Abstract
Human peptidoglycan recognition protein 2 (PGLYRP2) is an N-acetylmuramoyl-l-alanine amidase that hydrolyzes bacterial peptidoglycan and is constitutively produced in the liver and secreted into the blood. Here we demonstrate that PGLYRP2 was not expressed in healthy human skin and had low expression in the eye. However, upon exposure to gram-positive and gram-negative bacteria or cytokines, PGLYRP2 expression was highly induced in keratinocytes and to a lower level in corneal epithelial cells. Expression of PGLYRP2 was not induced in nonepithelial cells. Exposure of keratinocytes to bacteria induced keratinocyte differentiation and stress response and inhibited activation of signal transduction molecules involved in cell proliferation. Induction of PGLYRP2 expression correlated with expression of differentiation markers (cytokeratins and transglutaminase). Bacteria induced activation of p38 mitogen-activated protein kinase (MAPK) in keratinocytes, which was required for the induction of PGLYRP2 expression, because induction of PGLYRP2 transcription by bacteria was inhibited by SB203580 (a specific inhibitor of p38 MAPK) and by a dominant-negative p38 construct. Induction of PGLYRP2 expression by bacteria (in contrast to expression of human β-defensin-2) was not mediated by Toll-like receptor 2 or 4. PGLYRP2 may function in the skin and the eyes as an inducible scavenger of proinflammatory peptidoglycan.
Skin protects host tissues from invasion by microorganisms. Skin was once regarded as a merely mechanical barrier due to its thick layer of keratinized epithelium (49). While this function is important, in recent years it has become clear that skin is rich in antimicrobial peptides and proteins, such as β-defensins (20, 21, 45), dermcidin (44), cathelicidin (12, 48), RNases (22), psoriasin (18), bactericidal permeability-increasing protein (51), and others (32, 63, 64). Healthy intact skin contains low levels of antimicrobial peptides and proteins. Breakage of the keratinized layer, however, may expose keratinocytes to high numbers of microorganisms, which induce production of antimicrobial peptides and proteins. Antimicrobial peptides and proteins are also produced in the skin in response to proinflammatory cytokines released from macrophages and dendritic cells stimulated by microorganisms or in some inflammatory disease processes, such as psoriasis (6, 12, 13, 18, 22, 29, 38, 45, 48, 63, 64).
In addition to antimicrobial peptides and proteins, body secretions, such as sweat and tears, contain a bacteriolytic enzyme, lysozyme (EC 3.2.1.17). Lysozyme hydrolyzes the glycosidic bond between β(1-4)-linked N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) of peptidoglycan, a polymer uniquely present in the cell walls of virtually all bacteria (3, 50). Lysozyme by itself is bacteriolytic for only some gram-positive bacteria, but it acts synergistically with antimicrobial peptides enhancing their antibacterial effect. Digestion of peptidoglycan by lysozyme is also important in reducing peptidoglycan's proinflammatory activity (14).
Mammals also have another enzyme that digests peptidoglycan, N-acetylmuramoyl-l-alanine amidase (EC 3.5.1.28), which is primarily present in the serum and which hydrolyzes the amide bond between MurNAc and l-Ala and thus removes stem peptides from the peptidoglycan molecule (5, 24, 36, 56-58). Digestion of peptidoglycan with amidase reduces or eliminates biologic activities of polymeric peptidoglycan (23, 34).
We have recently demonstrated that human peptidoglycan recognition protein L (PGRP L) has N-acetylmuramoyl-l-alanine amidase activity (60). PGRPs are a family of pattern recognition molecules that were discovered first in insects (27, 62, 66) and then in mammals (7, 9, 27, 31). Insects have up to 17 different PGRP proteins that recognize peptidoglycan and bacteria and have several functions. They initiate activation of the prophenoloxidase cascade (which generates antimicrobial melanin and reactive oxygen species), activate Toll and Imd pathways (which induce production of antimicrobial peptides), participate in phagocytosis of bacteria, and are peptidoglycan-lytic enzymes (2, 7, 9, 27, 52, 62).
Mammals have a family of four PGRPs, which were initially named PGRP-S, PGRP-L, and PGRP-Iα and PGRP-Iβ, by analogy to insect PGRPs (31); they were recently renamed peptidoglycan recognition protein 1 (PGLYRP1) PGLYRP2, PGLYRP3, and PGLYRP4, respectively, by the Human Genome Organization Gene Nomenclature Committee.
Although mammalian PGLYRPs were initially thought of as pattern recognition receptors similar to insect PGRPs (7, 31), it is now becoming clear that they do not function as cell-surface receptors but more likely as effector molecules. Mammalian PGLYRP1 is present in granulocyte granules and has antibacterial properties (10, 30, 55), and mammalian PGLYRP2 is an N-acetylmuramoyl-l-alanine amidase (15, 60). PGLYRP2 is constitutively produced in the liver (31, 60) and is secreted into the bloodstream (68) but is not constitutively produced in other tissues. The aim of this study was to determine whether production of PGLYRP2 could be induced in other tissues that come in contact with bacteria and especially in the skin and the eyes.
MATERIALS AND METHODS
Cells and cell stimulation.
Human epidermal keratinocytes (from neonatal foreskin, unless otherwise indicated, or from adult skin) and human corneal epithelial cells (both from Cascade Biologics, Portland, OR) were grown in EpiLife medium with human keratinocyte growth supplement or human corneal growth supplement (Cascade Biologics), respectively. Human umbilical vein endothelial cells (HUVEC) were cultured in medium 200 with low serum growth supplement (from Cascade Biologics). The primary cells were used between the third and fifth passages and before each experiment were maintained in medium without the supplement for 18 to 24 h. Human U373 astrocytoma cell line (from the American Type Culture Collection, Rockville, MD) was cultured in RPMI 1640 medium with 10% fetal calf serum (HyClone, Logan, UT). Human peripheral blood monocytes from normal healthy donors were obtained, cultured, and stimulated as described previously (61). The experiments with human cells have been reviewed and approved by the Indiana University School of Medicine Institutional Review Board.
Cells were stimulated as indicated (see Results) for 4 h with 2 × 108/ml (unless otherwise indicated) of heat-killed (70°C, 30 min) Bacillus subtilis (ATCC 6633), Lactobacillus acidophilus (ATCC 4356), Staphylococcus aureus (clinical isolate Rb), Micrococcus luteus (ATCC 4698), Escherichia coli K12, Enterobacter cloacae (ATCC 13047), Pseudomonas aeruginosa (ATCC 39324), Candida albicans (ATCC 18804) (4 × 107/ml), lipopolysaccharide from Salmonella minnesota Re595 (61), interleukin 1β (IL-1β) (human recombinant) (from DuPont; obtained through the National Cancer Institute, Rockville, MD) (100 ng/ml), tumor necrosis factor alpha (TNF-α) (mouse recombinant expressed in E. coli) (Sigma, St. Louis, MO) (100 ng/ml), insulin-like growth factor I (human recombinant expressed in E. coli) (Sigma) (100 ng/ml), or transforming growth factor alpha (TGF-α) (human recombinant expressed in E. coli) (Sigma) (100 ng/ml). In experiments with kinase inhibitors, SB203580 (20 μM), PD98059 (50 μM), SP600125 (30 μM), wortmannin (100 nM), or the solvent dimethyl sulfoxide (all from Calbiochem, La Jolla, CA) was added to the keratinocyte cultures 1 h before the addition of the stimulants. Anti-human Toll-like receptor 2 (TLR2) monoclonal antibody (MAb) (clone 2392; Genentech, South San Francisco, CA), anti-human TLR4 MAb (clone HTA125; E-Bioscience, San Diego, CA), or control immunoglobulin G2a (IgG2a) (Pharmingen, San Diego, CA) was added at 20 μg/ml 30 min before the addition of the stimulants. The results were calculated as follows: percent IgG control = 100 × [mRNA expression in cultures with TLR]/[mRNA expression in cultures with control IgG].
RNA, real-time RT-PCR, and Northern blot analysis.
RNAs from normal human liver and skin were obtained from Clontech (Palo Alto, CA) and Stratagene (La Jolla, CA), respectively. Cornea and sclera were isolated from normal human eyes preserved in RNAlater (Ambion, Austin, TX) and obtained from the National Disease Research Interchange (Philadelphia, PA) or Central Florida Lions Eye and Tissue Bank (Tampa, FL). RNA was extracted from cornea, sclera, or cultured cells by use of TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative real-time reverse transcriptase PCR (RT-PCR) was done using TaqMan reagents and an ABI Prism 7000 sequence detection system as recommended by the manufacturer (Applied Biosystems, Foster City, CA). Briefly, first-strand cDNA was synthesized from 1 μg RNA by use of TaqMan reverse transcription reagents (Applied Biosystems) and random hexamers (Invitrogen), for 10 min at 25°C, followed by reverse transcription for 60 min at 37°C and reverse transcriptase inactivation for 5 min at 95°C. For quantitative real-time PCR, the comparative cycle threshold method was used with 18S RNA as an endogenous control. Each sample was assayed in duplicate with TaqMan Universal PCR Master Mix (Applied Biosystems), primer concentrations of 0.6 μM (experimental) and 0.2 μM (18S), and 0.1 μM probe concentrations (Table 1). The cycling conditions were as follows: uracil-DNA glycosylase incubation at 50°C for 2 min, AmpliTaqGold DNA polymerase activation at 95°C for 10 min, and 40 two-step cycles of 95°C for 15 s and 60°C for 60 s. Each experiment (including reverse transcription) was repeated at least three times. The results (Fig. 1) are ratios of the amounts of mRNA in tissues or in stimulated cells to unstimulated keratinocyte amounts. Northern blot analysis was done as previously described (31) with the following modifications: 10 μg RNA/lane was used, the PGLYRP2 (GenBank accession number AF384856) probe was the 518-nucleotide N-terminal fragment amplified with forward primer ACAATGGCCCAGGGTGTCCTCT and reverse primer CCTGGGGAGGAGGTGGCTCTTA, and the β-actin probe was from Clontech. The purified fragments were labeled with 32P by use of the RadPrime DNA labeling system (Invitrogen).
TABLE 1.
Primers and probes used for real-time RT-PCR
| mRNA | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| PGLYRP2 | CTGGATCCTACTCGGATTGCTACT | GCAGAAGCTGTGTGTCTGGTCTT | CCTGGCTGAGCTGGAGCAGAAAGTG |
| 18S | GCCGCTAGAGGTGAAATTCTTG | CATTCTTGGCAAATGCTTTCG | ACCGGCGCAAGACGGACCAG |
| HBD2 | TCCTCTTCTCGTTCCTCTTCATATTC | TTAAGGCAGGTAACAGGATCGC | ACCACCAAAAACACCTGGAAGAGGCA |
| IL-6 | CCAGGAGCCCAGCTATGAAC | CCCAGGGAGAAGGCAACTG | CCTTCTCCACAAGCGCCTTCGGT |
| TLR2 | GGCCAGCAAATTACCTGTGTG | AGGCGGACATCCTGAACCT | TCCATCCCATGTGCGTGGCC |
| TLR4 | CCAGTGAGGATGATGCCAGAAT | GCCATGGCTGGGATCAGAGT | TGTCTGCCTCGCGCCTGGC |
FIG. 1.
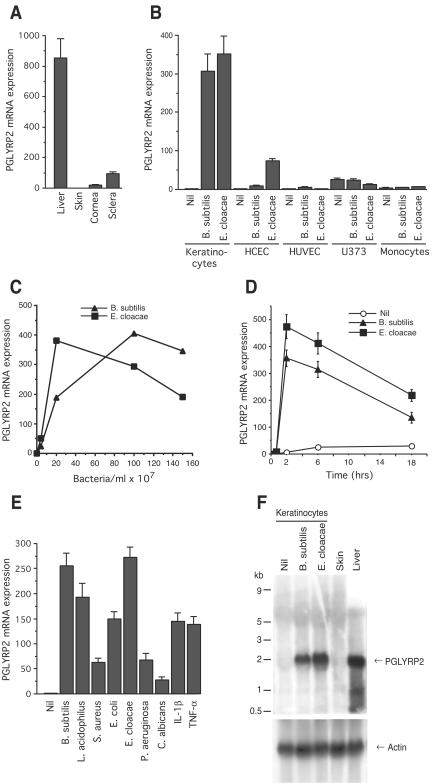
Bacteria induce expression of PGLYRP2 mRNA in human keratinocytes and corneal epithelial cells, shown by real-time RT-PCR (A to E) and Northern blotting (F). (A) Constitutive PGLYRP2 mRNA expression in normal human liver, skin, cornea, and sclera. (B) Induction of PGLYRP2 mRNA by bacteria in cultured human epidermal keratinocytes and human corneal epithelial cells (HCEC) but not in HUVEC, U373 astrocyte cell line, and peripheral blood monocytes. (C) Dose response and (D) time course of induction of PGLYRP2 mRNA expression by B. subtilis and E. cloacae in keratinocytes. Nil, no stimulus. (E) PGLYRP2 mRNA expression in keratinocytes is induced by a variety of bacteria, a yeast, and IL-1β and TNF-α. (F) Induction of 2-kb PGLYRP2 mRNA by B. subtilis and E. cloacae in keratinocytes. The results are means of three to four experiments ± standard errors (A, B, D, and E) or the averages of two experiments (C) or one out of two similar experiments (F).
IF.
Keratinocytes were grown on glass coverslips and stimulated as described above. Immunofluorescence (IF) staining of permeabilized cells was performed as described previously (60) with the following primary antibodies (Abs) (individually or in combination): (i) rabbit polyclonal anti-PGLYRP2 (1:100) obtained by immunization with PGLYRP2 cDNA and shown by IF and Western blotting to be specific for PGLYRP2 and not any other PGLYRP (68); (ii) a mixture of two mouse monoclonal anticytokeratin antibodies (clones AE1 and AE3; obtained from Dako, Carpinteria, CA), which react with 14 cytokeratins (cytokeratins 1 to 8, 10, 13 to 16, and 19); (iii) mouse monoclonal anti-human keratinocyte transglutaminase antibody (BT-621; obtained from Biomedical Technologies, Stoughton, MA); (iv) anti-human TLR2 MAb (clone 2392; from Genentech) (20 μg/ml); and (v) anti-human TLR4 MAb (clone HTA125; from E-Bioscience) (20 μg/ml). The secondary antibodies were goat anti-rabbit IgG-fluorescein isothiocyanate (1:320) and goat anti-mouse IgG-tetramethyl rhodamine isothiocyanate (1:150) (Sigma). The negative controls (cells stained with normal rabbit IgG or control IgG2a from Pharmingen and the secondary antibody) showed no specific fluorescence.
Detection of protein phosphorylation.
A phosphorylation screening that detects 49 phosphorylation sites associated with regulation of activity of 36 signal transduction proteins (Kinetworks KPSS-4.1 screen) was performed by Kinexus (Vancouver, Canada). This procedure is a qualitative and quantitative analysis of the expression and extent of phosphorylation of signal transduction proteins by use of a validated panel of antibodies specific to phosphorylation sites on these proteins that regulate the activity of these proteins, measured by chemiluminescence on Western blots. The results are normalized based on expression of known standards and expressed for each protein as percent untreated control = 100% × [normalized phosphorylation in a treated sample]/[normalized phosphorylation in an untreated control sample]. A change in the extent of phosphorylation of 25% or more is considered significant, based on the company's validation procedures. For the above-described phosphorylation screening, keratinocytes were left untreated (control) or were stimulated with E. cloacae for 10 or 30 min and then lysed at 1 mg soluble cellular protein/ml in a 20 mM MOPS (morpholinepropanesulfonic acid) buffer (pH 7.0) with 0.5% Triton X-100, 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 40 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenymethylsulfonyl fluoride, 3 mM benzamidine, 5 μM pepstatin A, and 10 μM leupeptin. The insoluble cytoskeleton was removed by centrifugation at 20,000 × g for 30 min at 4°C. The phosphorylation of p38 was further studied in keratinocyte lysates (prepared as described above) on Western blots with rabbit antibodies to phospho-p38 (specific for the T180/Y182 phosphorylation site) and to nonphosphorylated p38 (both antibodies obtained from Cell Signaling Technology, Beverly, MA), detected with anti-rabbit-peroxidase secondary antibody and an ECL enhanced chemiluminescence system (Amersham, Chicago Heights, IL). The bands were quantified using Kodak Image Station 440 and software, and the results were expressed as severalfold increases over the unstimulated control.
Activation of PGLYRP2 and HBD2 promoters.
PGLYRP2 (GenBank AF384856) 2-kb promoter, −15 to −2006 bp 5′ from the start codon, was cloned from genomic DNA (GenBank AC011492) into the KpnI and NheI sites of the luciferase reporter vector pGL3 basic (Promega, Madison, WI). Keratinocytes were cultured at 0.2 × 106/ml in 24-well plates (0.5 ml/well) in complete medium. Human HEK293 cells were cultured as described previously (59). Subconfluent cells were transfected with the following plasmids (individually or in combination): 2-kb PGLYRP2 promoter-luciferase reporter, human β-defensin 2 (HBD2) promoter-luciferase reporter (20), TLR2, TLR4, CD14 (46), and MD2 (47) (all at 0.4 to 0.8 μg/ml), and with 0.1 μg/ml of the following dominant-negative mutants: p38 (DNp38) (40), extracellular signal-regulated kinase 1 (ERK1) (dominant-negative ERK) (42), IκBΔN, MyD88, IL-1 receptor-associated kinase 1 (IRAK1), IRAK2 (59), or appropriate control vectors by use of Lipofectamine 2000 (Invitrogen). Following transfection, keratinocytes were maintained in medium without supplement for 18 to 24 h and then stimulated with bacteria (as described above) for 12 h. Luciferase activity that reflected induction of the PGLYRP2 or HBD2 transcription (activation of the PGLYRP2 or HBD2 promoters) was determined in the cell lysates as previously described (65). The results were expressed as severalfold increases over the unstimulated control.
Statistical analysis.
Differences between the groups were analyzed using Student's t test with GB-Stat PPC6.5.6 (Dynamic Microsystems, Silver Spring, MD), and the differences were considered significant at P ≤ 0.05.
RESULTS
Bacteria induce expression of PGLYRP2 in keratinocytes.
We have previously shown that PGLYRP2 mRNA had high constitutive expression in the liver and low expression in some parts of the intestinal tract (out of 76 tissues tested) (31). To further explore expression of PGLYRP2 in tissues that come in contact with the external environment, we tested whether PGLYRP2 is expressed in the skin and the eyes (which were not previously tested). PGLYRP2 mRNA was not expressed in normal human skin and had low expression in the cornea, and its expression was somewhat higher in the sclera but still ten times lower than in the liver, as determined by real-time RT-PCR (Fig. 1A). PGLYRP2 mRNA was also not expressed in cultured human neonatal (Fig. 1) and adult (not shown) keratinocytes, but high expression of PGLYRP2 mRNA was induced in cultures of human neonatal (Fig. 1B) and adult (not shown) keratinocytes following exposure to bacteria (B. subtilis and E. cloacae). Bacteria also induced expression of PGLYRP2 mRNA in cultured human corneal epithelial cells, but the level of PGLYRP2 mRNA expression in corneal cells was several times lower than in keratinocytes (Fig. 1B).
In contrast, exposure of HUVEC and other nonepithelial cells (U373 astrocytoma cell line or peripheral blood monocytes) to bacteria (Fig. 1B) or other stimuli, such as cytokines (IL-1β and TNF-α) or growth factors (IGF and TGF-α) (not shown), did not induce expression of PGLYRP2 mRNA (Fig. 1B), despite high induction of IL-6 mRNA (not shown).
Induction of PGLYRP2 mRNA expression in keratinocytes by bacteria was dose dependent (Fig. 1C) and rapid (Fig. 1D) and could be accomplished by both gram-positive and gram-negative bacteria, a yeast (C. albicans), and cytokines (IL-1β and TNF-α) (Fig. 1E). However, expression of PGLYRP2 mRNA in cultured keratinocytes was not induced by growth factors IGF and TGF-α (not shown) despite the previously shown ability of these growth factors to induce antimicrobial peptides in human keratinocytes (48). The size of the PGLYRP2 mRNA transcript induced by bacteria in keratinocytes (2 kb) was the same as that of the PGLYRP2 mRNA transcript constitutively produced in the liver, as determined by Northern blot analysis (Fig. 1F). The Northern blot analysis also confirmed the lack of constitutive expression of PGLYRP2 mRNA in normal human skin and in unstimulated keratinocytes (Fig. 1F). These results suggest that PGLYRP2 expression is selectively induced in epithelial cells by bacteria and other stimuli but is not induced in other cells, such as endothelial cells or monocytes.
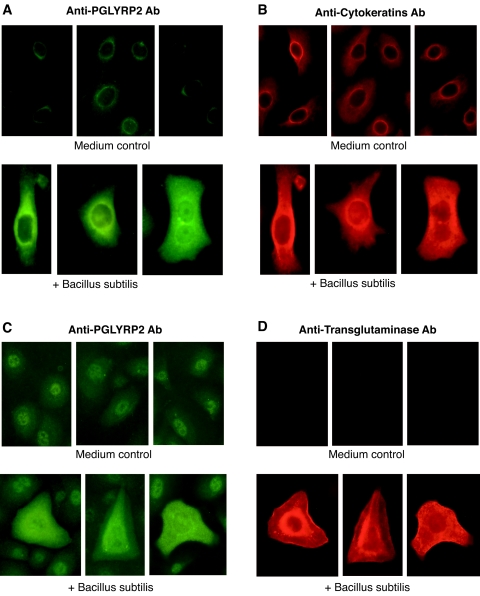
We then used IF to determine whether stimulation of keratinocytes with bacteria induced production of PGLYRP2 protein. Unstimulated keratinocytes showed low-intensity perinuclear staining with anti-PGLYRP2 Abs, whereas following exposure to bacteria, some keratinocytes showed intense cytoplasmic PGLYRP2 staining (Fig. 2A). The latter cells comprised approximately 5% of all keratinocytes in cultures, although all cells in our cultures were keratinocytes, as confirmed by their expression of cytokeratins, which are selective markers for keratinocytes (11, 49). These results indicate that only a subpopulation of keratinocytes in culture is activated to produce PGLYRP2.
FIG. 2.
Bacteria induce expression of PGLYRP2 protein in keratinocytes, which correlates with high expression of cytokeratins and transglutaminase. Keratinocytes were cultured in medium alone or with B. subtilis as indicated, and the expression of PGLYRP2 and cytokeratins or transglutaminase was determined by two-color IF. The results represent one out of two to three similar experiments. Similar results were obtained for keratinocyte cultures stimulated with E. cloacae (not shown).
High expression of PGLYRP2 following exposure to bacteria correlated with high expression of cytokeratins (i.e., all cells with high expression of PGLYRP2 had high expression of cytokeratins and vice versa) (Fig. 2A and B). These results suggested that PGLYRP2-expressing cells may represent more-differentiated keratinocytes. To further test this hypothesis, we performed double-IF staining of unstimulated and bacterially stimulated keratinocytes with anti-PGLYRP2 Ab and antitransglutaminase Ab (a marker for differentiated keratinocytes) (11, 54). Unstimulated keratinocytes were not stained with antitransglutaminase Ab, whereas keratinocytes stimulated with bacteria that showed increased cytoplasmic PGLYRP2 expression were also highly positive for transglutaminase (Fig. 2C and D). Most, but not all, PGLYRP2-positive cells were transglutaminase positive, whereas we did not detect any transglutaminase-positive PGLYRP2-negative cells. These results indicate that expression of PGLYRP2 correlates with differentiation of keratinocytes and suggest that PGLYRP2 may be expressed somewhat earlier than transglutaminase in the differentiation pathway of keratinocytes.
PGLYRP2 is induced in keratinocytes through a p38 MAPK-dependent pathway.
To gain insight into the pathway(s) through which bacteria induce expression of PGLYRP2 in keratinocytes, we next performed a phosphorylation screening (Kinetworks KPSS-4.1) that detects 49 phosphorylation sites associated with regulation of activity of 36 signal transduction proteins. Out of 36 proteins tested, 20 were detected in cultured keratinocytes, and 14 of these proteins showed a significant change in phosphorylation status: 10 showed decreased and 4 showed increased phosphorylation (Table 2). Although these phosphoproteins can have multiple functions in various cell types, the proteins whose phosphorylation was decreased following exposure to bacteria (cyclin-dependent kinase 1, ERK1, ERK2, mitogen-activated protein kinase [MAPK]/Erk kinase 1/2 [MEK1/2], p70 S6 kinase, protein kinase B [PKBα], protein kinase C α/β [PKCα/β], Raf1 60, Raf1 70, and retinoblastoma protein [Rb]) (Table 2) are activated by growth factors and are important in cell proliferation. On the other hand, the four proteins whose phosphorylation was increased (MAPK kinase 6 [MKK6], p38 MAPK, Lyn, and type 1 protein phosphatase alpha [PP1α]) (Table 2) are involved in stress responses, cell activation, and cell differentiation. Therefore, these results suggest that exposure of keratinocytes to bacteria inhibits their proliferation and induces their differentiation, which is consistent with increased expression of cytokeratins and keratinocyte transglutaminase in cultured keratinocytes following exposure to bacteria (Fig. 2).
TABLE 2.
Changes in phosphorylation status of proteins in keratinocytes following exposure to bacteria
| Protein | Abbreviation | Phosphorylated epitope | % Of unstimulated controla |
|---|---|---|---|
| Cyclin-dependent kinase 1 | CDK1 | Y15 | 53 |
| Extracellular signal-regulated kinase 1 | ERK1 | T202/Y204 | 50 |
| Extracellular signal-regulated kinase 2 | ERK2 | T185/Y187 | 43 |
| Lyn (44) | Lyn (44) | Y507 | 175 |
| MAPK/Erk kinase 1/2 | MEK1/2 | S217/221 | 70 |
| Mitogen-activated protein kinase kinase 6 | MKK6 | S207 | 218 |
| p38 mitogen-activated protein kinase | p38 MAPK | T180/Y182 | 209 |
| p70 S6 kinase | S6Ka p70 | T421/T424 | 61 |
| Protein kinase B | PKBα (Akt1) | T308 | 49 |
| Protein kinase C α/β | PKC α/β | T638 | 53 |
| Raf (60) | Raf1 (60) | S259 | 19 |
| Raf (70) | Raf1 (70) | S259 | 67 |
| Retinoblastoma protein | Rb | S807/S811 | 59 |
| Type 1 protein phosphatase α | PP1α | T320 | 165 |
Keratinocytes were left unstimulated or stimulated for 10 or 30 min with E. cloacae; percent unstimulated control = 100% × normalized phosphorylation in a stimulated sample/normalized phosphorylation in an unstimulated control sample; a change of 25% or more is considered significant.
The greatest increase in phosphorylation was noted in MKK6 and p38 MAPK. Since MKK6 is one of the kinases that phosphorylate and activate p38 MAPK (19, 41), we next investigated whether p38 MAPK was involved in induction of PGLYRP2 expression in keratinocytes activated by bacteria. We first confirmed increased phosphorylation of the activation motif of p38 MAPK (T180/Y182) in keratinocytes exposed to bacteria. Exposure of keratinocytes to B. subtilis or E. cloacae induced increased phosphorylation of T180/Y182, with a maximum at 30 min following addition of bacteria (Fig. 3).
FIG. 3.
Stimulation of keratinocytes with bacteria induces phosphorylation of p38 MAPK. Keratinocytes were cultured in medium alone or were stimulated with B. subtilis or E. cloacae, and the presence of phosphorylated p38 MAPK (P-p38) or total p38 MAPK (p38) in the cell lysates was determined on Western blots. The results are a representative blot (A) and quantification of average increases of P-p38 (expressed as the ratio to unstimulated time 0 group; means from four experiments).
We then used two approaches to test whether p38 MAPK was involved in the activation of PGLYRP2 expression by bacteria in keratinocytes. First, we took advantage of specific inhibitors of p38 MAPK (SB203580), ERK1/2 (PD98059, an inhibitor MKK1/2), JNK (SP600125), and phosphatidylinositol 3-kinase (wortmannin). When keratinocytes were stimulated with bacteria in the presence of these inhibitors, only SB203580, the inhibitor of p38 MAPK, significantly inhibited bacterially induced expression of PGLYRP2 mRNA (Fig. 4A).
FIG. 4.
Activation of p38 MAPK by bacteria is required for induction PGLYRP2 expression. (A) Induction PGLYRP2 expression in keratinocytes by B. subtilis or E. cloacae is inhibited by an inhibitor of p38 MAPK activation (SB203580) but not by inhibitors of ERK1 and ERK2 activation (PD98059), JNK activation (SP600125), and PI3 kinase activation (wortmannin). The results represent the means of four experiments ± standard errors (*, P ≤ 0.005 [treated versus untreated or dimethyl sulfoxide control]); all other differences were not significant (P > 0.05). DMSO, dimethyl sulfoxide. (B) Induction of transcription of PGLYRP2 promoter by bacteria in transiently transfected keratinocytes is inhibited by dominant-negative p38 (DNp38) but not by dominant-negative ERK (DNERK). The results represent means for four cultures from two experiments (*, P ≤ 0.015) (DNp38 versus control vector [CV]).
Because SB203580 also inhibits RICK kinase (in addition to p38 kinase) (25), in the second approach we tested whether a dominant-negative mutant of p38 inhibits induction of PGLYRP2 promoter. The PGLYRP2 promoter was activated in keratinocytes by bacteria, and a dominant-negative p38 construct, but not a dominant-negative ERK1 construct, inhibited activation of PGLYRP2 promoter in keratinocytes stimulated with bacteria (Fig. 4B). The results shown in Fig. 4, therefore, indicate that p38 MAPK is required for the induction of PGLYRP2 expression in keratinocytes stimulated with bacteria.
PGLYRP2 is not induced through TLR2 or TLR4.
We next tested whether expression of PGLYRP2 is induced by bacteria through TLR2 or TLR4, because (i) TLR2 and TLR4 are the primary receptors recognizing gram-positive and gram-negative bacteria in cells of myeloid origin (8, 46, 53, 67); (ii) TLR2 and TLR4 are expressed in keratinocytes (28, 33, 35, 39); and (iii) activation of keratinocytes by S. aureus or its peptidoglycan was shown to occur through TLR2 (28, 33, 35).
We detected in our keratinocytes TLR2 mRNA and a low level of TLR4 mRNA expression by use of real-time RT-PCR and a low level of protein expression by use of IF (not shown). However, anti-TLR2 MAb, anti-TLR4 MAb, and a combination of anti-TLR2 plus anti-TLR4 MAbs did not inhibit induction of PGLYRP2 mRNA expression by B. subtilis and E. cloacae in cultured keratinocytes (Fig. 5A). B. subtilis and E. cloacae induced expression of HBD2 mRNA in cultured keratinocytes to an extent similar to that seen with PGLYRP2 mRNA (not shown), and, by contrast, anti-TLR2 MAb or a combination of anti-TLR2 plus anti-TLR4 MAbs significantly inhibited induction of HBD2 mRNA expression by B. subtilis or E. cloacae, respectively (Fig. 5B). The dependence of E. cloacae-induced HBD2 expression on both TLR2 and TLR4 is consistent with the presence of both TLR2 stimulants (lipoproteins, peptidoglycan) and TLR4 stimulants (lipopolysaccharide) in gram-negative bacteria.
FIG. 5.
PGLYRP2, in contrast to HBD2, is not induced through TLR2 or TLR4. (A and B) Induction of PGLYRP2 mRNA expression in keratinocytes by B. subtilis or E. cloacae (A) is not inhibited by anti-TLR2 and anti-TLR4 antibodies, in contrast to HBD2 mRNA expression (B), which is significantly inhibited by anti-TLR2 antibodies or by a mixture of anti-TLR2 and anti-TLR4 antibodies (following stimulation with B. subtilis or E. cloacae, respectively). The results in panels A and B are means of four experiments; P values versus the IgG control are shown, and all other differences were not significant (P > 0.05). (C) Induction of transcription of PGLYRP2 promoter in transiently transfected keratinocytes following stimulation with B. subtilis or E. cloacae is not increased by cotransfection with TLR2 or TLR4. (D) Transcription of PGLYRP2 promoter in transiently transfected 293 cells following stimulation with B. subtilis or E. cloacae is not induced by cotransfection with TLR2 or TLR4, in contrast to transcription of the HBD2 promoter, which is induced by both B. subtilis and E. cloacae or by E. cloacae only (but not B. subtilis) in 293 cells transfected with TLR2 or TLR4, respectively. TLR2-dependent induction of HBD2 transcription by B. subtilis or E. cloacae is inhibited by dominant-negative (DN) IκB (DN-IκB), DN-MyD88, and DN-IRAK1, but not by DN-IRAK2. All cells transfected with TLR2 or TLR4 were also cotransfected with CD14 or CD14 plus MD-2, respectively. The results in panels C and D are means of three experiments. Nil, no stimulus.
We next assayed whether cotransfection of TLR2 and TLR4 (and their accessory molecules, CD14 and MD-2) would increase the induction of PGLYRP2 promoter transcription to test the hypothesis that the lack of TLR2 or TLR4 dependence of induction of PGLYRP2 might have been due to low expression of TLRs or their accessory molecules in keratinocytes. However, cotransfection of keratinocytes with a PGLYRP2 promoter construct together with TLR2 or TLR4 (and CD14 or CD14 plus MD-2, respectively) did not increase induction of PGLYRP2 promoter transcription by B. subtilis or E. cloacae above the level induced by bacteria without TLR2 or TLR4 (Fig. 5C). Moreover, cotransfection of HEK293 cells with PGLYRP2 promoter and TLR2 plus CD14 or TLR4 plus CD14 plus MD2 and stimulation with B. subtilis or E. cloacae did not result in any induction of PGLYRP2 promoter, in contrast to induction of HBD2 promoter by bacteria, which was fully dependent on TLR2 or TLR4 (Fig. 5D). This TLR2-dependent induction of HBD2 by bacteria was inhibited by cotransfection with dominant-negative IκBΔN, MyD88, and IRAK1 (which are components of the TLR2- and TLR4-activated signal transduction pathway) (59) but not IRAK2 (which is not activated by TLR2 and TLR4) (Fig. 5D).
These results demonstrate that induction of PGLYRP2 expression by bacteria is not mediated through TLR2 or TLR4, in contrast to the induction of HBD2, which is mediated through TLR2 or TLR4 and is dependent on the activation of the MyD88 → IRAK1 → NF-κB pathway.
DISCUSSION
Some innate immunity defenses are constitutive, and some are inducible. Here we demonstrate that PGLYRP2 (N-acetylmuramoyl-l-alanine amidase) is not constitutively expressed in normal human skin, but its expression is inducible in keratinocytes by exposure to bacteria or cytokines. The inducible expression of PGLYRP2 is limited to epithelial cells, correlates with differentiation of these cells, and requires activation of p38 MAPK.
Human epidermis consists primarily of epidermal keratinocytes that undergo an orderly process of differentiation from proliferating keratinocytes in the basal layer, through progressively more differentiated keratinocytes in the spinous and granular layers, to nonviable external horny layer (11, 49). This differentiation program can be modified by stress, injury, or invasion by microorganisms. In healthy (uninfected and not inflamed) skin, keratinocytes do not express or express low levels of antimicrobial factors. Exposure of keratinocytes to microorganisms (which can gain entrance into the skin through a breakage of the horny layer) or to cytokines or growth factors (released in the injured or infected skin) induces expression of several antimicrobial factors, such as β-defensins (13, 20, 21, 29, 37, 38, 45, 48), cathelicidin (6, 12, 38, 48), RNases (22), secretory leukocyte protease inhibitor (48, 63, 64), neutrophil gelatinase-associated lipocalin (32), and psoriasin (18).
Our results are consistent with this model and show that exposure of proliferating keratinocytes to bacteria inhibits keratinocyte proliferation (associated with a decrease in phosphorylation of signal transduction molecules involved in cell proliferation) (Table 2) and induces both keratinocyte differentiation (increased expression of cytokeratins and transglutaminase) and stress response mediated through activation of the p38 MAPK pathway.
MAPK cascades are ubiquitous signal transducers of many signals delivered by growth factors, hormones, mitogens, and environmental agents. p38 MAPK is one of the three main families of MAPKs and is involved in many cell functions, including responses to inflammatory signals and stress (11, 43). p38 MAPK is usually activated by upstream kinases MKK3 and/or MKK6 (19, 41, 43), although in keratinocytes it can also be activated by MKK7 (4). p38 activation involves dual phosphorylation of a TGY motif, following which p38 translocates into the nucleus and phosphorylates and activates several transcription factors, including ATF-2, Elk, Myc, MEF2, Stat1, and CHOP, which in turn results in activation of transcription of several genes (11, 19, 41, 43).
Our results show that exposure of keratinocytes to bacteria induces phosphorylation of MKK6 and p38 MAPK and that bacteria-induced activation of p38 MAPK is required for the induction of PGLYRP2 transcription (Fig. 4). There are four isoforms of p38 MAPK: α, β, γ, and δ (11, 43). Keratinocytes express p38α and p38β (which are ubiquitously expressed) and p38δ (which is selectively expressed in some tissues) (11). p38α and p38β are involved in keratinocyte responses to proinflammatory signals and stress, and p38δ is involved in keratinocyte differentiation (11). Exposure of keratinocytes to bacteria likely results in the activation of all three p38 isoforms, and all three are likely involved in the activation of PGLYRP2 transcription, because DNp38 construct, which inhibits activity of all p38 isoforms, completely inhibited bacterially induced activation of transcription of PGLYRP2 promoter (Fig. 4B). However, SB203580, a specific inhibitor of p38α and p38β, but not of p38δ (4, 11), caused profound but not complete inhibition of bacterially induced PGLYRP2 mRNA expression (Fig. 4A), which suggests some contribution from SB203580-insensitive p38δ. Our results are consistent with the recent report of p38 activation in cultured keratinocytes induced by another bacterium, S. aureus (33).
Constitutive expression of PGLYRP2 is limited to the liver (31), from which it is secreted into the bloodstream (68). Our current results demonstrate that the inducible expression of PGLYRP2 is limited to epithelial cells (keratinocytes and corneal epithelial cells), since it is not induced in other cells, namely, endothelial cells, monocytes, and cell lines such as U373 (Fig. 1A) or HEK293, HELA, and Cos7 (unpublished results). We have cloned the PGLYRP2 promoter and determined that this promoter is inducible only in epithelial cells but not in other cells, whereas it is constitutively active only in liver cells but not in other cells. Furthermore, different regions of the promoter regulate the constitutive expression of PGLYRP2 in liver cells and the inducible expression of PGLYRP2 in keratinocytes (D. Gupta, S. Wang, X. Li, and H. Wang, unpublished data).
It remains to be determined which receptor(s) is involved in the recognition of bacteria by keratinocytes and in the induction of PGLYRP2 expression. Our results show that TLR2 and TLR4 are not involved in the induction of PGLYRP2 expression by bacteria in keratinocytes, although they are involved in the induction of HBD2 expression by bacteria. CD14 by itself is likely not involved in the induction of PGLYRP2 expression either, because (i) consistent with a previous report (26), we did not detect any significant expression of CD14 mRNA by use of real-time RT-PCR or of protein by use of IF (H. Wang and R. Dziarski, unpublished); (ii) transfection of cells with CD14 did not enable or enhance induction of PGLYRP2 expression by bacteria; and (iii) CD14 alone does not function as a cell-activating receptor, because it is not a transmembrane molecule. The induction of PGLYRP2 expression by bacteria in keratinocytes, however, seems to be direct and not through secretion of cytokines, such as IL-β or TNF-α, because these cytokines were not induced by bacteria in our keratinocyte cultures (H. Wang and R. Dziarski, unpublished). Induction of PGLYRP2 expression is likely mediated through a different mechanism than the induction of antimicrobial peptides, because (i) expression of antimicrobial peptides was induced in cultured keratinocytes by growth factors that participate in wound healing (48), but the same growth factors (IGF and TGF-α) did not induce PGLYRP2 mRNA expression, and (ii), as mentioned above, induction of HBD2 expression by bacteria was mediated through TLR2 and TLR4, and induction of PGLYRP2 was not.
Therefore, the receptors and pathways that activate defense mechanisms in keratinocytes are likely different from the pathways that operate in immune cells of myeloid origin. Accordingly, although TLRs play a major role in immune cells of myeloid origin, they may not play a major role in keratinocytes: only a few TLRs are expressed in keratinocytes, their expression is low, and they induce activation of only few defense molecules, such as HBD2 and IL-8 (references 28, 35, and 39 and this paper). Induction of many other defense molecules in keratinocytes likely does not depend on TLRs, and, therefore, the future challenge will be to identify the receptors and signal transduction pathways that induce defense mechanisms in keratinocytes.
Activation of keratinocytes by bacteria could involve nucleotide oligomerization domain-containing proteins 1 and 2 (Nod1 and Nod2), which recognize bacterial peptidoglycan (1, 9, 16, 17). However, the expression and function of Nods in keratinocytes have not been studied and will require further investigation. Nods are located intracellularly, and are activated by peptidoglycan fragments in cytosol and, therefore, may not serve as initial recognition receptors for extracellular bacteria. Moreover, Nods recognize peptidoglycan, and PGLYPR2 expression is induced not only by peptidoglycan-containing bacteria but also by fungi and cytokines.
Digestion of peptidoglycan with PGLYRP2, due to its amidase activity, separates the stem peptide of peptidoglycan from the glycan chain. PGLYRP2 is identical with the previously identified serum amidase (68), and it is the only human N-acetylmuramoyl-l-alanine amidase. The significance of induction of PGLYRP2 expression by bacteria could be fourfold. First, digestion of peptidoglycan with amidase reduces or eliminates cell-activating proinflammatory activity of polymeric peptidoglycan (23, 34). In mammals, recognition of extracellular polymeric peptidoglycan occurs through Toll-like receptor 2 (8, 46, 53, 67), which likely requires glycan chains and stem peptides. Second, because recognition of intracellular peptidoglycan by Nod2 requires, at minimum, a muramyl dipeptide peptidoglycan fragment (17), digestion of peptidoglycan with amidase would be expected to abolish Nod2-activiating capacity of peptidoglycan. Third, since the minimum structure activating Nod1 is a tripeptide derived from the stem peptide of peptidoglycan from gram-negative bacteria (without the glycan) (1, 16), such a peptide could be generated from polymeric peptidoglycan by digestion with amidase. And fourth, PGLYRP2, similarly to lysozyme, could enhance the killing activity by antibacterial peptides. Therefore, digestion of peptidoglycan with amidase could have a scavenger function to reduce proinflammatory activity of peptidoglycan or could generate Nod1-activating peptides and could enhance antimicrobial defenses. These possibilities will be explored in future studies.
Acknowledgments
We are grateful to Roger J. Davis for DN p38 plasmid, to Melanie H. Cobb for DN ERK1 plasmid, to Carsten J. Kirschning for TLR2, TLR4, and CD14 plasmids, to Kensuke Miyake for MD-2 plasmid, to Marta Muzio for DN MyD88, IRAK1, and IRAK2 plasmids, to D. W. Ballard for IκBΔN plasmid, and to Jens-M. Schroder and Jurgen Harder for HBD2 plasmid.
This work was supported by U. S. Public Health Service grants AI2879 and AI56395 from the National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 7:702-707. [DOI] [PubMed] [Google Scholar]
- 2.Chang, C.-I., S. Pili-Floury, M. Herve, C. Parquet, Y. Chelliah, B. Lemaitre, D. Mengin-Lecreulx, and J. Deisenhofer. 2004. A Drosophila pattern recognition receptor contains a peptidoglycan docking groove and unusual L,D-carboxypeptidase activity. PLOS Biol. 2:1293-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chipman, D. M., and N. Sharon. 1967. Mechanism of lysozyme action. Science 165:454-464. [DOI] [PubMed] [Google Scholar]
- 4.Dashti, S. R., T. Efimova, and R. L. Eckert. 2001. MEK7-dependent activation of p38 MAP kinase in keratinocytes. J. Biol. Chem. 276:8059-8063. [DOI] [PubMed] [Google Scholar]
- 5.De Pauw, P., C. Neyt, E. Vanderwinkel, R. Wattiez, and P. Falmagne. 1995. Characterization of human serum N-acetylmuramyl-l-alanine amidase purified by affinity chromatography. Protein Expr. Purif. 6:371-378. [DOI] [PubMed] [Google Scholar]
- 6.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 7.Dziarski, R. 2004. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 40:877-886. [DOI] [PubMed] [Google Scholar]
- 8.Dziarski, R., and D. Gupta. 2005. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect. Immun. 73:5212-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziarski, R., and D. Gupta. Peptidoglycan recognition in innate immunity. J. Endotoxin Res., in press. [DOI] [PubMed]
- 10.Dziarski, R., K. A. Platt, E. Gelius, H. Steiner, and D. Gupta. 2003. Defect in neutrophil killing and increased susceptibility to infection with non-pathogenic Gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102:689-697. [DOI] [PubMed] [Google Scholar]
- 11.Eckert, R. L., T. Efimova, S. Balasubramanian, J. F. Crish, F. Bone, and S. Dashti. 2003. p38 mitogen-activated protein kinases on the body surface—a function for p38δ. J. Investig. Dermatol. 120:823-828. [DOI] [PubMed] [Google Scholar]
- 12.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 13.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 14.Ganz, T., V. Gabayan, H.-I. Liao, L. Liu, A. Oren, T. Graf, and A. M. Cole. 2002. Increased inflammation in lysozyme M-deficient mice in response to Micrococcus luteus and its peptidoglycan. Blood 101:2388-2392. [DOI] [PubMed] [Google Scholar]
- 15.Gelius, E., C. Persson, J. Karlsson, and H. Steiner. 2003. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-l-alanine amidase activity. Biochem. Biophys. Res. Commun. 306:988-994. [DOI] [PubMed] [Google Scholar]
- 16.Girardin, S. E., I. G. Boneca, L. A. M. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M.-K. Taha, A. Labigne, U. Zharinger, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 17.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 18.Glaser, R., J. Harder, H. Lange, J. Bartels, E. Christophers, and J.-M. Schroder. 2005. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 6:57-64. [DOI] [PubMed] [Google Scholar]
- 19.Han, J., J.-D. Lee, Y. Jiang, Z. Li, L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 20.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 21.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 22.Harder, J., and J.-M. Schroder. 2002. RNase 7, a novel innate immune defense antimicrobial protein of human healthy skin. J. Biol. Chem. 277:46779-46784. [DOI] [PubMed] [Google Scholar]
- 23.Hoijer, M. A., M.-J. Melief, R. Debets, and M. P. Hazenberg. 1997. Inflammatory properties of peptidoglycan are decreased after degradation with human N-acetylmuramyl-l-alanine amidase. Eur. Cytokine Netw. 8:375-381. [PubMed] [Google Scholar]
- 24.Hoijer, M. A., M.-J. Melief, W. Keck, and M. P. Hazenberg. 1996. Purification and characterization of N-acetylmuramyl-l-alanine amidase from human plasma using monoclonal antibodies. Biochim. Biophys. Acta 1289:57-64. [DOI] [PubMed] [Google Scholar]
- 25.Hollenbach, E., M. Vieth, A. Roessner, M. Neumann, P. Malfertheiner, and M. Naumann. 2005. Inhibition of RICK/nuclear factor-κB and p38 signaling attenuates the inflammatory response in murine model of Crohn disease. J. Biol. Chem. 280:14981-14988. [DOI] [PubMed] [Google Scholar]
- 26.Hunyadi, J., M. Simon, A. S. Kenderessy, J. Olah, and A. Dobozy. 1992. Expression of adhesion molecules and monocyte markers on cultured human keratinocytes. Eur. J. Investig. Dermatol. 2:50-55. [Google Scholar]
- 27.Kang, D., G. Liu, A. Lundstrom, E. Gelius, and H. Steiner. 1998. A peptidoglycan recognition protein in innate immunity conserved from insects to mammals. Proc. Natl. Acad. Sci. USA 95:10078-10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai, K., H. Shimura, N. Minagawa, A. Ito, K. Tomiyama, and M. Ito. 2002. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J. Dermatol. Sci. 30:185-194. [DOI] [PubMed] [Google Scholar]
- 29.Liu, A. Y., D. Destoumieux, A. V. Wong, C. H. Park, E. V. Valore, L. Liu, and T. Ganz. 2002. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Investig. Dermatol. 118:275-281. [DOI] [PubMed] [Google Scholar]
- 30.Liu, C., E. Gelius, G. Liu, H. Steiner, and R. Dziarski. 2000. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 275:24490-24499. [DOI] [PubMed] [Google Scholar]
- 31.Liu, C., Z. Xu, D. Gupta, and R. Dziarski. 2001. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 276:34686-34694. [DOI] [PubMed] [Google Scholar]
- 32.Mallbris, L., K. P. O'Brien, A. Hulthen, B. Sandstedt, J. B. Cowland, N. Borregaard, and M. Stahle-Backdahl. 2002. Neutrophil gelatinase-associated lipocalin is a marker for dysregulated keratinocyte differentiation in human skin. Exp. Dermatol. 11:584-591. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara, M., D. Harada, H. Manabe, and K. Hasegawa. 2004. Staphylococcus aureus peptidoglycan stimulates granulocyte macrophage colony-stimulating factor production from human epidermal keratinocytes via mitogen-activated protein kinases. FEBS Lett. 566:195-200. [DOI] [PubMed] [Google Scholar]
- 34.Mellroth, P., J. Karlsson, and H. Steiner. 2003. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 278:7059-7064. [DOI] [PubMed] [Google Scholar]
- 35.Mempel, M., V. Voelcker, G. Kollisch, C. Plank, R. Rad, M. Gerhard, C. Schnopp, P. Fraunberger, A. K. Walli, J. Ring, D. Abeck, and M. Ollert. 2003. Toll-like receptor expression in human keratinocytes: nuclear factor κB controlled gene activation by Staphylococcus aureus is Toll-like receptor 2 not Toll-like receptor 4 or platelet activating factor receptor dependent. J. Investig. Dermatol. 121:1389-1396. [DOI] [PubMed] [Google Scholar]
- 36.Mollner, S., and V. Braun. 1984. Murein hydrolase (N-acetyl-muramyl-l-alanine amidase) in human serum. Arch. Microbiol. 140:171-177. [DOI] [PubMed] [Google Scholar]
- 37.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Droschner, V. Pestonjamasp, J. Piralno, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 38.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 39.Pivarcsi, A., L. Bodai, B. Rethi, A. Kennderessy-Szabo, A. Koreck, M. Szell, Z. Beer, Z. Bata-Csorgo, M. Magocsi, E. Rajnavolgyi, A. Dobozy, and L. Kemeny. 2003. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 15:721-730. [DOI] [PubMed] [Google Scholar]
- 40.Raingeaud, J., S. Gupta, M. Dickens, and J. Han. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 41.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins, D. J., E. Zhen, H. Owaki, C. A. Vanderbilt, D. Ebert, T. D. Geppert, and M. H. Cobb. 1993. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 268:5097-5106. [PubMed] [Google Scholar]
- 43.Saklatvala, J. 2004. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 4:372-377. [DOI] [PubMed] [Google Scholar]
- 44.Schittek, B., R. Hipfel, B. Sauer, J. Bauer, H. Kalbacher, S. Stevanovic, M. Schirle, K. Schroeder, N. Blin, F. Meier, G. Rassner, and C. Garbe. 2001. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2:1133-1137. [DOI] [PubMed] [Google Scholar]
- 45.Schroeder, J.-M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 46.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 47.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorensen, O. E., J. B. Cowland, K. Theilgaard-Monch, L. Liu, T. Ganz, and N. Borregaard. 2003. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170:5583-5589. [DOI] [PubMed] [Google Scholar]
- 49.Stenn, K. S. 1983. The skin, p. 540-573. In L. Weiss (ed.), Cell and tissue biology. Urban & Schwarzenberg, Baltimore, MD.
- 50.Stryer, L. 1988. Mechanisms of enzyme action, p. 201-223. In L. Stryer (ed.), Biochemistry, 3rd ed. W. H. Freeman, New York, N.Y.
- 51.Takahashi, M., Y. Horiuchi, and T. Tezuka. 2004. Presence of bactericidal/permeability-increasing protein in human and rat skin. Exp. Dermatol. 13:55-60. [DOI] [PubMed] [Google Scholar]
- 52.Takehana, A., T. Yano, S. Mita, A. Kotani, Y. Oshima, and S. Kurata. 2004. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 23:4690-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 54.Thacher, S. M., and R. H. Rice. 1985. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell 40:685-695. [DOI] [PubMed] [Google Scholar]
- 55.Tydell, C. C., N. Yount, D. Tran, J. Yuan, and M. E. Selsted. 2002. Isolation, characterization, and antimicrobial properties of bovine oligosaccharide-binding protein. J. Biol. Chem. 277:19658-19664. [DOI] [PubMed] [Google Scholar]
- 56.Valinger, Z., B. Ladesic, and J. Tomasic. 1982. Partial purification and characterization of N-acetylmuramyl-l-alanine amidase from human and mouse serum. Biochim. Biophys. Acta 701:63-71. [DOI] [PubMed] [Google Scholar]
- 57.Vanderwinkel, E., P. De Pauw, D. Philipp, J.-P. T. Have, and K. Bainter. 1995. The human N-acetylmuramyl-l-alanine amidase: distribution, action on different bacterial peptidoglycans, and comparison with the human lysozyme activities. Biochem. Mol. Med. 54:26-32. [DOI] [PubMed] [Google Scholar]
- 58.Vanderwinkel, E., M. De Vlieghere, P. De Pauw, N. Cattalini, V. Ledoux, D. Gigot, and J.-P. ten Have. 1990. Purification and characterization of N-acetylmuramoyl-l-alanine amidase from human serum. Biochim. Biophys. Acta 1039:331-338. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Z.-M., X. Li, R. R. Cocklin, M. Wang, M. Wang, K. Fukase, S. Inamura, S. Kusumoto, D. Gupta, and R. Dziarski. 2003. Human peptidoglycan recognition protein-L is an N-acetylmuramoyl-l-alanine amidase. J. Biol. Chem. 278:49044-49052. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Z.-M., C. Liu, and R. Dziarski. 2000. Chemokines are the main pro-inflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J. Biol. Chem. 275:20260-20267. [DOI] [PubMed] [Google Scholar]
- 62.Werner, T., G. Liu, D. Kang, S. Ekengren, H. Steiner, and D. Hultmark. 2000. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97:13772-13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiedow, O., J. Harder, J. Bartels, V. Streit, and E. Christophers. 1998. Antileukoprotease in human skin: an antibiotic peptide constitutively produced by keratinocytes. Biochem. Biophys. Res. Commun. 248:904-909. [DOI] [PubMed] [Google Scholar]
- 64.Wingens, M., B. H. van Bergen, P. S. Hiemstra, J. F. Meis, I. M. van Vlijmen-Willems, P. L. Zeeuwen, J. Mulder, H. A. Kramps, F. van Ruissen, and J. Schalkwijk. 1998. Induction of SLPI (ALP/HUSI-I) in epidermal keratinocytes. J. Investig. Dermatol. 111:996-1002. [DOI] [PubMed] [Google Scholar]
- 65.Xu, Z., R. Dziarski, Q. Wang, K. Swartz, K. M. Sakamoto, and D. Gupta. 2001. Bacterial peptidoglycan-induced tnf-α transcription is mediated through the transcription factors Egr-1, Elk-1, and NF-κB. J. Immunol. 167:6975-6982. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida, H., K. Kinoshita, and M. Ashida. 1996. Purification of peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 271:13854-13860. [DOI] [PubMed] [Google Scholar]
- 67.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 68.Zhang, Y., L. van der Fits, J. S. Voerman, M.-J. Melief, J. D. Laman, M. Wang, H. Wang, M. Wang, X. Li, C. D. Walls, D. Gupta, and R. Dziarski. 2005. Identification of serum N-acetylmuramoyl-l-alanine amidase as liver peptidoglycan recognition protein 2. Biochim. Biophys. Acta 1752:34-46. [DOI] [PubMed] [Google Scholar]