Abstract
Fosmidomycin acts through inhibition of 1-deoxy-d-xylulose 5-phosphate (DOXP) reductoisomerase, a key enzyme of the nonmevalonate pathway of isoprenoid biosynthesis. It possesses potent antimalarial activity in vitro and in murine malaria. In a recent clinical study, fosmidomycin was effective and well tolerated in the treatment of patients with acute uncomplicated Plasmodium falciparum malaria but resulted in an unacceptably high rate of recrudescence. In order to identify a potential combination partner, the interaction of fosmidomycin with a number of antimalarial drugs in current use was investigated in a series of in vitro experiments. Synergy was observed between fosmidomycin and the lincosamides, lincomycin and clindamycin. The efficacy of a combination of fosmidomycin and clindamycin was subsequently demonstrated in the Plasmodium vinckei mouse model.
In humans, isoprenoids are synthesized via the mevalonate pathway. In contrast, they are synthesized by the nonmevalonate pathway (the 1-deoxy-d-xylulose 5-phosphate [DOXP] pathway, also called the MEP pathway) in a number of bacterial species and inside the plastides of algae and higher plants (22). Similarly, the enzymes of the nonmevalonate pathway are located inside the plastide-like organelle (apicoplast) of malaria parasites (7, 27). The antibiotic fosmidomycin, originally isolated from Streptomyces lavendulae, represents a potent inhibitor of DOXP reductoisomerase, a key enzyme of the nonmevalonate pathway (15, 28). Recently, it was demonstrated that fosmidomycin possesses potent antimalarial activity in vitro and in murine malaria (7). FR900098, a fosmidomycin derivative, was found to be twice as effective, while the prodrug derivatives had increased oral bioavailability in the mouse model (21).
In a recent clinical study conducted in Gabon and Thailand, 20 patients with acute uncomplicated Plasmodium falciparum malaria were treated with fosmidomycin administered orally (B. Lell, R. Ruangweerayut, J. Wiesner, M. Missinou, A. Schindler, T. Baranek, M. Hintz, D. Hutchinson, H. Jomaa, and P. Kremsner, unpublished data). The treatment was well tolerated and resulted in rapid parasite and fever clearance times, comparable to those obtained with conventional quinoline antimalarial agents. All patients were clinically and parasitologically cured by day 7. By day 28, however, 9 out of 18 evaluable patients experienced recrudescence. A similarly high rate of recrudescence had been observed previously when the hydroxynaphthoquinone antimalarial agent atovaquone was evaluated as a single entity (17). Subsequently, proguanil was identified as a partner for atovaquone on the basis of in vitro synergistic activity, resulting in a highly effective and well-tolerated fixed drug combination, approved and marketed as Malarone (3). Using a similar approach, we have investigated the interaction of fosmidomycin with most antimalarial agents in clinical use.
MATERIALS AND METHODS
Materials.
Blood components were provided by the local Institute of Clinical Immunology and Transfusion Medicine. Chloroquine, quinine, artemisinin, doxycycline, ciprofloxacin, rifampin, and lincomycin were purchased from Sigma. Mefloquine and halofantrine were gifts from Reto Brun (Basel, Switzerland). Atovaquone was a gift from Peter Kremsner (Tübingen, Germany). Proguanil was a gift from Wallace Peters and Brian Robinson (Harrow, Middlesex, United Kingdom). Lumefantrine was provided by Welding GmbH & Co. (Hamburg, Germany). Azithromycin was extracted from Zithromax tablets (Pfizer). Clindamycin was purchased from Sigma, ICN, and Welding GmbH & Co. The P. falciparum laboratory strains used were 3D7 (The Netherlands), HB3 (Honduras), Dd2 (Indochina), and A2 (Gambia). The Plasmodium vinckei strain was provided by Henri Vial (Montpellier, France).
In vitro antimalarial activity.
P. falciparum was cultivated in RPMI 1640 medium (Life Technologies) supplemented with 10% human type O+ serum and 25 mM HEPES. Human type O+ erythrocytes served as host cells (24). Cultures were kept at 37°C under an atmosphere of 5% O2, 3% CO2, and 92% N2. In vitro drug sensitivity assays were carried out on 96-well microtitration plates (1, 4). Fosmidomycin was dissolved in complete culture medium and sterilized by filtration. The other drugs were dissolved in dimethyl sulfoxide and prediluted with complete culture medium. Infected erythrocytes (0.15 ml per well with 2% hematocrit and 0.4% parasitemia) were incubated in duplicate with a twofold serial dilution of each drug for 48 h. After addition of 0.8 μCi of [3H]hypoxanthine (Amersham Pharmacia) in 50 μl of medium per well, the plates were incubated for another 24 h. Parasites were collected on glass fiber filters with a cell harvester (Micromate 196; Packard), and incorporated radioactivity was measured using a β-counter (Matrix 9600; Packard). Growth inhibition was expressed as percent 3H incorporation compared with untreated controls. Values were plotted on semilogarithmic paper for extrapolation of 50% inhibitory concentrations (IC50s).
In vitro drug interaction.
Drug interaction studies were performed as previously described (3). Initially, the IC50s of the test drugs alone were determined. Subsequently, drug solutions were diluted with culture medium to initial concentrations of 80 times the predetermined IC50s. These solutions were combined in ratios of 1:5, 1:2, 2:1, and 5:1. Single and combination drug solutions were then introduced into 96-well plates to give duplicate rows of fosmidomycin alone, the test drug, and the four combinations. Finally, the IC50s of the two test drugs alone and in combination were determined. For data interpretation, the IC50s of the drugs in combination were expressed as fractions of the IC50s of the drugs alone normalized to 1. Isobolograms were constructed by plotting the IC50 of one drug against the IC50 of the other for each of the four drug ratios, with a concave curve indicating synergy, a straight line indicating addition, and a convex curve indicating antagonism. To obtain numeric values for the kind of interaction, results were expressed as the sum of the fractional inhibitory concentrations (sum FIC), calculated as (IC50 of drug A in mixture/IC50 of drug A alone) + (IC50 of drug B in mixture/IC50 of drug B alone). Sum FIC values indicate the kinds of interactions as follows: <0.5, synergy; 0.5 to 1, addition; 1 to 2, indifferent interaction; >2, antagonism. Sum FIC values were calculated for the drug ratio resulting in the point closest to the middle of the isobologram.
For determination of growth inhibition by fosmidomycin in the presence of constant clindamycin concentrations, 20-ml aliquots of the suspension of infected erythrocytes in culture medium were adjusted to the desired clindamycin concentration from a 2 mM stock solution in dimethyl sulfoxide before being loaded in triplicated rows onto the 96-well plate. Then a dilution series of fosmidomycin was prepared on the plate.
In vivo drug interaction.
For in vivo drug testing, mice were inoculated by intraperitoneal injection with approximately 5 × 107 infected erythrocytes from a donor mouse. Fosmidomycin was dissolved in phosphate-buffered saline and administered orally (75 mg/kg of body weight). Clindamycin hydrochloride was dissolved in distilled water and administered by intraperitoneal injection (5 mg/kg). Four mice were used for each treatment group, and three mice were used for the control group. Parasitemia was monitored by Giemsa staining of blood smears. Mice were sacrificed when parasitemia exceeded 40%. The animal experiments complied with all relevant federal guidelines and institutional policies.
RESULTS
In vitro drug interaction.
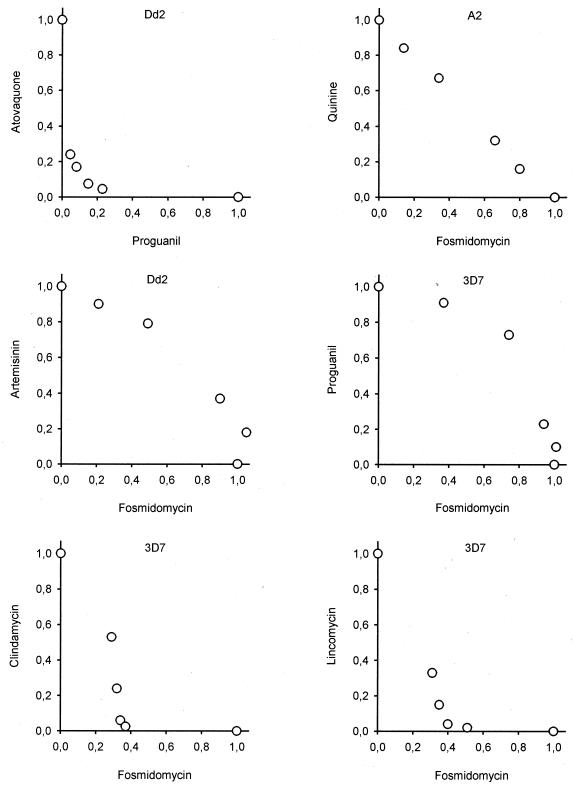
Before we embarked on the identification of a therapeutic partner for fosmidomycin, a control experiment was performed to validate the methodology through assessment of the interaction between atovaquone and proguanil, a proven synergistic drug combination. As expected, the IC50s of the individual drugs in the four different mixtures were significantly lower than the IC50s of the drugs alone, resulting in a concave curve in the isobologram (Fig. 1). Subsequently, fosmidomycin was tested in combination with most of the currently used antimalarial drugs (Fig. 1). The antifolate drugs, pyrimethamine and cycloguanil, were excluded from the study because of the existence of highly resistant P. falciparum strains in the field (2). The study was performed with four different strains of P. falciparum including the multidrug-resistant strain Dd2 (resistant to chloroquine, pyrimethamine, and cycloguanil). The absolute IC50s of the drugs used in the study for the different strains are listed in Table 1. In the interaction experiments, there was no apparent specific tendency for any strain. Typical isobolograms are shown in Fig. 1, and the sum FIC values for all drug combinations tested are summarized in Table 2. The interaction of fosmidomycin with all quinoline and aryl-amino-alcohol antimalarial drugss was indifferent, with the exception of quinine, for which the interaction was additive. Also, artemisinin, atovaquone, and proguanil had indifferent effects. In addition, a triple combination of fosmidomycin, proguanil, and atovaquone was tested but also resulted in an indifferent effect (data not shown). Among the antibiotics with known antimalarial activity, doxycycline and azithromycin were additive, and ciprofloxacin and rifampin were indifferent. Synergy was observed only with clindamycin and its natural precursor, lincomycin. Remarkably, the shapes of the corresponding isobolograms were asymmetric, in contrast to the curve obtained in the control experiment with atovaquone and proguanil.
FIG. 1.
Representative isobolograms of the interaction of fosmidomycin with quinine, artemisinin, proguanil, clindamycin, or lincomycin. The interaction of proguanil with atovaquone was assessed as a control experiment (upper left panel). The P. falciparum strain used for each experiment is indicated.
TABLE 1.
In vitro IC50s of the test drugs determined for P. falciparum strains 3D7, HB3, Dd2, and A2
| Drug | IC50 (ng/ml)a for strain:
|
|||
|---|---|---|---|---|
| 3D7 | HB3 | Dd2 | A2 | |
| Fosmidomycin | 150 (100-240) (n = 22) | 71 (46-140) (n = 24) | 170 (120-260) (n = 24) | 150 (70-260) (n = 10) |
| Chloroquine | 6.4 (n = 1) | 7.0 (6.4-8.0) (n = 3) | 56 (54-64) (n = 3) | 14 (10-22) (n = 4) |
| Quinine | 31 (22-49) (n = 3) | 100 (65-130) (n = 3) | 120 (120-130) (n = 2) | 75 (45-130) (n = 3) |
| Mefloquine | 9.1 (7.6-11) (n = 2) | 14 (14-15) (n = 2) | 21 (15-25) (n = 3) | ND |
| Halofantrine | 5.0 (4.3-5.8) (n = 3) | 4.3 (3.3-5.8) (n = 2) | 4.7 (3.8-5.8) (n = 2) | ND |
| Lumefantrine | 18 (15-21) (n = 2) | 21 (20-23) (n = 3) | 23 (16-34) (n = 2) | 16 (15-17) (n = 3) |
| Artemisinin | 6.8 (5.6-7.9) (n = 2) | 5.9 (n = 1) | 5.1 (n = 1) | 7.9 (n = 1) |
| Atovaquone | 0.37 (0.35-0.37) (n = 3) | 0.18 (n = 1) | 0.37 (0.37-0.37) (n = 3) | ND |
| Proguanil | 1,700 (940-2,500) (n = 3) | 1,300 (990-1,800) (n = 2) | 1,200 (510-2,200) (n = 4) | ND |
| Doxycycline | ND | 4,400 (n = 1) | 3,000 (2,700-3,300) (n = 2) | 2,700 (n = 1) |
| Azithromycin | 10,000 (n = 1) | 7,400 (5,200-10,000) (n = 2) | 4,100 (3,000-5,600) (n = 2) | 19,000 (n = 1) |
| Ciprofloxacin | 5,300 (n = 1) | 13,000 (n = 1) | 6,000 (n = 1) | ND |
| Rifampin | 2,100 (n = 1) | 520 (n = 1) | 2,500 (n = 1) | ND |
| Clindamycin | 24,000 (23,000-25,000) (n = 2) | 28,000 (27,000-30,000) (n = 2) | 18,000 (13,000-27,000) (n = 3) | ND |
| Lincomycin | 45,000 (n = 1) | 33,000 (n = 1) | 45,000 (41,000-53,000) (n = 2) | ND |
Values are geometric means, with ranges of observed IC50s in parentheses. The number of experiments with each drug-strain combination is also given in parentheses. ND, not determined.
TABLE 2.
Interaction of fosmidomycin with other antimalarial drugs against P. falciparum in vitro
| Drug | Sum FICa | Interaction |
|---|---|---|
| Chloroquine | 1.46 ± 0.17 (n = 6) | Indifferent |
| Quinine | 0.93 ± 0.11 (n = 8) | Additive |
| Mefloquine | 1.42 ± 0.17 (n = 4) | Indifferent |
| Halofantrine | 1.23 ± 0.08 (n = 6) | Indifferent |
| Lumefantrine | 1.25 ± 0.17 (n = 10) | Indifferent |
| Artemisinin | 1.33 ± 0.08 (n = 5) | Indifferent |
| Atovaquone | 1.39 ± 0.13 (n = 4) | Indifferent |
| Proguanil | 1.40 ± 0.21 (n = 4) | Indifferent |
| Doxycycline | 0.93 ± 0.09 (n = 4) | Additive |
| Azithromycin | 0.84 ± 0.04 (n = 5) | Additive |
| Ciprofloxacin | 1.04 ± 0.06 (n = 2) | Indifferent |
| Rifampin | 1.19 ± 0.10 (n = 3) | Indifferent |
| Clindamycin | 0.43 ± 0.02 (n = 6) | Synergistic |
| Lincomycin | 0.46 ± 0.02 (n = 2) | Synergistic |
Values are means ± standard deviations from several independent experiments. The number of experiments is given in parentheses. Data obtained with different strains were pooled.
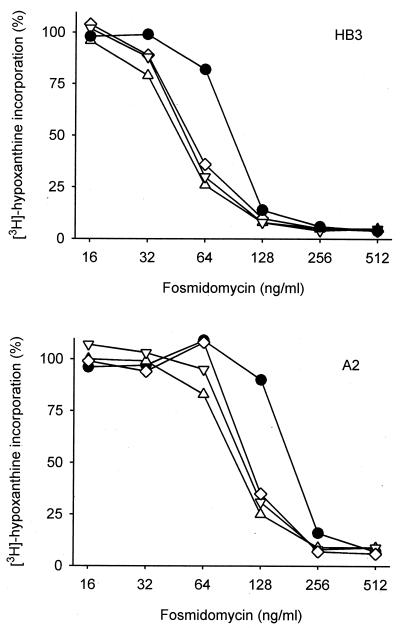
To assess whether the synergy of fosmidomycin and clindamycin is of clinical relevance, it should be noted that clindamycin is an effective but very slow acting antimalarial drug. Therefore, the absolute IC50s of clindamycin obtained under our assay conditions were comparatively high (Table 1). At lower concentrations of clindamycin, the parasites develop normally in the first cycle after exposure, and reinvasion of new host erythrocytes takes place. Growth inhibition finally occurs at the end of the second cycle (the so-called “delayed kill effect”) (6). Consistently, for patients treated with clindamycin, amelioration of symptoms is observed as late as the fourth day of treatment (10). Fosmidomycin, in contrast, kills the parasites at the end of the first cycle. Consequently, we have investigated whether the synergy of fosmidomycin and clindamycin remains apparent under pharmacologically achievable concentrations of clindamycin. It has been reported that during the course of standard low-dose clindamycin therapy with 5 mg/kg every 8 h, minimal plasma drug levels of 150 to 800 ng/ml are achieved (16, 18). The broad range reported possibly depends on the methods used for drug determination. Therefore, we investigated the sensitivity of P. falciparum to fosmidomycin in the presence of clindamycin concentrations between 42 ng/ml (0.1 μM) and 850 ng/ml (2 μM). Parasite growth was not affected by these concentrations of clindamycin alone within the assay time, but the parasites were killed when the incubation time was extended to 4 days (data not shown). In the presence of an 850-ng/ml concentration of clindamycin, the IC50 of fosmidomycin for P. falciparum strain HB3 changed from 82 to 48 ng/ml (Fig. 2). Remarkably, in the presence of a 42-ng/ml concentration of clindamycin, the IC50 of fosmidomycin was still reduced to 55 ng/ml. In an independent experiment using P. falciparum strain A2, a similar shift to lower IC50s was observed in the presence of clindamycin (Fig. 2). These data clearly demonstrate that an increased therapeutic response can be expected from a combination of fosmidomycin and clindamycin.
FIG. 2.
Dose response of P. falciparum growth to fosmidomycin in the presence of different constant clindamycin concentrations. P. falciparum-infected erythrocytes were incubated with a serial dilution of fosmidomycin in the absence (filled circles) or in the presence of clindamycin at 43 ng/ml (diamonds), 210 ng/ml (inverted triangles), or 850 ng/ml (triangles). Parasite growth was monitored by radioactive hypoxanthine incorporation. The clindamycin concentrations selected did not have intrinsic antimalarial activity under the assay conditions. Results obtained by independent experiments with P. falciparum strains HB3 (upper panel) and A2 (lower panel) are presented.
In vivo drug interaction.
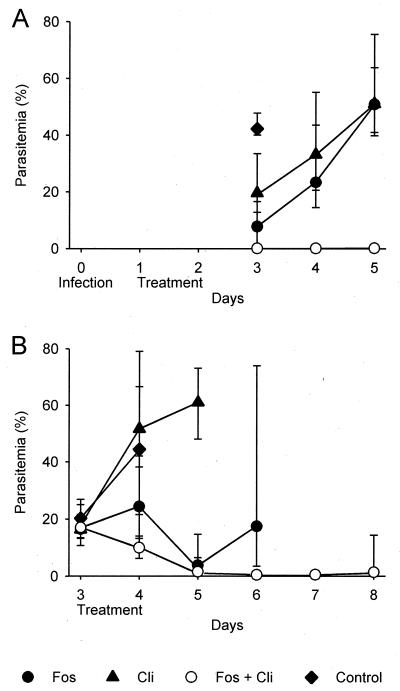
Next, the efficacy of fosmidomycin plus clindamycin was investigated in the P. vinckei mouse model. Mice were treated with each drug, administered in doses that were calculated to result in a partial reduction of parasitemia. In parallel, mice were treated with a combination of these drugs in subtherapeutic doses. A relatively high dose of fosmidomycin was chosen because of the anticipated short half-life in plasma (25). In the first experiment, the mice were infected on day 0 and treated on days 1 and 2. To assess efficacy, parasitemia was monitored on days 3, 4, and 5. On day 3, the parasitemia of mice treated with 75 mg of fosmidomycin/kg or 5 mg of clindamycin/kg was 7.8 or 20%, respectively, in comparison to 42% in untreated control mice (Fig. 3). When the mice were treated with a combination of 75 mg of fosmidomycin/kg and 5.0 mg of clindamycin/kg, the parasitemia was approximately 0.1% on day 3, increasing to 0.2% on day 5. A combination of 75 mg of fosmidomycin/kg and 2.5 mg of clindamycin/kg was equally efficacious (data not shown). However, efficacy decreased significantly when the clindamycin dose was further reduced to 1.3 mg/kg (data not shown).
FIG. 3.
In vivo efficacy of fosmidomycin plus clindamycin in P. vinckei-infected mice. (A) For suppressive treatment, drugs were administered on days 1 and 2 postinfection and parasitemia was monitored on days 3 to 5. (B) For curative treatment starting with high parasitemia, drugs were administered on days 3 and 4 and parasitemia was monitored from days 3 to 8. Geometric mean values and ranges of observed values are indicated. Fos, fosmidomycin; Cli, clindamycin.
An additional study was designed to investigate whether a combination of fosmidomycin and clindamycin would be effective when treatment is initiated in the presence of high parasitemia. This was of particular interest because clindamycin alone, even at high doses, is not able to rescue mice under such conditions. Therefore, treatment was started on day 3 after infection at a parasitemia of approximately 20%. Following treatment with fosmidomycin alone, parasitemia continued to increase for 24 h but then fell to 3.9% by day 5. By day 6, parasitemia had reached a range between 3.6 and 74%, and all mice, even those with only moderate parasitemia, had symptoms of severe anemia, a condition known as postschizontal anemia. Treatment with clindamycin alone did not stop the rise of parasitemia. When fosmidomycin and clindamycin were administered in combination, there was a constant reduction in the level of parasitemia to 0.5% on day 6. Again, a combination of 75 mg of fosmidomycin/kg plus 2.5 mg of clindamycin/kg was as effective as 75 mg of fosmidomycin/kg plus 5.0 mg of clindamycin/kg (data not shown).
DISCUSSION
In our in vitro experiments, combining fosmidomycin with commonly used antimalarial drugs resulted in an indifferent effect in most cases, with sum FIC values between 1.04 and 1.46. An antagonistic effect, resulting in sum FIC values higher than 2, refers to loss of activity when drugs are used in combination, indicating that higher concentrations of the individual drugs would be required to produce the same effect as when the drugs are administered singly. Such conditions, however, were not observed in this study. Therefore, even the drugs associated with an indifferent effect in vitro may prove to be useful therapeutic partners for fosmidomycin. In this regard, a combination of fosmidomycin with an artemisinin derivative may be of particular interest. Artemisinin derivatives such as artesunate and artemether have been successfully employed in various drug combinations, since they are active against multidrug-resistant parasites, display a favorable safety profile, and rapidly reduce the parasite load within one replication cycle (26). The additive effect observed with quinine may become relevant in severe malaria, where intravenous quinine, commonly given in combination with doxycycline, is still the first choice of treatment. Since the use of doxycycline is not appropriate for young children and pregnant women, there would seem to be a case for replacing it with fosmidomycin in view of the more rapid action of the latter.
Our studies have provided conclusive evidence of potent in vitro synergistic activity between fosmidomycin and the lincosamide antibiotics lincomycin and clindamycin. This has also been established in a malaria mouse model, even when treatment was delayed pending the development of high parasitemia. While lincomycin as the natural precursor of clindamycin is of only historical interest, clindamycin is widely used for treating infections with gram-positive or anaerobic bacteria. In addition, it is active against parasites of the phylum Apicomplexa, which includes Plasmodium, Theileria, and Toxoplasma (5). Clindamycin is believed to target the prokaryote-like ribosomes of the apicoplast (6, 9). By this means, self-replication of the organelle is inhibited, leading to the death of the parasite in the second replication cycle. The fact that fosmidomycin inhibits DOXP reductoisomerase, an enzyme that is localized in the apicoplast, may provide an explanation for the observed synergy with the lincosamides. However, it is not yet clear why other antibiotics that are also believed to impair apicoplast function do not exhibit such synergy. Possibly, clindamycin facilitates the transport of fosmidomycin into the parasite or the apicoplast by an unknown mechanism.
Early studies on the effectiveness of clindamycin as an antimalarial agent were very promising (10). However, in view of its slow onset of action, the use of clindamycin as a single entity is restricted to the treatment of asymptomatic or uncomplicated P. falciparum malaria. It is also useful as a therapeutic partner in antimalarial-drug combinations. In Gabon, all of 38 adult patients receiving 5 mg of clindamycin/kg twice daily for 5 days were cured, with only 1 patient developing a recurrent parasitemia which may have been due to reinfection (11). The same regimen led to a 100% cure rate for 35 patients in Brazil (12). A quinine-clindamycin combination was effective against multidrug-resistant malaria in Thailand (20). Furthermore, a 3-day course of clindamycin plus quinine was curative in the treatment of uncomplicated P. falciparum malaria, compared to 7 days of treatment with quinine alone (19). Since clindamycin has been used only for a relatively small number of malaria patients, it is not expected that resistant parasites have developed in the field. As a therapeutic partner for fosmidomycin, clindamycin has the advantage of having a similarly short half-life in plasma (16, 18). As a consequence, repeated dosing will be necessary, but the parasites will be exposed to subtherapeutic drug concentrations for a short time only, thereby deterring the emergence of resistance. Furthermore, the safety of clindamycin as an antibacterial agent has been substantiated through 35 years of clinical experience (5, 8, 23).
In addition to its potential use as an effective and affordable medication for uncomplicated malaria, fosmidomycin plus clindamycin may be of particular value for the treatment of severe malaria when patients are not able to tolerate oral medication. Conventional treatment with intravenous quinine may be life-threatening when the required dose is infused too rapidly. Parenteral administration of several highly potent drugs such as mefloquine, halofantrine, and atovaquone is precluded by their poor solubility. Fosmidomycin, in contrast, is freely water soluble, and bolus infusions of as much as 2 g were well tolerated in a phase I volunteer study (13, 14). Also, clindamycin, in the form of its phosphonic acid ester, is available as an intravenous formulation. Therefore, development of an intravenous formulation of a combination of fosmidomycin and clindamycin for treatment of severe malaria should be technically feasible. However, the role of the combination of fosmidomycin and clindamycin in the treatment of acute uncomplicated P. falciparum malaria will first be established through an extended program of phase II studies. The first clinical studies are currently ongoing in Gabon and Thailand.
Acknowledgments
We thank Matthias Eberl for critical reading of the manuscript, Steffen Borrmann for helpful discussions, and our colleagues for kindly providing some of the antimalarial test compounds.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Basco, L. K., and P. Ringwald. 2000. Molecular epidemiology of malaria in Yaounde, Cameroon. VI. Sequence variations in the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene and in vitro resistance to pyrimethamine and cycloguanil. Am. J. Trop. Med. Hyg. 62:271-276. [DOI] [PubMed] [Google Scholar]
- 3.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhawan, V. K., and H. Thadepalli. 1982. Clindamycin: a review of fifteen years of experience. Rev. Infect. Dis. 4:1133-1153. [DOI] [PubMed] [Google Scholar]
- 6.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 7.Jomaa, H., J. Wiesner, S. Sanderbrand, B. Altincicek, C. Weidemeyer, M. Hintz, I. Turbachova, M. Eberl, J. Zeidler, H. K. Lichtenthaler, D. Soldati, and E. Beck. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573-1576. [DOI] [PubMed] [Google Scholar]
- 8.Klainer, A. S. 1987. Clindamycin. Med. Clin. N. Am. 71:1169-1175. [DOI] [PubMed] [Google Scholar]
- 9.Kohler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. Wilson, J. D. Palmer, and D. S. Roos. 1997. A plastid of probable green algal origin in Apicomplexan parasites. Science 275:1485-1489. [DOI] [PubMed] [Google Scholar]
- 10.Kremsner, P. G. 1990. Clindamycin in malaria treatment. J. Antimicrob. Chemother. 25:9-14. [DOI] [PubMed] [Google Scholar]
- 11.Kremsner, P. G., S. Winkler, C. Brandts, W. Graninger, and U. Bienzle. 1993. Curing of chloroquine-resistant malaria with clindamycin. Am. J. Trop. Med. Hyg. 49:650-654. [DOI] [PubMed] [Google Scholar]
- 12.Kremsner, P. G., G. M. Zotter, H. Feldmeier, W. Graninger, R. L. Westerman, and R. M. Rocha. 1989. Clindamycin treatment of falciparum malaria in Brazil. J. Antimicrob. Chemother. 23:275-281. [DOI] [PubMed] [Google Scholar]
- 13.Kuemmerle, H. P., T. Murakawa, H. Sakamoto, N. Sato, T. Konishi, and F. De Santis. 1985. Fosmidomycin, a new phosphonic acid antibiotic. Part II. 1. Human pharmacokinetics. 2. Preliminary early phase IIa clinical studies. Int. J. Clin. Pharmacol. Ther. Toxicol. 23:521-528. [PubMed] [Google Scholar]
- 14.Kuemmerle, H. P., T. Murakawa, K. Soneoka, and T. Konishi. 1985. Fosmidomycin: a new phosphonic acid antibiotic. Part I. Phase I tolerance studies. Int. J. Clin. Pharmacol. Ther. Toxicol. 23:515-520. [PubMed] [Google Scholar]
- 15.Kuzuyama, T., T. Shizimu, S. Takahashi, and H. Seto. 1998. Fosmidomycin, a specific inhibitor of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway of isoprenoid biosynthesis. Tetrahedron Lett. 39:7913-7916. [Google Scholar]
- 16.Leigh, D. A. 1981. Antibacterial activity and pharmacokinetics of clindamycin. J. Antimicrob. Chemother. 7(Suppl. A):3-9. [DOI] [PubMed] [Google Scholar]
- 17.Looareesuwan, S., J. D. Chulay, C. J. Canfield, D. B. Hutchinson, et al. 1999. Malarone (atovaquone and proguanil hydrochloride): a review of its clinical development for treatment of malaria. Am. J. Trop. Med. Hyg. 60:533-541. [DOI] [PubMed] [Google Scholar]
- 18.Mazur, D., B. S. Schug, G. Evers, V. Larsimont, H. Fieger-Buschges, W. Gimbel, A. Keilbach-Bermann, and H. H. Blume. 1999. Bioavailability and selected pharmacokinetic parameters of clindamycin hydrochloride after administration of a new 600 mg tablet formulation. Int. J. Clin. Pharmacol. Ther. 37:386-392. [PubMed] [Google Scholar]
- 19.Parola, P., S. Ranque, S. Badiaga, M. Niang, O. Blin, J. J. Charbit, J. Delmont, and P. Brouqui. 2001. Controlled trial of 3-day quinine-clindamycin treatment versus 7-day quinine treatment for adult travelers with uncomplicated falciparum malaria imported from the tropics. Antimicrob. Agents Chemother. 45:932-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pukrittayakamee, S., A. Chantra, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 44:2395-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichenberg, A., J. Wiesner, C. Weidemeyer, E. Dreiseidler, S. Sanderbrand, B. Altincicek, E. Beck, M. Schlitzer, and H. Jomaa. 2001. Diaryl ester prodrugs of FR900098 with improved in vivo antimalarial activity. Bioorg. Med. Chem. Lett. 11:833-835. [DOI] [PubMed] [Google Scholar]
- 22.Rohmer, M. 1999. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16:565-574. [DOI] [PubMed] [Google Scholar]
- 23.Soper, D. E. 1992. Clindamycin. Obstet. Gynecol. Clin. N. Am. 19:483-496. [PubMed] [Google Scholar]
- 24.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya, T., K. Ishibashi, M. Terakawa, M. Nishiyama, N. Itoh, and H. Noguchi. 1982. Pharmacokinetics and metabolism of fosmidomycin, a new phosphonic acid, in rats and dogs. Eur. J. Drug Metab. Pharmacokinet. 7:59-64. [DOI] [PubMed] [Google Scholar]
- 26.White, N. J., and P. Olliaro. 1998. Artemisinin and derivatives in the treatment of uncomplicated malaria. Med. Trop. 58:54-56. [PubMed] [Google Scholar]
- 27.Wiesner, J., M. Hintz, B. Altincicek, S. Sanderbrand, C. Weidemeyer, E. Beck, and H. Jomaa. 2000. Plasmodium falciparum: detection of the deoxyxylulose 5-phosphate reductoisomerase activity. Exp. Parasitol. 96:182-186. [DOI] [PubMed] [Google Scholar]
- 28.Zeidler, J., J. Schwender, C. Müller, J. Wiesner, C. Weidemeyer, E. Beck, H. Jomaa, and H. K. Lichtenthaler. 1998. Inhibition of the non-mevalonate 1-deoxy-d-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z. Naturforsch. Sect. C 53:980-986. [Google Scholar]