Abstract
Initial field malaria prophylaxis trials with azithromycin revealed insufficient efficacy against falciparum malaria to develop azithromycin as a single agent. The objective of this in vitro study was to determine the best drug combination(s) to evaluate for future malaria treatment and prophylaxis field trials. In vitro, azithromycin was tested in combination with chloroquine against 10 representative Plasmodium falciparum isolates. Azithromycin was also assessed in combination with eight additional antimalarial agents against two or three multidrug-resistant P. falciparum isolates. Parasite susceptibility testing was carried out with a modification of the semiautomated microdilution technique. The incubation period was extended from the usual 48 h to 68 h. Fifty percent inhibitory concentrations (IC50s) were calculated for each drug alone and for drugs in fixed combinations of their respective IC50s (1:1, 3:1, 1:3, 4:1, 1:4, and 5:1). These data were used to calculate fractional inhibitory concentrations and isobolograms. Chloroquine-azithromycin studies revealed a range of activity from additive to synergistic interactions for the eight chloroquine-resistant isolates tested, while an additive response was seen for the two chloroquine-sensitive isolates. Quinine, tafenoquine, and primaquine were additive to synergistic with azithromycin, while dihydroartemisinin was additive with a trend toward antagonism. The remaining interactions appeared to be additive. These results suggest that a chloroquine-azithromycin combination should be evaluated for malaria prophylaxis and that a quinine-azithromycin combination should be evaluated for malaria treatment in areas of drug resistance.
The emergence of Plasmodium falciparum malaria resistant to standard antimalarial drugs is a major threat to public health (9). Chloroquine treatment is now ineffective in most areas of the world. The usual replacement, pyrimethamine-sulfadoxine, is rapidly losing efficacy in many areas (15). Compliance with quinine is challenging, especially in the 7- to 10-day dosing regimens commonly used for pregnant women and children in Southeast Asia. Mefloquine remains efficacious in most areas of the world but is associated with stillbirth in pregnancy (13), and concerns about neuropsychiatric reactions (19) may limit its use. Artesunate requires 5- to 7-day treatment regimens to achieve acceptable cure rates, and it is therefore usually combined with mefloquine (23). Toxicity seen in animals (7) raises concerns about the use of artemisinin derivatives in childhood and pregnancy. Ultimately, the choice of antimalarial drugs depends on a cost and risk benefit analysis for each of the alternatives.
Pregnant women and young children bear the brunt of malaria-induced morbidity and mortality. They constitute a particularly difficult treatment problem because the drugs commonly added to the standard agents to augment efficacy, such as doxycycline and mefloquine, are potentially hazardous for these populations. Azithromycin is approved for use in children (Zithromax product information, 1999; Pfizer, Inc., New York, N.Y.), and a growing database also suggests safety for pregnant women (8, 21).
Combination therapy has become the standard of care for several infectious diseases where drug resistance is a problem (e.g., tuberculosis and human immunodeficiency virus) and should also become standard for malaria (22). Tetracycline derivatives have proven to be very effective for combination treatment of malaria (20). Azithromycin, an antibiotic with activity similar to that of tetracyclines against malaria in vitro (24) and in vivo (1), has clear advantages for malaria-related indications.
Three field malaria prophylaxis trials with azithromycin as a single agent (250 mg/day or 1 g/week) have been completed in the last 7 years (2, 16; D. G. Heppner, personal communication). Although showing high efficacy for Plasmodium vivax, azithromycin had less than acceptable protective efficacy for Plasmodium falciparum (∼70 to 90%). Based on these results and the clear safety advantages of azithromycin, we believed that an assessment of drugs to use in combination with azithromycin was warranted.
Canfield and colleagues established a paradigm for combination antimalarial development that rescued atovaquone as an antimalarial agent and ultimately led to the atovaquone-proguanil combination (Malarone) (4). With that paradigm, this in vitro assessment was intended to choose the best partner drugs for azithromycin in proof-of-concept malaria treatment trials.
MATERIALS AND METHODS
Drugs.
Azithromycin was tested in combination with chloroquine against 10 P. falciparum isolates. Azithromycin was also tested in combination with eight additional antimalarial agents against two or three multidrug-resistant P. falciparum isolates to screen for interactions. The eight additional drugs were quinine, mefloquine, desbutylhalofantrine, dihydroartemisinin, proguanil, ciprofloxacin, primaquine, and tafenoquine (WR238605). All of these drugs were obtained from the Experimental Therapeutics Chemical Information System, Walter Reed Army Institute of Research. Azithromycin was a gift from Pfizer, Inc.
Parasites and drug susceptibility testing.
The Sierra Leone I (D6) parasite and Indochina I (W2; Vietnam) parasite clones were used as reference standards. D6 is sensitive to the drugs tested (with the possible exception of mefloquine), and W2 is resistant to chloroquine, pyrimethamine, and proguanil. Eight isolates collected in the last decade were also assessed (Papua, Indonesia: I3, I14, A8121, and A9123; Thailand: C2A and C2B; Kenya: MF and KS021). C2A and C2B represent a pair of primary and recrudescent isolates from a patient who failed to respond to atovaquone treatment. The cutoff values used for drug resistance are as follows: chloroquine, 10 ng/ml; quinine, 20 ng/ml; and mefloquine, 10 to 20 ng/ml. There are no defined cutoff values for the other drugs tested.
All isolates were maintained in continuous cultures by a modification of the methods of Trager and Jensen (18). Each culture was maintained in 50-ml sealed flasks in an atmosphere of 90% nitrogen, 3 to 5% oxygen, and 2.5 to 4.0% CO2 (premixed bottled gas; Potomac Airgas, Hyattsville, Md.). Each flask was filled with 5 ml of culture medium supplemented with 10% pooled human plasma and A+ red blood cells at a hematocrit of 6%.
Parasite susceptibility testing was done with a modification of the semiautomated microdilution technique (6) in which the hematocrit was 1.5% and the initial parasitemia was 0.5 to 0.8% (>70% ring forms). All drugs were initially dissolved in 70% ethanol and then diluted to the desired starting concentration in culture medium containing 10% human serum. Dilutions from ethanol were always greater than 1:40 to avoid a carryover effect. The starting concentration (for serial dilutions across the microtiter plate) was assigned so that the 50% inhibitory concentration (IC50) of each drug would be in the center of the plate. Each drug was tested alone and at fixed ratios of its IC50 (azithromycin/test drug ratios of 0.5:0.5, 0.75:0.25, 0.25:0.75, 0.8:0.2, 0.2:0.8, and 0.83:0.27 [1:1, 3:1, 1:3, 4:1, 1:4, and 5:1, respectively]). Suspensions of the drugs and parasites were incubated in 96-well microtiter plates at 37°C. Because antibiotics have a delayed onset of action, the incubation period was extended from the usual 48 h to 68 h. Radiolabel (3H-hypoxanthene) was added to the suspension at 48 h. Each combination was assessed usually twice and up to four times (on separate days) to confirm reproducibility.
Data analysis.
IC50s were determined for each drug alone and for drugs in fixed concentration ratios by fitting a logistic dose-response equation to the concentration-response curves (TableCurve 2D; SPSS Science, Chicago, Ill.). IC50s were used to calculate 50% fractional inhibitory concentrations (FIC50s) as previously described (3, 14). FIC50s can be expressed with the following equation (14): FIC50 = (IC50 of drug A in combination/IC50 of drug A alone) + (IC50 of drug B in combination/IC50 of drug B alone). FIC50s of drug A and drug B at different concentration ratios were used to plot isobolograms, which represent a plane through the center of the three-dimensional dose-response surface. Sums of the FIC50s of drug A and drug B were used to generate the data presented in Tables 1 and 2. A sum of 1.0 represents the line of additivity on the isobologram, a sum of less than 1.0 represents a trend toward synergy, and a sum of greater than 1.0 represents a trend toward antagonism. No absolute breakpoints have been characterized for P. falciparum synergy testing. Ninety percent inhibitory concentrations (IC90s) were calculated from the IC50s and the slope of the dose-response curve by using the following equation: IC90 = IC50[(0.9/0.1)(1/slope)]. Ninety percent fractional inhibitory concentrations (FIC90s) and sums of FIC90s were then calculated by using the same methods as those described above for FIC50s. All data generated are presented unless a clear reason for assay failure was identified. Assay exclusion criteria were as follows: bacterial contamination, IC50s incorrectly aligned, low counts in control wells, and inability to accurately fit the logistic dose-response equation. Instead of presenting individual representative isobolograms, we plotted all data points to represent the complete range of interactions seen at the concentration ratios evaluated (including repeat assessments of the same isolate on separate days). Isobolograms can be estimated from these data points.
TABLE 1.
Results for chloroquine-azithromycin combinationa
| Source | Parasite clone | Sum (1:1 ratio)b
|
IC50 (ng/ml) of:
|
||
|---|---|---|---|---|---|
| FIC50s | FIC90s | Chloroquine | Azithromycin | ||
| Sierra Leone | D6c | 1.02 ± 0.19 | 1.24 ± 0.16 | 3.5 ± 3.5 | 5,499.3 ± 2,944.0 |
| Kenya | MFc | 1.18 ± 0.17 | 1.12 ± 0.03 | 4.0 ± 1.1 | 7,763.0 ± 3,544.0 |
| KS021 | 0.63 ± 0.02 | 0.55 ± 0.22 | 15.2 ± 3.4 | 2,229.5 ± 499.9 | |
| Thailand | C2A | 0.68 ± 0.10 | 0.62 ± 0.15 | 27.0 ± 7.6 | 827.2 ± 132.6 |
| C2B | 0.78 ± 0.02 | 0.64 ± 0.09 | 29.7 ± 10.4 | 741.0 ± 394.6 | |
| Indonesia | I3 | 0.78 ± 0.01 | 1.01 ± 0.53 | 34.2 ± 5.0 | 3,586.5 ± 1,518.2 |
| A8I21 | 0.90 ± 0.13 | 0.69 ± 0.17 | 39.7 ± 9.2 | 5,328.0 ± 2,261.7 | |
| I14 | 0.88 ± 0.17 | 0.99 ± 0.18 | 55.3 ± 9.9 | 4,991.0 ± 1,128.5 | |
| A9123 | 0.86 ± 0.01 | 0.76 ± 0.12 | 65.9 ± 14.3 | 10,207.0 ± 4,594.8 | |
| Vietnam | W2 | 0.89 ± 0.26 | 0.71 ± 0.31 | 57.9 ± 14.4 | 620.4 ± 595.3 |
Values are reported as means and standard deviations for assays run in duplicate on different days.
Synergy, <1; additivity, 1; antagonism, >1.
Chloroquine sensitive; 10 ng/dl is the cutoff in our laboratory.
TABLE 2.
Results for Antimalarial agents in combination with azithromycina
| Test drug | Source | Parasite isolate | Sum (1:1 ratio)b
|
IC50 (ng/ml) of:
|
||
|---|---|---|---|---|---|---|
| FIC50 | FIC90 | Test drug | Azithromycin | |||
| Quinine | Thailand | C2B | 0.51 ± 0.02 | 0.37 ± 0.05 | 64.8 ± 20.9 | 1,335.0 ± 1,325.1 |
| C2A | 0.71 ± 0.04 | 0.73 ± 0.27 | 43.2 ± 18.0 | 763.0 ± 409.1 | ||
| Vietnam | W2 | 1.00 ± 0.06 | 0.63 ± 0.12 | 24.2 ± 2.6 | 467.3 ± 165.8 | |
| Tafenoquine | Thailand | C2B | 0.83 ± 0.02 | 0.96 ± 0.01 | 64.6 ± 22.6 | 1,463.2 ± 837.8 |
| Vietnam | W2 | 0.63 ± 0.02 | 0.81 ± 0.01 | 110.4 ± 55.3 | 816.0 ± 339.4 | |
| Primaquine | Thailand | C2B | 0.67 ± 0.18 | 1.20 ± 0.34 | 242.7 ± 40.7 | 1,762.9 ± 1,469.4 |
| Vietnam | W2 | 0.80 ± 0.07 | 1.10 ± 0.37 | 146.1 ± 14.7 | 332.5 ± 61.5 | |
| Dihydroartemisinin | Thailand | C2B | 1.32 ± 0.30 | 1.02 ± 0.23 | 0.2 ± 0.0 | 886.0 ± 246.1 |
| Vietnam | W2 | 1.03 ± NA | 0.79 ± NA | 0.1 ± 0.1 | 424.0 ± 407.3 | |
| Mefloquine | Thailand | C2B | 0.96 ± 0.35 | 0.85 ± 0.12 | 5.7 ± 4.6 | 675.6 ± 538.0 |
| Vietnam | W2 | 1.10 ± 0.20 | 1.02 ± 0.17 | 1.7 ± 0.6 | 795.0 ± 855.6 | |
| Desbutylhalofantrine | Thailand | C2B | 0.88 ± 0.25 | 0.78 ± 0.17 | 3.0 ± 0.1 | 2,103.1 ± 1,796.2 |
| Vietnam | W2 | 0.97 ± 0.08 | 0.90 ± 0.08 | 0.3 ± 0.1 | 930.0 ± 1038.0 | |
| Ciprofloxacin | Thailand | C2B | 1.12 ± 0.26 | 1.02 ± 0.19 | 1,401.6 ± 1,897.8 | 987.6 ± 16.4 |
| Vietnam | W2 | 0.93 ± 0.38 | 1.04 ± 0.16 | 390.0 ± 408.7 | 833.0 ± 869.7 | |
| Proguanil | Thailand | C2B | 1.13 ± 0.15 | 1.01 ± 0.16 | 58.6 ± 18.0 | 640.5 ± 153.4 |
| Vietnam | W2 | 0.82 ± 0.04 | 0.60 ± 0.06 | 1,753.9 ± 1,099.9 | 357.0 ± 216.4 | |
Values are reported as means and standard deviations for assays run on different days.
Synergy, <1; additivity, 1; antagonism, >1.
Mean FICs and ICs for each malaria parasite were used for statistical comparisons (Tables 1 and 2). Correlation of variation was calculated as the standard deviation divided by the mean for each parasite FIC or IC. Ninety-five percent confidence intervals (CI) for mean FICs and ICs were computed. The one-sample t test was used to assess whether FICs were less than one. Unpaired t tests and the Mann-Whitney U test were used to compare chloroquine-sensitive and -resistant isolate FICs. The paired t test was used to compare mean coefficients of variation. Pearson correlation was used to assess the relationship between ICs as well as to compare the coefficients of variation between ICs and FICs. Multiple linear regression was used to assess factors associated with IC variability. Minitab 13 (Minitab Inc., State College, Pa.), SPSS (SPSS Science), or CrossGraphs 2.0 (PPD Informatics, Wilmington, N.C.) was used for statistical calculations or graphics.
RESULTS
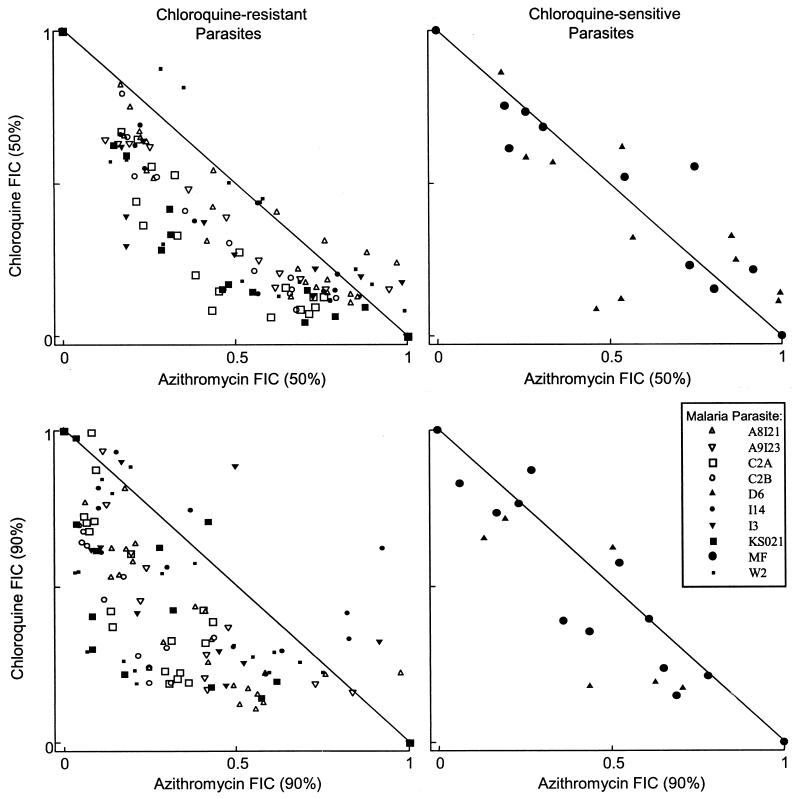
The chloroquine-azithromycin combinations are illustrated in Table 1 and Fig. 1. Both the table and the figure illustrate a range of activity from additive to synergistic for the eight chloroquine-resistant parasites (mean FIC50, 0.80, and 95% CI, 0.72 to 0.89; mean FIC90, 0.75, and 95% CI, 0.61 to 0.89) but additive for the two chloroquine-sensitive parasites (mean FIC50, 1.1, and 95% CI, 0.08 to 2.1; mean FIC90, 1.2, and 95% CI, 0.42 to 1.94). The FIC estimates for the chloroquine-resistant isolates were significantly less than one (FIC50, P = 0.001; FIC90, P = 0.004). Despite there being only two chloroquine-sensitive isolates, a statistically significant difference was found between the chloroquine-sensitive and -resistant isolates with the Mann-Whitney U test (FIC50, P = 0.05; FIC90, P = 0.05) but only for FIC90 with the unpaired t test (FIC50, P = 0.18; FIC90, P = 0.01). There was no correlation between azithromycin sensitivity and chloroquine sensitivity (r = 0.09, P = 0.80) or the degree of synergy with chloroquine sensitivity (FIC50, r = 0.23, P = 0.52; FIC90, r = 0.34, P = 0.33) (Table 1). A trend was present for lower FICs for isolates for which azithromycin IC50s were lower (FIC50, r = 0.58, P = 0.08; FIC90, r = 0.48, P = 0.15).
FIG. 1.
Azithromycin and chloroquine FIC50s and FIC90s at various concentration ratios. All data points from successful assays are presented. Isobolograms can be visually fit through the data points. A concave isobologram is consistent with synergy, a convex one is consistent with antagonism, and a straight line is consistent with additivity. Axes are IC50s and IC90s normalized to 1.
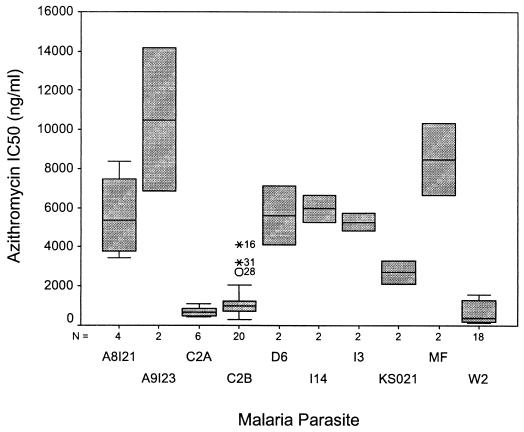
The azithromycin sensitivity of the parasites is illustrated in Table 1 and Fig. 2. The intraparasite variability in azithromycin IC50s could be accounted for largely by the percentage of schizonts at the start of the assay and minor variations in the time when the radiolabel was added (data not shown; R2 = 0.95). Between-day variability was slightly higher with azithromycin than with chloroquine (mean coefficients of variation: azithromycin IC50, 0.44; chloroquine IC50, 0.32 [P = 0.23]). Between-day FIC variability was lower than azithromycin IC50 variability (mean coefficients of variation: FIC50, 0.12 [P = 0.001]; FIC90, 0.25 [P = 0.04]).
FIG. 2.
Azithromycin sensitivity of parasites. Boxes represent the interquartile range, and error bars represent the range. Error bars are not present for some boxes because only two isolates are represented (see number of isolates indicated below the x axis). Data points outside of the range represent outliers.
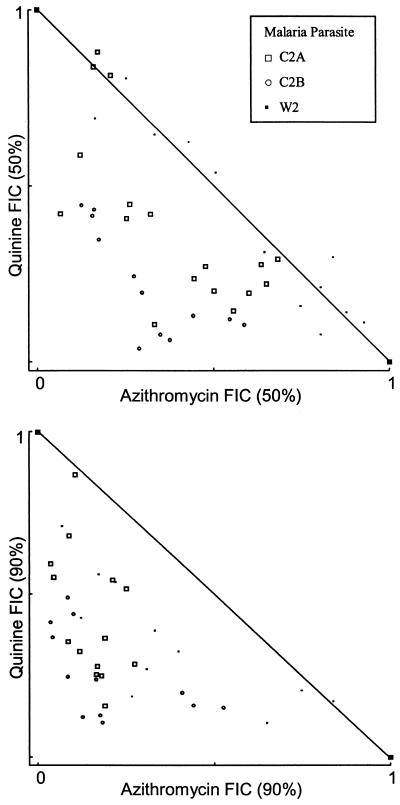
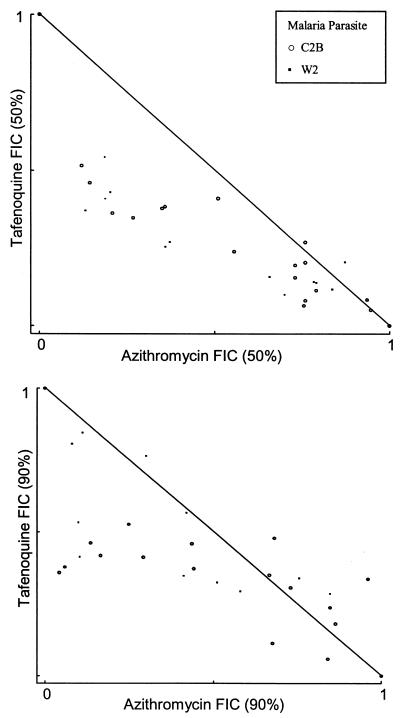
Additivity with a trend toward synergy was identified with quinine (mean FIC50, 0.74, and 95% CI, 0.13 to 1.35; mean FIC90, 0.58, and 95% CI, 0.115 to 1.038) and possibly with primaquine (mean FIC50, 0.74, and 95% CI, −0.09 to 1.56; mean FIC90, 1.15, and 95% CI, 0.52 to 1.79) and tafenoquine (mean FIC50, 0.73, and 95% CI, −0.54 to 2.0; mean FIC90, 0.89, and 95% CI, −0.07 to 1.84) (Table 2 and Fig. 3 and 4). Interpretation and statistical analyses of these data are limited by the sample size. Only the quinine FIC90 approached significance (P = 0.06). With quinine, more synergism was present at the FIC90, but this was not the case with primaquine or tafenoquine (Fig. 3 and 4). Less strong trends were seen with the remaining drugs tested (Table 2).
FIG. 3.
Azithromycin and quinine FIC50s and FIC90s at various concentration ratios.
FIG. 4.
Azithromycin and tafenoquine FIC50s and FIC90s at various concentration ratios.
DISCUSSION
The in vitro data reported here suggest that azithromycin will have clinical utility in combination with other antimalarial agents. This in vitro work was inspired by the safety of azithromycin and the partial activity that this drug demonstrated as a single agent for malaria prophylaxis.
Canfield and colleagues rescued atovaquone as an antimalarial drug with in vitro combination testing (4) following the rapid emergence in vivo of P. falciparum resistant to this drug (11). Proguanil showed moderate synergy and tetracycline showed mild to moderate synergy in vitro; both proved to be efficacious combinations in vivo. Proguanil was chosen as the partner drug because of its safety and pharmacokinetic profile. This combination is now successfully marketed as Malarone. The in vitro evaluations of azithromycin presented in this report were designed to model the same paradigm for antimalarial drug development.
In vitro sensitivity assessment of drug combinations for malaria is used to help predict which combinations will be clinically useful. Theoretically, if synergy is found in vitro, less than 50% of each of the components should achieve 100% cure rates. The greater the synergy, the less of each drug needed. Lower doses of one or both drugs may lead to increased tolerability and safety, more practical dosing regimens, and/or decreased cost. Additionally, synergy may allow two drugs, both less than 50% efficacious, to be combined to achieve 100% efficacy. On the other hand, if antagonism is found in vitro, more than 50% of each of the components will be needed to achieve 100% cure rates. The greater the antagonism, the more of each drug needed.
Many other factors also need to be considered when combining antimalarial agents. First and foremost, drugs with different mechanisms of action used in combination greatly reduce the probability that resistance will emerge (22). Ideally, to prevent the emergence of drug resistance, fully curative regimens of each agent alone should be used. Some antimalarial agents act rapidly and never have early (RIII) treatment failures (e.g., artesunate and quinine), while others act slowly (e.g., doxycycline and azithromycin) or have high-grade treatment failures in areas of resistance (chloroquine and pyrimethamine-sulfadoxine). These factors must be taken into consideration when designing treatment regimens in order to prevent early (RIII) treatment failures (17). For drugs that have dose-related toxicity (e.g., quinine), combination treatment could result in relatively lower or fewer doses without a loss of efficacy. Synergistic or additive toxicity must also be evaluated when combining drugs in vivo. Finally, a drug regimen’s complexity will affect compliance and must also be considered.
The antimicrobial activity of azithromycin in a Streptococcus pneumoniae infection model correlated best with the ratio of the area under the serum concentration-time curve to the MIC (5). It is unknown which pharmacokinetic parameter of azithromycin correlates with efficacy against malaria. It is clear that with weekly dosing, substantial activity was maintained for a week in a semi-immune population (2). Future studies should elucidate pharmacokinetic-pharmacodynamic profiles for azithromycin against malaria in order to optimize dosing regimens and to characterize the activity-time profile. Since azithromycin appears synergistic at all concentration ratios (symmetric isobolograms), doses used in clinics should not affect the level of synergy present.
Parasites from the various locations showed substantial variability. The two Thai isolates appear to have significantly lower IC50s (Fig. 2). We suspect that this finding occurred only by chance due to the small sample size. Malaria prophylaxis studies have revealed similar protective efficacies of azithromycin (250 mg/day) against P. falciparum in Thailand (69%; 95% CI, 0 to 89%), Indonesia (72%; 95% CI, 50 to 84%), and Kenya (83%; 95% CI, 68.5 to 91.1%) (2, 16; Heppner, personal communication).
In vitro sensitivity assays are limited in general by substantial between-day and between-laboratory variabilities in the IC50 or IC90 results reported. IC50s or IC90s have not been shown to predict treatment failure with most antimalarial drugs in a given individual. They do, however, reflect population trends toward drug resistance. The intent of assessing antimalarial combinations is to help select combinations that will be clinically useful. While this paradigm needs further validation, it was successful with atovaquone-proguanil (4). We have attempted to achieve a high standard for data reporting in this article, including duplicate assays on separate days and presentation of all of the data points instead of just representative assays. We encourage uniformity in the conduct and reporting of in vitro combination studies so that pooled data can be compared with results of clinical trials. The data presented in this article contribute to a growing body of evidence suggesting that in vitro combination results will help predict in vivo outcomes.
Three trials have assessed the in vivo efficacy of azithromycin in combination with artemisinin derivatives for falciparum malaria (10, 12; P. I. de Vries, N. H. Le, T. D. Le, P. L. Ho, V. N. Nguyen, K. A. Trinh, and P. A. Kager, Letter, Trop Med. Int. Health 4:407-408, 1999). All revealed very limited efficacy of azithromycin at doses of between 50 and 500 mg daily for 3 days. Azithromycin-artesunate was found to be additive with a trend toward antagonism in vitro in this study (Table 2).
We have recently completed phase II dose-ranging studies with larger doses of azithromycin (1,000 to 1,500 mg/day for 3 days) in combination with additive to synergistic antimalarial agents (chloroquine and quinine). These phase II studies were initiated based on the results presented in this report. Preliminary results with 3-day regimens revealed very high cure rates for quinine in Thailand and chloroquine in India (Robert Scott Miller and Michael Dunne, personal communication). Ultimately, we plan to evaluate the best combinations in phase III clinical trials with pregnant women and children, patient groups that bear the brunt of the morbidity and mortality attributed to P. falciparum infections.
Acknowledgments
This study was supported by Pfizer, Inc., and by the U.S. Army Research and Materiel Command.
The views expressed here are those of the authors and not necessarily those of the U.S. Army or the U.S. Department of Defense.
REFERENCES
- 1.Andersen, S. L., A. Ager, P. McGreevy, B. G. Schuster, D. Wesche, R. Kuschner, C. Ohrt, W. Ellis, R. Rossan, and J. Berman. 1995. Activity of azithromycin as a blood schizonticide against rodent and human plasmodia in vivo. Am. J. Trop. Med. Hyg. 52:159-161. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, S. L., A. J. Oloo, D. M. Gordon, O. B. Ragama, G. M. Aleman, J. D. Berman, D. B. Tang, M. W. Dunne, and G. D. Shanks. 1998. Successful double-blinded, randomized, placebo-controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in western Kenya. Clin. Infect. Dis. 26:146-150. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 4.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. 1997. Postantibiotic effects and the dosing of macrolides, azalides, and streptogramins, p. 27-38. In S. H. Zinner, L. S. Young, J. F. Acar, and H. C. Neu (ed.), Expanding indications for the new macrolides, azalides, and streptogramins. Marcel Dekker, Inc., New York, N.Y.
- 6.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese, R. F., D. B. Newman, K. A. Gordon, and T. G. Brewer. 1999. Acute high dose arteether toxicity in rats. Neurotoxicology 20:851-859. [PubMed] [Google Scholar]
- 8.Gray, R. H., F. Wabwire-Mangen, G. Kigozi, N. K. Sewankambo, D. Serwadda, L. H. Moulton, T. C. Quinn, K. L. O'Brien, M. Meehan, C. Abramowsky, M. Robb, and M. J. Wawer. 2001. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am. J. Obstet. Gynecol. 185:1209-1217. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood, B., and T. Mutabingwa. 2002. Malaria in 2002. Nature 415:670-672. [DOI] [PubMed] [Google Scholar]
- 10.Krudsood, S., U. Silachamroon, P. Wilairatana, P. Singhasivanon, W. Phumratanaprapin, K. Chalermrut, N. Phophak, and C. Popa. 2000. A randomized clinical trial of combinations of artesunate and azithromycin for treatment of uncomplicated Plasmodium falciparum malaria in Thailand. Southeast Asian J. Trop. Med. Public Health 31:801-807. [PubMed] [Google Scholar]
- 11.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 12.Na-Bangchang, K., T. Kanda, P. Tipawangso, A. Thanavibul, K. Suprakob, M. Ibrahim, Y. Wattanagoon, and J. Karbwang. 1996. Activity of artemether-azithromycin versus artemether-doxycycline in the treatment of multiple drug resistant falciparum malaria. Southeast Asian J. Trop. Med. Public Health 27:522-525. [PubMed] [Google Scholar]
- 13.Nosten, F., M. Vincenti, J. Simpson, P. Yei, K. L. Thwai, A. de Vries, T. Chongsuphajaisiddhi, and N. J. White. 1999. The effects of mefloquine treatment in pregnancy. Clin. Infect. Dis. 28:808-815. [DOI] [PubMed] [Google Scholar]
- 14.Saiman, L., F. Mehar, W. W. Niu, H. C. Neu, K. J. Shaw, G. Miller, and A. Prince. 1996. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin. Infect. Dis. 23:532-537. [DOI] [PubMed] [Google Scholar]
- 15.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 16.Taylor, W. R., T. L. Richie, D. J. Fryauff, H. Picarima, C. Ohrt, D. Tang, D. Braitman, G. S. Murphy, H. Widjaja, E. Tjitra, A. Ganjar, T. R. Jones, H. Basri, and J. Berman. 1999. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin. Infect. Dis. 28:74-81. [DOI] [PubMed] [Google Scholar]
- 17.Taylor, W. R., H. Widjaja, T. L. Richie, H. Basri, C. Ohrt, Tjitra, E. Taufik, T. R. Jones, K. C. Kain, and S. L. Hoffman. 2001. Chloroquine/doxycycline combination versus chloroquine alone, and doxycycline alone for the treatment of Plasmodium falciparum and Plasmodium vivax malaria in northeastern Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 64:223-228. [DOI] [PubMed] [Google Scholar]
- 18.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 19.van Riemsdijk, M. M., M. M. van der Klauw, J. A. van Heest, F. R. Reedeker, R. J. Ligthelm, R. M. Herings, and B. H. Stricker. 1997. Neuro-psychiatric effects of antimalarials. Eur. J. Clin. Pharmacol. 52:1-6. [DOI] [PubMed] [Google Scholar]
- 20.Watt, G., L. Loesuttivibool, G. D. Shanks, E. F. Boudreau, A. E. Brown, K. Pavanand, H. K. Webster, and S. Wechgritaya. 1992. Quinine with tetracycline for the treatment of drug-resistant falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 47:108-111. [DOI] [PubMed] [Google Scholar]
- 21.Wawer, M. J., N. K. Sewankambo, D. Serwadda, T. C. Quinn, L. A. Paxton, N. Kiwanuka, F. Wabwire-Mangen, C. Li, T. Lutalo, F. Nalugoda, C. A. Gaydos, L. H. Moulton, M. O. Meehan, S. Ahmed, R. H. Gray, et al. 1999. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet 353:525-535. [DOI] [PubMed] [Google Scholar]
- 22.White, N., and P. Olliaro. 1996. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol. Today 12:399-401. [DOI] [PubMed] [Google Scholar]
- 23.White, N. J., and P. Olliaro. 1998. Artemisinin and derivatives in the treatment of uncomplicated malaria. Med. Trop. 58:54-56. [PubMed] [Google Scholar]
- 24.Yeo, A. E., and K. H. Rieckmann. 1995. Increased antimalarial activity of azithromycin during prolonged exposure of Plasmodium falciparum in vitro. Int. J. Parasitol. 25:531-532. [DOI] [PubMed] [Google Scholar]