Abstract
We identified the genes encoding the AcrA-AcrB-TolC efflux pump in Enterobacter aerogenes and constructed acrAB and tolC mutants from a multidrug-resistant isolate. Both derivatives were more susceptible to antibiotics than the parental strain. Sequence analysis and complementation experiments revealed that the multidrug-resistant isolate is an acrR mutant.
For the last decade, Enterobacter aerogenes, a commensal gram-negative bacterium of human intestinal flora, has been rapidly emerging as an important nosocomial pathogen (14, 18). Of concern is the increasing frequency of E. aerogenes isolates that are resistant to antibiotics and antiseptics (3).
Several types of systems have evolved in gram-negative bacteria to pump deleterious molecules out of the cytosol (13, 16). Among these, the resistance-nodulation-division family of systems bypasses the periplasm and provides efflux across both the inner membrane and the outer membrane (OM). Such systems require three partners: an inner membrane transporter, a periplasmic membrane fusion protein, and an OM channel (13). These pumps utilize the energy of the proton motive force to extrude dyes, detergents, disinfectants, solvents, and antibiotics from the cell. The Escherichia coli AcrAB-TolC and the Pseudomonas aeruginosa MexAB-OprM efflux systems have been the most extensively studied (13, 16). The AcrAB-TolC pump provides E. coli with a natural resistance to bile salts (19).
Active antibiotic efflux has been detected in some E. aerogenes multidrug-resistant (MDR) clinical isolates (11). As a first step to identify the efflux systems and their regulation, we cloned the E. aerogenes genes involved in such mechanisms by complementation of E. coli mutants.
The strains and plasmids used in this work are listed in Table 1. MICs were determined according to the standard twofold dilution method of the French Society for Microbiology (11). EA27 is an MDR isolate that exhibits active efflux of norfloxacin (11). EAEP289, the Kans derivative of EA27, was obtained after growth of the isolate in successive cultures in the presence of mitomycin C (0.4 μg/ml). No differences were observed between EA27 and EAEP289 in their plasmid, protein, or lipopolysaccharide profiles (data not shown).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant featuresa | Source or reference |
|---|---|---|
| E. coli strains | ||
| AG100 | K-12 | 15 |
| AG100A | AG100 ΔacrAB::Kanr | 15 |
| BW5104 | Mu-1 Δlac169 creB510 hsdR514 | 10 |
| LBB1296 | AG100 tolC::Tn10 | 4 |
| EP661 | BW5104 ΔacrAB::Kanr | This study |
| EP663 | BW5104 tolC::Tn10 | This study |
| E. aerogenes strains | ||
| BW16627 | ATCC 15038 rpsL | 10 |
| BW16662 | BW16627 (pREG2-1, pEG5166S) | 10 |
| BW16665 | BW16627 (pREG2-1, pEG5166S) | 10 |
| EA27 | MDR clinical isolate; Kanr Ampr Chlr Nalr Strr Tetr | 11 |
| EAEP289 | Kans derivative of EA27 | This study |
| EAEP294 | EAEP289 acrA::Kanr (pEP755 integration) | This study |
| EAEP298 | EAEP289 tolC::Kanr (pEP786 integration) | This study |
| Plasmids | ||
| Mu dI5166 | Mini-Mu for in vivo cloning; Chlr | 6 |
| pEP676 | Mu dI5166 bearing acrRAB on a 6-kb insert | This study |
| pEP685 | Mu dI5166 bearing tolC on an 8-kb insert | This study |
| pBCSK+ | High-copy-number vector; Chlr | Stratagene |
| pEP709 | pBCSK+ bearing acrRAB on a 7-kb BamHI-HindIII fragment from pEP676, 1 kb is from Mu dI5166 | This study |
| pEP710 | pBCSK+ bearing tolC on a 9-kb BamHI-HindIII fragment from pEP685, 1 kb is from Mu dI5166 | This study |
| pVIK108 | pir-dependent plasmid, oriR6K; Kanr | 7 |
| pEP755 | pVIK108 bearing ′acrA′ on a 0.8-kb XhoI-EcoRI fragment | This study |
| pEP786 | pVIK108 bearing ′tolC′ on a 1-kb NruI-SacI fragment | This study |
| pBBR1MCS | Medium-copy-number vector; Chlr | 9 |
| pEP787 | pBBR1MCS bearing tolC on a 2.2-kb SacII fragment oriented opposite Plac | This study |
| pJQ254 | High-copy-number vector; Kanr | 17 |
| pEP805 | pJQ254 bearing acrR from EA27 cloned under Plac | This study |
| pEP806 | pJQ254 bearing acrR from EA27 cloned opposite Plac | This study |
| pEP808 | pJQ254 bearing acrR from BW16627 cloned under Plac | This study |
| pEP809 | pJQ254 bearing acrR from BW16627 cloned opposite Plac | This study |
Ampr, Chlr, Kanr, Nalr, Strr, and Tetr, resistance to ampicillin, chloramphenicol, kanamycin, nalidixic acid, streptomycin, and tetracycline, respectively.
Primers acrR1 (5′-GCGAATAGCGGCAGAGA-3′) and acrR2 (5′-GAGAGCATCAGAACGACCG-3′) were used to amplify acrR and its promoter. PCR products were cloned into the SmaI site of pJQ254 (17).
Whole-membrane extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel. Proteins were electroblotted onto nitrocellulose membranes and probed with polyclonal antibodies raised against E. coli AcrA (22) or TolC (5), and the immunoblots were developed by colorimetric detection with alkaline phosphatase-conjugated secondary antibodies.
Cloning and identification of the E. aerogenes acr and tolC loci.
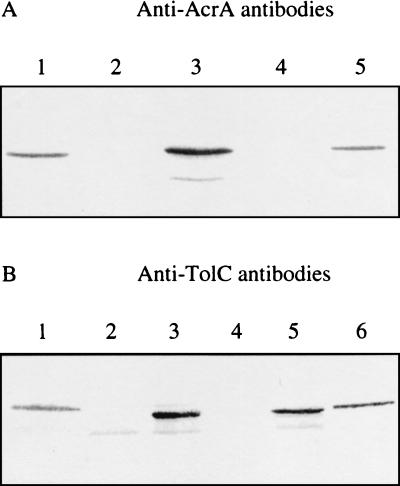
We used a genomic library in the form of a Mu dI5166 phage lysate to complement E. coli ΔacrAB or tolC mutants for growth on plates supplemented with 0.1% SDS (6, 10). From pEP676, which complemented the E. coli ΔacrAB mutant, a 6-kb genomic DNA fragment was cloned into pBCSK+ to generate pEP709. From pEP685, which complemented the E. coli tolC mutant, an 8-kb DNA insert was subcloned into pBCSK+ to generate pEP710. Whole-membrane extracts of E. coli AG100 (AcrAB+ TolC+), AG100A ΔacrAB, AG100A(pEP709), AG100 tolC, and AG100 tolC bearing pEP710 were analyzed by immunoblotting. An immunoreactive protein was detected in AG100A(pEP709) with antibodies raised against E. coli AcrA (22) (Fig. 1A), while an anti-TolC immunoreactive protein was detected in AG100 tolC(pEP710) (5) (Fig. 1B).
FIG. 1.
Immunoblots of whole-membrane extracts with antibodies raised against E. coli AcrA (A) or E. coli TolC (B). (A) Lane 1, E. coli AG100 (AcrA+); lane 2, E. coli AG100A (AcrA−); lane 3, E. coli AG100A(pEP709); lane 4, E. aerogenes EAEP294 (AcrA−); lane 5, E. aerogenes EAEP289 (AcrA+). (B) Lane 1, E. coli AG100 (TolC+); lane 2, E. coli LBB1296 (TolC−); lane 3, E. coli LBB1296(pEP710); lane 4, E. aerogenes EAEP298 (TolC−); lane 5, E. coli LBB1296(pEP787); lane 6, E. aerogenes EAEP289 (TolC+).
Sequence analysis of the insert in pEP709 revealed that the 6-kb E. aerogenes acrRAB locus organization is identical to that of E. coli. First, acrR and acrA are transcribed divergently. The sequences of the acrA promoter and AcrR repressor binding sites identified in E. coli are strictly conserved in E. aerogenes. Second, the 22-bp acrA-acrB intergenic sequences are identical in both species, and thus the E. aerogenes genes are probably also transcribed as an operon.
E. aerogenes and E. coli AcrR proteins share 78% overall sequence identity in a 215-amino-acid (aa) overlap. The sequence of the predicted N-terminal helix-turn-helix DNA binding motif is even more conserved (90% identity in a 31-aa overlap). Both AcrA proteins exhibit 85% identity in a 399-aa overlap. E. aerogenes AcrB and its E. coli homolog present 87% sequence identity in a 1,049-aa overlap.
Several subclones of pEP710 were constructed, and they enabled us to localize E. aerogenes tolC in a 2.2-kb SacII fragment carried by pEP787. Sequence analysis of this fragment revealed that TolC proteins from E. aerogenes and E. coli share 82% sequence identity in a 495-aa overlap. E. aerogenes TolC is slightly smaller as it contains a 6-aa deletion in a cell-surface-exposed loop and a 3-aa deletion in the C-terminal region.
AcrAB and TolC contribute to drug resistance in an MDR E. aerogenes isolate.
We constructed acrA and tolC mutants from EAEP289 via chromosomal integration of Kanr suicide plasmids pEP755 and pEP786, respectively (Table 1). The absence of AcrA or TolC production in the resulting Kanr clones, EAEP294 and EAEP298, was confirmed by immunoblotting (Fig. 1). EAEP294 and EAEP298 were unable to grow on plates supplemented with the bile salt deoxycholate (data not shown), with SDS, or with novobiocin and showed reduced resistance to all drugs tested (Table 2).
TABLE 2.
Susceptibilities of E. aerogenes strains to antimicrobials
| Strain and relevant phenotype(s) | MIC (μg/ml) ofa:
|
Growthb
|
||||||
|---|---|---|---|---|---|---|---|---|
| CHL | NOR | CIP | TET | MC | AF | SDS | NOV | |
| BW16627 AcrA+ TolC+ | 4 | <0.125 | <0.125 | 1 | 1 | 64 | + | − |
| EA27 AcrA+ TolC+ | >256 | 256 | 32 | 8 | 2 | 256 | + | + |
| EAEP289 AcrA+ TolC+ | >256 | 256 | 32 | 8 | 4 | 256 | + | + |
| EAEP294 AcrA− TolC+ | 32 | 64 | 16 | <0.25 | <0.125 | 32 | − | − |
| EAEP298 AcrA+ TolC− | 32 | 16 | 4 | <0.25 | <0.125 | 32 | − | − |
| EAEP294(pEP709) AcrA+ TolC+ | >256 | 256 | 32 | 8 | 2 | 256 | + | + |
| EAEP298(pEP787) AcrA+ TolC+ | >256 | 128 | 32 | 8 | 1 | 256 | + | + |
| EA289(pJQ254) AcrR− | >256 | 256 | 32 | 8 | 4 | 256 | + | + |
| EA289(pEP805) AcrR− | >256 | 256 | 32 | 8 | 4 | 256 | + | + |
| EA289(pEP806) AcrR− | >256 | 256 | 64 | 16 | 4 | 256 | + | + |
| EA289(pEP808); AcrR overproducer | 128 | 64 | 8 | 2 | 1 | 64 | − | + |
| EA289 (pEP809); AcrR producer | >256 | 256 | 32 | 8 | 4 | 256 | + | + |
Abbreviations: CHL, chloramphenicol; NOR, norfloxacin; CIP, ciprofloxacin; TET, tetracycline; MC, mitomycin C; AF, acriflavine.
Growth (+) or absence of growth (−) on LBA plates containing 0.1% SDS or 30 μg of novobiocin (NOV) per ml.
To determine that the observed phenotypes did not result from polar effects on downstream genes, we transformed EAEP294 acrA::Kanr with pEP709 and EAEP298 tolC::Kanr with pEP787. The transformants were selected on Luria broth agar-kanamycin plates containing 0.05% cetyltrimethylammonium bromide, a detergent, since selection on plates containing SDS or chloramphenicol was not efficient. EAEP294(pEP709) and EAEP298(pEP787) were able to grow on plates containing SDS and novobiocin (Table 2). The MICs of all drugs for both transformants were increased 2- to 16-fold, compared to those for the nontransformed strains.
Overexpression of acrR reduces drug resistance in an MDR E. aerogenes isolate.
The acr and tolC loci of EA27 were PCR amplified and sequenced. Comparison of the EA27 and BW16627 tolC sequences indicated no difference in the promoter regions. The detected 31 base changes generated only 11 aa substitutions, and most of them were conservative or involved residues located in loops based on the E. coli TolC tridimensional structure (8).
The 21 base substitutions observed in the EA27 acr locus sequence were silent. However, a frameshift mutation due to a 1-bp deletion was detected in codon 47 of acrR. To confirm that EAEP289 is an acrR null mutant, we transformed it with pJQ254 (17) bearing acrR amplified from the BW16627 (AcrR+) or EA27 (AcrR−) genome. In pEP805 and pEP808, acrR is in the same orientation as Plac, while in pEP806 and pEP809, acrR is cloned opposite Plac. EAEP289 containing pJQ254, pEP805, pEP806, or pEP809 exhibited identical resistance levels for all the antimicrobials tested (Table 2). In contrast, EAEP289(pEP808) was SDS susceptible and its susceptibility to all drugs was reduced fourfold. However, EAEP289(pEP808) was able to grow on Luria broth agar-novobiocin plates, suggesting that residual pump activity was sufficient to discharge novobiocin. In EAEP289(pEP808), overproduced AcrR may repress acrAB transcription, while in EAEP289(pEP809), AcrR production may be insufficient to repress acrAB. This could result from acrR autoregulation.
In E. coli, upon derepression of marA transcription, the MarA regulator mediates MDR by activating acrAB and tolC and by downregulating the synthesis of the major OmpF porin (1, 2). EA27 does not synthesize the major E. aerogenes porin (11), and complementary sequence data obtained in this laboratory indicated the absence of a mutation in the EA27 mar locus. Our results suggest that in a porin-deficient E. aerogenes strain, acrAB derepression is sufficient to generate high resistance levels in the absence of the MarA activator. These observations are in accordance with several recent studies of E. coli isolates that reveal the role of acrR mutations in high-level fluoroquinolone resistance in the absence of mar mutations (12, 20, 21).
Nucleotide sequence accession numbers.
The nucleotide sequences of the E. aerogenes BW16627 acrRAB and tolC and the E. aerogenes EA27 tolC loci have been assigned EMBL accession nos. AJ306389, AJ306390, and AJ421426, respectively.
Acknowledgments
We thank Joe Fralick, Hiroshi Nikaido, Rajeev Misra, Barry Wanner, and Stephen Winans for their generous gifts of bacterial strains, plasmids, and antisera. We acknowledge Aurélie Thiolas and the IMTSSA for technical assistance with automatic sequencing. We are grateful to Françoise Jacob-Dubuisson for critical reading of this manuscript, and to Ruth Winter for checking English usage. We thank Carl Schnaitman for his cheerful encouragement and enlightening comments.
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Université de la Méditerranée, and the Région Marseille-Métropole.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. [DOI] [PubMed] [Google Scholar]
- 3.Bosi, C., A. Davin-Regli, C. Bornet, M. Mallea, J. M. Pages, and C. Bollet. 1999. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J. Clin. Microbiol. 37:2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.German, G. J., and R. Misra. 2001. The TolC protein of Escherichia coli serves as a cell-surface receptor for the newly characterized TLS bacteriophage. J. Mol. Biol. 308:579-585. [DOI] [PubMed] [Google Scholar]
- 6.Groisman, E. A., and M. J. Casadaban. 1986. Mini-Mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J. Bacteriol. 168:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 8.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 9.Kovac, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad host range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 10.Lee, K. S., W. W. Metcalf, and B. L. Wanner. 1992. Evidence for two phosphonate degradative pathways in Enterobacter aerogenes. J. Bacteriol. 174:2501-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallea, M., J. Chevalier, C. Bornet, A. Eyraud, A. Davin-Regli, C. Bollet, and J. M. Pages. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 12.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2000. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 44:3441-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido, H. 1998. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 27(Suppl. 1):S32-S41. [DOI] [PubMed] [Google Scholar]
- 14.NNIS. 1999. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1990-May 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 15.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 18.Sanders, W. E., Jr., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, H., J. L. Dzink-Fox, M. Chen, and S. B. Levy. 2001. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: role of acrR mutations. Antimicrob. Agents Chemother. 45:1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webber, M. A., and L. J. Piddock. 2001. Absence of mutations in marRAB or soxRS in acrB-overexpressing fluoroquinolone-resistant clinical and veterinary isolates of Escherichia coli. Antimicrob. Agents Chemother. 45:1550-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zgurskaya, H. I., and H. Nikaido. 1999. AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285:409-420. [DOI] [PubMed] [Google Scholar]