Abstract
Vaginal epithelial cell (VEC) anti-Candida albicans activity, despite being measured in vitro, is considered an innate host defense mechanism. This was supported further by the fact that women protected from symptomatic infection following a live intravaginal Candida challenge had increased VEC anti-Candida activity compared to those who acquired a symptomatic infection.
Vulvovaginal candidiasis (VVC), caused primarily by Candida albicans, is an opportunistic fungal infection that affects approximately 75% of women of childbearing age (10). Factors that predispose women to VVC include hormonal fluctuations (i.e., pregnancy, luteal phase of menstrual cycle, oral contraceptives, and hormone replacement therapy) or antibiotic usage. Another 5 to 10% of seemingly healthy women suffer from recurrent VVC (RVVC) (more than three episodes per annum) without any known predisposing factors (10). An undefined innate and/or adaptive immune deficiency or dysfunction is thought to enhance susceptibility to RVVC and possibly VVC as well.
Cell-mediated immunity is considered the predominant host defense mechanism against mucosal candidal infections. However, during the past 2 decades, research using animal models, as well as clinical studies, has suggested that adaptive immunity does not play a role in protection against VVC as a result of several putative immunoregulatory mechanisms (reviewed in reference 8). Instead, in a major paradigm shift, innate immunity is now considered to be associated with both resistance and susceptibility to VVC or RVVC (5, 6). Relative to resistance, epithelial cells from the vaginas of mice, humans, and macaques have been shown to inhibit the growth of C. albicans in vitro (2, 13, 14). Moreover, vaginal epithelial cell (VEC) anti-Candida activity in women with RVVC was reduced compared to that of healthy women, suggesting a possible role for VECs in protection against infection (2). This VEC activity is fungistatic and requires cell contact through an acid-labile mechanism, with no role for soluble factors (9, 12). Polymorphonuclear neutrophils (PMNs), on the other hand, while playing a protective role against Candida infection in blood (4), have little to no protective role against vaginal Candida infection (3, 6, 7).
Recently, our laboratory initiated an intravaginal live Candida challenge to evaluate the natural history of local immune responsiveness to Candida at the vaginal mucosa. Women with no history of VVC were generally protected against symptomatic infection, although asymptomatic infection occurred in some women. In contrast, women with an infrequent history of VVC (susceptible to infection while taking antibiotics, high-estrogen oral contraceptives, or hormone replacement therapy) were more susceptible to symptomatic infection (6). Symptomatic infection was associated with an infiltration of PMNs and high fungal burden, whereas protection against symptomatic infection was associated with a noninflammatory process and a lack of PMN infiltration. These results suggested that VVC was due to an aggressive innate response by PMNs rather than any deficiency in adaptive immunity. It is postulated that signals elicited by VECs following the interaction with Candida initiate in susceptible women the PMN response that is responsible for the signs and symptoms associated with vaginitis (6). On the other hand, protection against infection is associated with the lack of such signals and possibly with stronger VEC anti-Candida activity. The purpose of the present study was to determine whether VEC antifungal activity correlates with resistance to infection by using the live Candida challenge model.
(This work was presented in part at the World Conference on Vaginitis, Playa Herradura, Costa Rica, 10 to 13 January 2004.)
Eighteen healthy, nonpregnant women between the ages of 21 and 49 (70% African-American, 30% Caucasian) were enrolled at the Obstetrics and Gynecology Clinic at the Louisiana State University Health Sciences Center between April 2001 and October 2004 as part of a live intravaginal Candida challenge study (inoculated with a clinical vaginal isolate, DB579.94, as previously described [6]). Informed consent was obtained from all participants, and all procedures in the conduct of clinical research were followed in accordance with the Institutional Review Board at the Louisiana State University Health Sciences Center, New Orleans. Specimens (vaginal lavage and vaginal swab) were collected and processed, as previously described (6), from all women prior to intravaginal Candida inoculation. Prior to inoculation, seven women had multiple visits in which a vaginal lavage and a vaginal swab were collected. All women were confirmed free of detectable Candida colonization by vaginal swab culture on Sabouraud dextrose agar (Becton-Dickinson), although we do not rule out the possibility of some level of colonization detectable by more-sensitive techniques (i.e., PCR). After inoculation, the status of each woman, relative to signs and symptoms of VVC (vaginal discharge, vulvar or vaginal erythema and edema, burning, itching, or soreness), was determined over a 14-day period. Ten of the women inoculated became symptomatically infected (KOH test, positive yeast culture with C. albicans, presence of hyphae in lavage fluid, and visible signs and symptoms), and eight women became asymptomatically infected (positive yeast culture with C. albicans in the absence of any hyphae or symptoms/signs).
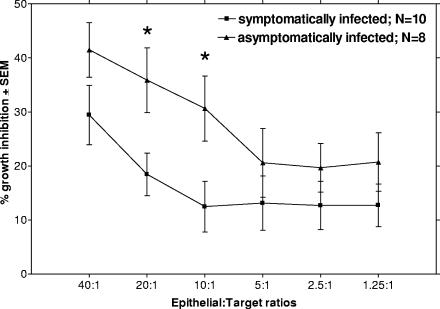
During the protocol, VECs were isolated from vaginal lavage as previously described (6). At the time of the assay, the VEC sample was thawed and the cellular fraction was enriched for epithelioid cells by density gradient centrifugation as previously described (14). To assess the growth inhibition potential of enriched viable VECs for each patient, an established modified [3H]glucose uptake assay was performed (13). Briefly, C. albicans strain 3153A grown to stationary phase was added to individual wells of a microtiter plate at 1 × 105 cells/ml in a volume of 100 μl of Phytone-peptone-10% fetal bovine serum medium along with 100 μl of epithelial-enriched cells in Phytone-peptone-10% fetal bovine serum at various effector-to-target (E:T) ratios, beginning at 40:1. The culture was then incubated at 37°C, 5% CO2 for 9 h in the presence of 1 μCi [3H]glucose. Following incubation, sodium perchloride (bleach) was added to each well, the cell extracts were harvested, and the incorporated [3H]glucose was measured by liquid scintillation (Beckman Instruments). Percent growth inhibition was calculated as previously described (13). A mean baseline for inhibition was determined for patients with multiple samplings prior to inoculation (whose data were determined to be normally distributed). Figure 1 shows that VECs collected from women who would become asymptomatically infected had generally higher levels of growth inhibitory activity than VECs from those who would become symptomatically infected, with statistically higher levels at both 20:1 and 10:1 E:T ratios (20:1, P = 0.0231; 10:1, P = 0.0279; Student's t test). Furthermore, vaginal fungal burden was found to be higher in women who became symptomatically infected than in those who became asymptomatically infected (P < 0.0001; Student's t test) (Table 1).
FIG. 1.
VEC growth inhibition of C. albicans. VECs were collected from women participating in a live intravaginal Candida challenge. The women were divided into groups, symptomatically infected and asymptomatically infected (the matched counterpart), based on the outcome of the challenge. The cells were evaluated for anti-Candida activity by a [3H]glucose uptake assay prior to challenge. Results are expressed as percent inhibition of total glucose uptake by C. albicans at various E:T ratios with epithelial cells. The figure shows the means ± standard errors of the means (SEM) for individual tests. *, P < 0.05.
TABLE 1.
Mean Candida CFU for women symptomatically and asymptomatically infected
| Status postinoculation (total no. of women) | Mean Candida CFU ± SEMa |
|---|---|
| Symptomatically infected (10) | 428 ± 45 |
| Asymptomatically infected (8) | 108 ± 20 |
Based on lateral swab and growth after 48 h on Sabouraud dextrose agar and Chromagar.
The moderate levels of activity by VECs from all women evaluated in this study are similar to those from other reports for human VEC anti-Candida activity, as was the dose response observed for the various E:T ratios (1, 2). Based on the trend toward a similar difference between the two groups at the 40:1 E:T ratio, we contend that statistical significance would be attained at this higher E:T ratio with a large sample size, whereas the activities at the lower E:T ratios would remain insignificant due to the lower overall levels of activity. Another important correlate was between vaginal fungal burden postinoculation and VEC activity. The high fungal burdens in those who acquired the symptomatic infection correlated well to the lower VEC anti-Candida activities. The converse was true as well, whereby the lower fungal burdens in those who became asymptomatically infected correlated well with their higher VEC anti-Candida activities. This was supported by a linear regression model incorporating data from both groups at a 20:1 E:T ratio, where a significant inverse correlation between fungal burden and VEC activity (r = 0.5; P = 0.0346) was confirmed. Also observed in this cohort was the fact that women with an infrequent history of VVC (defined as fewer than three infections in the past year and of known cause; antibiotic usage, high-estrogen oral contraception, hormone replacement therapy, or pregnancy) (n = 10) had lower levels of VEC antifungal activity than those with no history of VVC (n = 8), although the level of significance was not as high as that for infected versus protected women (data not shown). This is consistent with the fact that small numbers of women with an infrequent history of VVC were protected against the infection and small numbers of women with no history of VVC became symptomatic.
Together, these data support the concept that VEC antifungal activity as an innate immune mechanism could be associated with protection from candidiasis and that women with a history of VVC may be more susceptible to infection due to reduced levels of this innate anti-Candida activity. We realize, however, that the growth inhibition assay was in vitro and was evaluated prior to inoculation, making suggestions for cause and effect (activity to infection) difficult. However, under the circumstances, this is the best means to evaluate the role of VECs against Candida infection, since women with high vaginal fungal burden cannot be tested due to existing Candida-VEC interactions that interfere with the controls necessary for the assay. Furthermore, the results for inhibitory activity correlated with the natural history of infection. It will be interesting to conduct longitudinal studies for anti-Candida activity in order to better understand any subject's VEC activity and the effects on resistance to infection in the live Candida challenge model.
Previous studies examining VEC anti-Candida activity in specific cohorts showed that there were no differences in the levels of activity at various stages of the menstrual cycle (2) but that women with RVVC had reduced levels of anti-Candida activity compared to controls (2), which potentially contributed to recurrence. Interestingly, women who became symptomatically infected in the present cohort had levels of anti-Candida activity similar to those from women with RVVC (2), thus serving as another clinical correlate of activity to protection/susceptibility to vaginitis.
The mechanism of antifungal activity, while not completely understood, requires cell contact with intact but not necessarily live epithelial cells, has no role for any soluble factors, is fungistatic, and is acid labile (9, 12, 15). Thus, antifungal activity is garnered by the simple interaction of Candida with VECs, with a putative signal for inhibition being manifested within Candida. However, it is clear that the VEC activity is substantially weaker than that of oral epithelial cells (11). This together with the lack of any evidence for an adaptive response against Candida at the vaginal mucosa (reviewed in reference 8) is consistent with the high frequency of vaginitis compared to that of oral candidiasis in otherwise healthy women. Furthermore, VEC antifungal activity is reduced in acidic environments (pH < 5) (15).
In conclusion, despite several inherent limitations in testing the VECs in vitro, there is now considerable evidence for a possible association between VEC anti-Candida activity and protection against symptomatic vaginitis. Continued studies to identify the role of VEC antifungal activity will be important to the overall understanding of resistance and susceptibility to VVC and/or RVVC.
Acknowledgments
This work was supported by Public Health Service grant AI-41693.
There are no conflicts of interest.
Editor: A. Casadevall
REFERENCES
- 1.Barousse, M., B. J. Van Der Pol, D. Fortenberry, D. Orr, and P. L. Fidel, Jr. 2004. Vaginal yeast colonization, prevalence of vaginitis, and associated local immunity in adolescents. Sex. Transm. Infect. 80:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barousse, M. M., C. Steele, K. Dunlap, T. Espinosa, D. Boikov, and J. D. Sobel. 2001. Growth inhibition of Candida albicans by human vaginal epithelial cells. J. Infect. Dis. 184:1489-1493. [DOI] [PubMed] [Google Scholar]
- 3.Black, C. A., F. M. Eyers, A. Russell, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1998. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 66:1273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du, C., R. Calderone, J. Richert, and D. Li. 2005. Deletion of the SSK1 response regulator gene in Candida albicans contributes to enhanced killing by human polymorphonuclear neutrophils. Infect. Immun. 73:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidel, P. L., Jr. 2004. History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 12:220-227. [DOI] [PubMed] [Google Scholar]
- 6.Fidel, P. L., Jr., M. Barousse, T. Espinosa, C. Camaratti, M. Ficarra, and D. H. A. Martin. 2004. Live intravaginal Candida challenge in humans reveals new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect. Immun. 72:2939-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel, P. L., Jr., W. Luo, C. Steele, J. Chabain, M. Baker, and F. L. Wormley. 1999. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect. Immun. 67:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel P. L., Jr., and J. D. Sobel. 1996. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomanbhoy, F., C. Steele, J. Yano, and P. L. Fidel, Jr. 2002. Vaginal and oral epithelial cell anti-Candida activity. Infect. Immun. 70:7081-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14(Suppl. 1):S148-S153. [DOI] [PubMed] [Google Scholar]
- 11.Steele, C., J. E. Leigh, R. K. Swoboda, and P. L. Fidel, Jr. 2000. Growth inhibition of Candida by human oral epithelial cells. J. Infect. Dis. 182:1479-1485. [DOI] [PubMed] [Google Scholar]
- 12.Steele, C., J. Leigh, R. Swoboda, H. Ozenci, and P. L. Fidel, Jr. 2001. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect. Immun. 69:7091-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele, C., H. Ozenci, W. Luo, M. Scott, and P. L. Fidel, Jr. 1999. Growth inhibition of Candida albicans by vaginal cells from naive mice. Med. Mycol. 37:251-260. [PubMed] [Google Scholar]
- 14.Steele, C., M. Ratterree, and P. L. Fidel, Jr. 1999. Differential susceptibility to experimental vaginal candidiasis in macaques. J. Infect. Dis. 180:802-810. [DOI] [PubMed] [Google Scholar]
- 15.Yano, J., E. Lilly, C. Steele, D. Fortenberry, and P. L. Fidel, Jr. 2005. Oral and vaginal epithelial cell anti-Candida activity is acid-labile and does not require live epithelial cells. Oral Microbiol. Immunol. 20:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]