the vast majority of human cholera is caused by the O1 and O139 serogroups. The O1 serogroup of V. cholerae is subclassified into two biotypes (classical and El Tor) and two major serotypes (Inaba and Ogawa) (11). Due to variations in the predominating serogroup, biotype, and serotype in circulation, the epidemiology of cholera is in constant flux. In the 1960s, the V. cholerae O1 El Tor biotype emerged as a major cause of cholera, ultimately replacing the classical biotype. In 1992, the V. cholerae O139 serogroup first appeared; after briefly predominating in South Asia, it now persists in this region, but at much lower levels than V. cholerae O1 El Tor.
Susceptibility to infection with V. cholerae is dependent on both adaptive immune responses induced by previous infection and innate host factors. The best-studied correlate of adaptive immunity to V. cholerae is the serum vibriocidal antibody, a complement-fixing bactericidal antibody. Seroepidemiologic studies in areas of endemicity have shown that vibriocidal antibody titers increase with age and that risk of disease is inversely proportional to the vibriocidal antibody titer (9, 16, 17). However, there is no threshold vibriocidal antibody titer above which complete protection from infection is achieved, and it is hypothesized that the vibriocidal antibody is a surrogate marker for a protective mucosal immune response (24). Systemic antibodies to cholera toxin have not been found to correlate with protection from cholera (9). Furthermore, infection with V. cholerae O1 does not confer protection from V. cholerae O139, even though both serogroups produce identical cholera toxins (1, 22).
Among the intrinsic host factors that influence susceptibility to cholera, the ABH histo-blood group antigens are the most studied. Multiple case-control studies in areas of cholera endemicity have demonstrated that individuals with blood group O (the blood group phenotype associated with the H antigen) are at increased risk of hospitalization due to classical and El Tor V. cholerae O1 as well as V. cholerae O139 (3, 4, 5, 7, 26). A study of North American volunteers also demonstrated increased purging in blood group O subjects infected with a high inoculum of classical V. cholerae O1 (13). It has been hypothesized that V. cholerae infection is the evolutionary force behind the low prevalence of the O blood group in the Ganges River Delta, which is a historic and current global epicenter of cholera (10, 11).
Two previous studies have addressed the question of whether blood group O is also associated with an increased risk of asymptomatic infection or mild illness due to V. cholerae in addition to the increased risk of severe illness (10, 26). Both studies concluded that there was no difference in the overall risk of infection with V. cholerae among persons with blood group O compared to those with non-blood group O antigens. A prospective study of household contacts of cholera patients in Bangladesh performed between 1980 and 1982 found that while blood group O was associated with an increased risk of moderate to severe cholera, there was no association between blood group and the risk of overall infection (defined microbiologically) due to V. cholerae O1 El Tor (10). Similar findings were reported in a retrospective evaluation of cholera patients performed during the first outbreak of V. cholerae O1 El Tor in Trujillo, Peru (26). In this study, individuals with blood group O did not have increased evidence of recent infection (defined serologically) but were more than eight times as likely to require hospitalization for cholera.
Although the mechanism by which blood group influences susceptibility to cholera is undefined, the association is of significant public health importance. During the epidemic of cholera in the 1990s in South America, where there is a high prevalence of blood group O, the requirements for hospitalization, intravenous fluids, and oral rehydration therapy were greater than seen in outbreaks in other regions (26). The blood group distribution in a population may also influence the efficacy of cholera vaccination programs, although current data are conflicting. A trial of a cholera toxin B subunit-killed oral vaccine in Bangladesh showed a lower protective efficacy in individuals with blood group O (5). In contrast, a live oral attenuated cholera vaccine, CVD 103-HgR, resulted in higher vibriocidal antibody responses in Indonesian individuals with blood group O (12). Although CVD 103-HgR had an overall protective efficacy of only 14% over the 4-year surveillance period, there was a trend toward greater efficacy in individuals with blood group O, in whom the protective efficacy was 46% (23).
To understand the factors that mediate susceptibility to symptomatic and asymptomatic infection with V. cholerae, we undertook a prospective, observational study of a cohort of cholera patients and their household contacts in Dhaka, Bangladesh. Here, we present novel findings regarding the association between blood group, the vibriocidal antibody response, and the risk of V. cholerae infection in a current area of endemicity.
MATERIALS AND METHODS
Study design and subject enrollment.
The International Centre for Diarrheal Disease Research (ICDDR,B) hospital cares for approximately 10,000 to 20,000 cholera patients annually, the majority of whom are residents of Dhaka city. A random sample of patients over the age of 6 months presenting to the ICDDR,B hospital with severe acute watery diarrhea, dark-field positive stool, and no significant comorbid conditions was selected for inclusion in this study. Within 4 to 6 h of presentation of the index case, a field team discussed enrollment with household contacts of the index patient, defined as individuals sharing the same cooking pot. Blood specimens for ABO typing and baseline vibriocidal and anti-cholera toxin antibody titers were collected immediately upon enrollment from consenting contacts. Contacts were visited by the field team on each of the next 6 days and again on days 14 and 21 after presentation of the index case. During these visits, contacts were questioned about diarrheal symptoms, and rectal swabs were obtained for V. cholerae culture. Follow-up blood samples for vibriocidal antibody titers were obtained from contacts on study days 4 and 21. Patients and contacts were excluded from the analysis if they did not complete 21 days of follow-up. Household contacts with diarrhea during the week prior to enrollment or with a positive rectal swab culture for V. cholerae upon enrollment in the study were excluded from the analysis of baseline immunologic characteristics.
Informed consent for participation in this research was obtained from patients or their guardians. The human experimentation guidelines of the U.S. Department of Health and Human Services were followed in the conduct of this research. Approval for this human study was obtained from the Institutional Review Board of the Massachusetts General Hospital and the ICDDR,B Research and Ethical Review Committees.
Confirmation of bacterial strains.
All index cases of cholera were confirmed by culturing stool for V. cholerae on taurocholate-tellurite-gelatin agar. After overnight incubation of plates, serological confirmation of suspected vibrio colonies was carried out by slide agglutination (18, 21). Rectal swab specimens from household contacts were collected in Cary-Blair transport medium for subsequent plating on taurocholate-tellurite-gelatin agar and colony identification as described above.
Measurement of vibriocidal and anti-cholera toxin antibody responses in serum.
Vibriocidal antibody assays were performed with methodology previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 or O139 as the target organism (19). The concentrations of complement and bacteria have been separately optimized for determining the vibriocidal antibody responses to V. cholerae O1 and V. cholerae O139. Serum antibodies specific to the cholera toxin B subunit, of both immunoglobulin G (IgG) and IgA isotypes, were measured using a previously described ganglioside GM1 capture enzyme-linked immunosorbent assay (25).
Definition of outcomes in household contacts.
Among household contacts, infection was defined as shedding of V. cholerae in the stool and/or a fourfold or greater rise in vibriocidal antibody titer during the 21 days of follow-up. Symptomatic illness was defined as infection associated with any diarrheal symptoms; for contacts with a positive rectal swab culture, diarrheal symptoms had to occur within 3 days of the positive result. With the prompt medical care that was afforded by close follow-up of study participants, none of the household contacts required hospitalization or intravenous hydration. Therefore, diarrheal frequency (measured as the number of stools per 24-h period) was used as a marker of disease severity in this group. Severe symptoms among household contacts were defined as eight or more large-volume watery stools per 24-h period, which represented the top quartile of symptomatic household contacts. Because the frequency of stools was recorded on daily home visits only on study days 2 through 7, individuals whose symptoms began more than 7 days after enrollment were not classified for disease severity.
Statistical analysis.
Analysis was performed using Stata version 8.0 (Stata Corporation, Inc., College Station, Texas) and R version 1.5.0 (http://www.R-project.org) (6). The Student t test was used to compare log10-transformed vibriocidal antibody responses in the index patients. Characteristics of the infected and uninfected household contacts were compared using generalized estimating equations, with an exchangeable correlation matrix, and the reported odds ratios (OR) and P values were adjusted for clustering based on household (29). Multivariate analysis was performed using a clustered logistic regression model using the generalized estimating equations, with the final model selected based on backwards elimination. Means are reported in the text and tables with their standard deviation. All reported P values are two tailed.
RESULTS
Characteristics of the study population.
Three hundred index patients and 912 of their household contacts were enrolled in the study between January 2001 and September 2004. Of these, 269 patients and 793 contacts completed 21 days of follow-up and were included in this analysis.
The demographic, microbiologic, clinical, and immunologic features of the index cases and household contacts are shown in Table 1. Fifty-eight percent of the index patients with cholera were female, and the mean age was 25 years. Fifty-seven (21%) of the index patients were infected with V. cholerae O139, while 212 (79%) were infected with V. cholerae O1 El Tor (83 were infected with serotype Ogawa and 129 with serotype Inaba). Forty-three percent of index patients were blood group O; this was significantly higher than the prevalence of blood group O (33%) in the household contacts (P = 0.005), despite the familial relatedness. Additionally, the prevalence of blood group O among index cases in our study was significantly higher than that of a randomly selected cohort of individuals from a neighborhood of Dhaka in which cholera is highly endemic who were enrolled in a recent cholera vaccine study at the ICDDR,B (43% in our cohort versus 29% [89 were blood group O out of 310 subjects] in the vaccine study, P < 0.001) (20).
TABLE 1.
Demographic, microbiologic, clinical, and immunologic features of cholera patients and their household contacts
| Characteristic | Index patients (N = 269) | Household contacts
|
||
|---|---|---|---|---|
| Infectedb (N = 260) | Not infected (N = 533) | P (for infected versus uninfected household contacts) | ||
| Demographic data | ||||
| Age | 25 (±14) | 18 (±15) | 21 (±15) | 0.03 |
| Female | 155 (58%) | 132 (51%) | 267 (50%) | 0.8 |
| Blood group | ||||
| O | 115 (43%) | 74 (28%) | 190 (36%) | 0.03 |
| A | 60 (22%) | 67 (26%) | 136 (26%) | 0.9 |
| B | 72 (27%) | 85 (33%) | 155 (29%) | 0.4 |
| AB | 22 (8%) | 34 (13%) | 52 (10%) | 0.1 |
| Stool or rectal swab culture result | ||||
| Any | 269 (100%) | 185 (72%) | NA | |
| O1 Inaba | 129 (48%) | 83 (32%) | NA | |
| O1 Ogawa | 83 (31%) | 53 (20%) | NA | |
| O139 | 57 (21%) | 49 (19%) | NA | |
| Severe dehydration | 241 (90%) | 0 (0%) | NA | |
| Immunologic dataa | ||||
| Baseline log10 vibriocidal antibody titer | 1.8 (±0.7) | 1.6 (±0.9) | 1.9 (±0.9) | 0.001 |
| Day 21 log10 vibriocidal antibody titer | 3.3 (±0.6) | 2.6 (±0.9) | 1.9 (±0.9) | <0.001 |
| Baseline log10 anti-CT IgG | 2.0 (±0.3) | 1.9 (±0.3) | 2.0 (±0.4) | 0.5 |
| Day 21 log10 anti-CT IgG | 2.4 (±2.4) | 2.2 (±0.3) | 2.0 (±0.3) | <0.001 |
| Fourfold rise in vibriocidal antibody titer | 265 (99%) | 190 (73%) | NA | |
The baseline (day 2) immunologic features presented for household contacts exclude those individuals who were symptomatic or had a positive rectal swab for V. cholerae upon enrollment in the study (n included = 593).
Infection was defined microbiologically (72% of infected contacts), or in the absence of a positive culture, infection was defined serologically by a fourfold increase in vibriocidal antibody titer (28%). NA, not applicable.
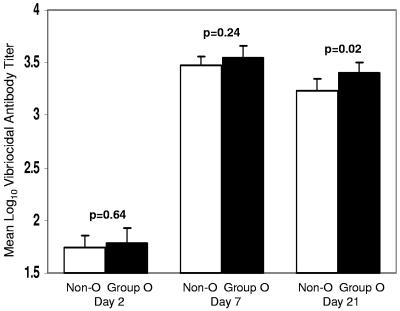
The patients admitted to the ICDDR,B and enrolled as index patients in this study had uniformly severe disease regardless of blood group. More than 90% of patients had severe dehydration and received intravenous fluid and antibiotics, and >99% had a fourfold increase in the vibriocidal titer over the 21 days of follow-up. Figure 1 shows the magnitude of the vibriocidal response among index cases, stratified by blood group. Index patients with blood group O had significantly higher vibriocidal titers on day 21 (P = 0.02) after the onset of illness than those with other blood groups. Furthermore, in index cases with a baseline vibriocidal antibody titer of ≤80, individuals with blood group O had a higher median increase (n-fold) in vibriocidal titer than non-blood group O individuals (median increase, 128- versus 64-fold, respectively, P = 0.01).
FIG. 1.
Vibriocidal antibody responses in index patients with cholera by blood group. Bars represent the mean log10-transformed vibriocidal titers, and the error bars represent the 95% CI of the mean. P values refer to the differences in mean titer between blood group O and non-blood group O patients on days 2, 7, and 21.
As shown in Table 1, both infected and uninfected household contacts were younger than index patients. The majority of infected household contacts (72%) were identified by the isolation of V. cholerae from rectal swab culture; a smaller proportion (28%) was identified by a fourfold increase in the vibriocidal titer without a positive rectal swab culture. Compared to uninfected household contacts, those who developed V. cholerae infection were younger, had lower baseline vibriocidal titers, and were less likely to be blood group O. With the prompt provision of medical care by the visiting field team, none of the infected household contacts required hospitalization or intravenous hydration. However, of the 260 infected household contacts, 150 (58%) developed watery diarrhea. The mean number of diarrheal stools among symptomatic household contacts was 5.5 ± 4.2 per day.
Relationship between blood group O and risk of V. cholerae infection among household contacts.
Table 2 shows additional data on the relationship between blood group and the risk of V. cholerae infection in our cohort of household contacts of cholera patients. Household contacts with blood group O were less likely to be infected with V. cholerae than individuals with other blood groups (OR, 0.80; 95% confidence interval [CI], 0.65 to 0.98; P = 0.03). However, once infected, severe symptoms were more likely to occur in household contacts with blood group O (OR, 2.4; 95% CI, 1.2 to 4.7; P = 0.01). The mean number of stools in symptomatic contacts with blood group O was 7.8 ± 6.5 per day compared with 5.1 ± 4.0 per day among those with other blood groups (P = 0.01).
TABLE 2.
Risk of infection and severe diarrhea if infected in a cohort of household contacts of index patients with Vibrio cholerae O1 and O139 infection
| V. cholerae serogroup in index patient | Blood group of household contact | Infection in household contacta | OR for infection (95% CI) | P | Severe diarrhea in household contact, if infectedb | OR for severe diarrhea (95% CI) | P |
|---|---|---|---|---|---|---|---|
| O1 & O139 combined | O | 74/264 (28%) | 0.80 (0.65-0.98) | 0.03 | 14/66 (21%) | 2.4 (1.2-4.7) | 0.01 |
| Non-O | 186/529 (35%) | 15/173 (9%) | |||||
| O1 | O | 49/208 (24%) | 0.67 (0.53-0.85) | 0.0008 | 8/43 (19%) | 2.3 (0.98-5.6) | 0.055 |
| Non-O | 151/423 (36%) | 11/139 (8%) | |||||
| O139 | O | 25/56 (45%) | 1.2 (0.80-1.9) | 0.32 | 6/23 (26%) | 2.3 (0.86-6.2) | 0.10 |
| Non-O | 35/106 (33%) | 4/34 (12%) |
Number of household contacts infected/number exposed.
Number with severe diarrhea/number infected. Severe diarrhea was defined as eight or more large-volume watery stools per 24 hours. Infected patients were excluded if the onset of symptoms was 8 or more days after enrollment.
Table 2 also shows the association between blood group O and the risk of infection stratified by the serogroup of V. cholerae to which the contacts were exposed. We found that the association between blood group O and protection from infection was serogroup dependent. While blood group O was associated with protection from infection with V. cholerae O1 (OR, 0.67; 95% CI, 0.53 to 0.85; P = 0.0008), there was no such protection from infection on exposure to V. cholerae O139. However, the associations between blood group O and an increased risk of developing severe diarrheal symptoms when infected were similar for both serogroups of V. cholerae. Stratification of the above V. cholerae O1 analyses by infecting serotype (Inaba or Ogawa) in contacts of patients with V. cholerae O1 showed no significant effect (data not shown).
Multivariate analysis.
To assess whether the association between blood group O and protection from infection among household contacts was confounded by other factors, such as preexisting adaptive immunity to V. cholerae, we performed a stepwise, clustered, multivariate analysis that included baseline vibriocidal antibody titer, baseline anti-cholera toxin antibody titer, blood group, age, and gender. Gender and antitoxin antibody levels were not predictive of the risk of infection and thus were removed by backwards elimination from the final model. Our final model demonstrated that the baseline vibriocidal antibody titer and blood group O were independent predictors of protection from infection with V. cholerae O1. Although in the unadjusted analysis, increasing age was associated with protection from infection (Table 1), age was not a significant predictor after adjustment for other variables in the final model. For each log10 increase in the baseline vibriocidal antibody titer, there was a 27% decrease in the odds of infection with V. cholerae O1 (OR, 0.73; 95% CI, 0.59 to 0.90; P = 0.003). Among individuals with blood group O, the odds of infection were half that of non-blood group O individuals in the multivariate model, independent of vibriocidal titer (OR, 0.50; 95% CI, 0.35 to 0.71; P = 0.0001). Using the same multivariate model, no significant predictors were found for the risk of infection with V. cholerae O139 (data not shown).
DISCUSSION
The ABH histo-blood group antigens are a set of cellular and secreted glycolipids and glycopeptides that are abundant and widely distributed throughout the body. The ABO phenotype is determined genetically by activity of specific glycosyltransferases and has been shown to be an important determinant of human susceptibility to a number of important gastrointestinal pathogens, including norovirus (14), Helicobacter pylori (2), and V. cholerae.
Previous studies have demonstrated that blood group O is associated with an increased risk of severe cholera due to the O1 serogroup of V. cholerae. In our present cohort, we observed an increased severity of cholera among patients with blood group O infected with both the O1 and O139 serogroups of V. cholerae. Additionally, we made the new observation that individuals in Bangladesh with blood group O are protected from infection with V. cholerae O1, despite the increased severity of disease once infected. This finding may provide insight into the mechanism of the association between histo-blood group antigens and susceptibility to cholera.
Current models of cholera pathogenesis include two key steps: colonization of the intestine by the organism and the elaboration of cholera toxin (CT), which causes secretory diarrhea. Because the risk of infection with V. cholerae O1 is lower in individuals with blood group O, the increased severity of disease once these individuals are infected is unlikely to reflect an increased susceptibility to colonization, as has previously been hypothesized (5). Rather, the increased severity of disease likely reflects a greater susceptibility to later stages of disease pathogenesis, such as an enhanced susceptibility to the secretory effects of CT.
In vitro studies have suggested a biologic mechanism that is consistent with our epidemiologic data, namely, that the A and B histo-blood group glycoconjugates present in non-blood group O individuals may interfere with the binding of CT to its intestinal receptor, the ganglioside GM1 (15). If this model is correct, then individuals of blood group O should be more susceptible to the pathological effects of CT after colonization, and increased disease severity would be seen in patients infected with either V. cholerae O1 or O139, as we have observed.
This interaction between the histo-blood group glycoconjugates and CT is distinct from the interaction with the heat-labile enterotoxin (LT) of enterotoxigenic Escherichia coli, despite the homology of the two toxins. While both CT and LT bind to the GM1 receptor, LT is additionally capable of utilizing the blood group A antigen as an alternate receptor through which the activation of cyclic AMP can be induced (8). This in vitro observation may explain the fact that individuals with blood group O are more susceptible than non-blood group O individuals to severe cholera but not to enterotoxigenic Escherichia coli-associated diarrhea (27) as well as provide a potential reason for the evolutionary divergence of the two enterotoxins (28).
Notably, the biological interaction between histo-blood group antigens and CT does not explain why individuals with blood group O are preferentially protected from infection with V. cholerae O1 rather than V. cholerae O139. Adaptive immunity may account for this observation. In particular, it is possible that the greater severity of V. cholerae infection in patients with blood group O may lead to more-potent adaptive immune responses after initial infection and hence enhanced protection from intestinal colonization on subsequent exposure. In support of this, we found that index patients with blood group O in our cohort had significantly higher vibriocidal antibody titers at day 21 following infection.
The hypothesis that adaptive immune responses from prior infection mediate the protection from V. cholerae infection conferred by blood group O may also explain our failure to observe an association between blood group O and protection from V. cholerae O139 infection. Immunity to V. cholerae is serogroup specific, and although substantial preexisting immunity to V. cholerae O1 exists in the population in Bangladesh, there is less preexisting immunity to the more recently emerged V. cholerae O139 (1). A similar circumstance existed in the early 1980s when the El Tor biotype of V. cholerae O1 first appeared and was replacing the classical biotype as the predominant cause of cholera in Bangladesh. The absence of population-level immunity to the El Tor biotype during that time period may account for the failure to detect an association between blood group O and protection from infection in the study from the early 1980s by Glass et al. (10), compared to our present results, in which protection was seen. Similarly, this may explain why no protection from infection was seen in subjects with blood group O when V. cholerae O1 El Tor was first introduced into Peru in the 1990s (26).
The prevalence of blood group O among our index cholera patients is lower than that described in previous studies, which reported prevalences ranging from 57 to 64% among patients hospitalized for infection with V. cholerae O1 in Bangladesh (5, 7, 10). This may be explained by the fact that after the introduction of a new strain of V. cholerae to which the population is naive, the proportion of cholera patients with blood group O would be expected to decrease over time, as greater adaptive immunity is conferred by previous exposure. Proof of this hypothesis would require further longitudinal studies of a V. cholerae-naive population, particularly very young children. A focused analysis of the relationship between blood group and risk of infection with V. cholerae among children was not possible in the present study, as there were few young children enrolled in the cohort of household contacts. However, findings reported here do have important implications for the study of infectious disease susceptibility. Specifically, if individuals with an intrinsically increased susceptibility to a disease are also more likely to develop protective adaptive immune responses following exposure, then this enhanced susceptibility may not be identified in the population of an area in which the disease is endemic.
Given our hypothesis regarding the role of adaptive immunity, we anticipated that a multivariate analysis of the risk of cholera infection would demonstrate that the association between blood group O and protection from infection with V. cholerae O1 was confounded by the vibriocidal antibody titer on exposure. In fact, our results revealed that blood group O and the baseline vibriocidal antibody titer were independent predictors of protection from V. cholerae O1 infection. Although this result may indicate that preexisting adaptive immunity does not explain the relationship between blood group and the risk of V. cholerae infection, an alternative explanation is that the vibriocidal antibody titer, which is relatively short-lived after infection, is not the optimal marker of protective immunity following previous infection. Indeed, in a previous analysis of this cohort, we showed that the vibriocidal antibody was an incomplete marker of protection from V. cholerae O1 infection (24). We hypothesize that the vibriocidal antibody response may be a surrogate marker for other immune responses that develop following infection and that actually confer protection on subsequent exposure and that these other immune responses are longer lasting than the measured serum vibriocidal antibody titer. Further definition of the specific mechanisms of immunity to V. cholerae may allow an improved understanding of the association between blood group and the risk of cholera infection and disease.
Acknowledgments
This research was supported by ICDDR,B: Centre for Health and Population Research; grant D43 TW05572 from the Fogarty International Center (S.B.C.); grant U01 AI58935 from the National Institute of Allergy and Infectious Diseases (S.B.C.); grant AI40725 from the National Institute of Allergy and Infectious Diseases (E.T.R.); and grant K01 TW07144 from the Fogarty International Center (R.C.L.). R.C.L. was also supported by an Ellison Medical Foundation Postdoctoral Fellowship in International Infectious Diseases from the Infectious Diseases Society of America. J.B.H. is an NICHD fellow of the Pediatric Scientist Development Program (NICHD grant award K12-HD00850).
We thank the study participants as well as the dedicated field and laboratory workers of the Cholera Immune Response Study at the ICDDR,B.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Albert, M. J., A. K. Siddique, M. S. Islam, A. S. Faruque, M. Ansaruzzaman, S. M. Faruque, and R. B. Sack. 1993. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341:704. [DOI] [PubMed] [Google Scholar]
- 2.Aspholm-Hurtig, M., G. Dailide, M. Lahmann, A. Kalia, D. Ilver, N. Roche, S. Vikstrom, R. Sjostrom, S. Linden, A. Backstrom, C. Lundberg, A. Arnqvist, J. Mahdavi, U. J. Nilsson, B. Velapatino, R. H. Gilman, M. Gerhard, T. Alarcon, M. Lopez-Brea, T. Nakazawa, J. G. Fox, P. Correa, M. G. Dominguez-Bello, G. I. Perez-Perez, M. J. Blaser, S. Normark, I. Carlstedt, S. Oscarson, S. Teneberg, D. E. Berg, and T. Boren. 2004. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305:519-522. [DOI] [PubMed] [Google Scholar]
- 3.Barua, D., and A. S. Paguio. 1977. ABO blood groups and cholera. Ann. Hum. Biol. 4:489-492. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri, A., and C. R. DasAdhikary. 1978. Possible role of blood-group secretory substances in the aetiology of cholera. Trans. R. Soc. Trop. Med. Hyg. 72:664-665. [DOI] [PubMed] [Google Scholar]
- 5.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, M. R. Khan, S. Huda, F. Ahmed, J. Gomes, M. R. Rao, and A. M. Svennerholm. 1989. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J. Infect. Dis. 159:770-773. [DOI] [PubMed] [Google Scholar]
- 6.Dalgaard, P. 2002. Introductory statistics with R. Springer, New York, N.Y.
- 7.Faruque, A. S., D. Mahalanabis, S. S. Hoque, and M. J. Albert. 1994. The relationship between ABO blood groups and susceptibility to diarrhea due to Vibrio cholerae O139. Clin. Infect. Dis. 18:827-828. [DOI] [PubMed] [Google Scholar]
- 8.Galvan, E. M., C. D. Diema, G. A. Roth, and C. G. Monferran. 2004. Ability of blood group A-active glycosphingolipids to act as Escherichia coli heat-labile enterotoxin receptors in HT-29 cells. J. Infect. Dis. 189:1556-1564. [DOI] [PubMed] [Google Scholar]
- 9.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236-242. [DOI] [PubMed] [Google Scholar]
- 10.Glass, R. I., J. Holmgren, C. E. Haley, M. R. Khan, A. M. Svennerholm, B. J. Stoll, K. M. Belayet Hossain, R. E. Black, M. Yunus, and D. Barua. 1985. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am. J. Epidemiol. 121:791-796. [DOI] [PubMed] [Google Scholar]
- 11.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagos, R., A. Avendano, V. Prado, I. Horwitz, S. Wasserman, G. Losonsky, S. Cryz, Jr., J. B. Kaper, and M. M. Levine. 1995. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect. Immun. 63:707-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine, M. M., D. R. Nalin, M. B. Rennels, R. B. Hornick, S. Sotman, G. Van Blerk, T. P. Hughes, S. O'Donnell, and D. Barua. 1979. Genetic susceptibility to cholera. Ann. Hum. Biol. 6:369-374. [DOI] [PubMed] [Google Scholar]
- 14.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 15.Monferran, C. G., G. A. Roth, and F. A. Cumar. 1990. Inhibition of cholera toxin binding to membrane receptors by pig gastric mucin-derived glycopeptides: differential effect depending on the ABO blood group antigenic determinants. Infect. Immun. 58:3966-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosley, W. H., A. S. Benenson, and R. Barui. 1968. A serological survey for cholera antibodies in rural East Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull. W. H. O. 38:335-346. [PMC free article] [PubMed] [Google Scholar]
- 17.Mosley, W. H., S. Ahmad, A. S. Benenson, and A. Ahmed. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull. W. H. O. 38:777-785. [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, M. S. Alam, K. Zaman, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, D. A. Sack, and Peru-15 Study Group. 2005. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J. Infect. Dis. 192:573-579. [DOI] [PubMed] [Google Scholar]
- 21.Rahman, M., D. A. Sack, S. Mahmood, and A. Hossain. 1987. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J. Clin. Microbiol. 25:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karasawa, H. Kurazano, and A. Pal. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in Southern and Eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 23.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399-2410. [DOI] [PubMed] [Google Scholar]
- 24.Saha, D., R. C. LaRocque, A. I. Khan, J. B. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 25.Svennerholm, A. M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 26.Swerdlow, D. L., E. D. Mintz, M. Rodriguez, E. Tejada, C. Ocampo, L. Espejo, T. J. Barrett, J. Petzelt, N. H. Bean, and L. Seminario. 1994. Severe life-threatening cholera associated with blood group O in Peru: implications for the Latin American epidemic. J. Infect. Dis. 170:468-472. [DOI] [PubMed] [Google Scholar]
- 27.van Loon, F. P., J. D. Clemens, D. A. Sack, M. R. Rao, F. Ahmed, S. Chowdhury, J. R. Harris, M. Ali, J. Chakraborty, and M. R. Khan. 1991. ABO blood groups and the risk of diarrhea due to enterotoxigenic Escherichia coli. J. Infect. Dis. 163:1243-1246. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto, T., T. Gojobori, and T. Yokota. 1987. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J. Bacteriol. 169:1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeger, S. L., and K. Y. Liang. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121-130. [PubMed] [Google Scholar]