Abstract
MCC-478 is a newly synthesized 2-amino-6-arylthio-9-phosphonomethoxyethylpurine bis(2,2,2-trifluoroethyl) ester derivative. MCC-478 showed a substantially higher (ca. 80-fold) anti-hepatitis B virus (HBV) activity than that of lamivudine, despite no significant anti-human immunodeficiency virus activity. Since the bis(2,2,2-trifluoroethyl) ester group was used to improve the oral bioavailability of the phosphonomethoxyethylpurine derivatives, two monoester derivatives and one phosphonic acid derivative were also evaluated. It was suggested that these hydrolyzed derivatives, which appeared in animals given MCC-478, have enough anti-HBV activity to contribute to efficacy in vivo. Furthermore, no apparent cytotoxic effects or reductions of mitochondrial DNA content by MCC-478 and its derivatives were observed. These results indicated that MCC-478 may be a new promising anti-HBV agent.
Hepatitis B virus (HBV) is a small, partially double-stranded DNA virus and a prototype member of the hepadnavirus family. HBV is a causative agent of both acute and chronic hepatitis. The World Health Organization lists hepatitis B as one of the 10 leading killer diseases and estimates that 350 million people are chronically infected with HBV. Until 1999 the only therapy approved for chronic HBV infection was alpha interferon, and this treatment was useful only for a small minority of Asian patients (13). The hepadnavirus has a unique replication cycle. Following entry into a hepatocyte, the viral genome matures to a single covalently closed circular DNA by an unknown enzyme derived from the host cell and/or hepadnavirus polymerase. The covalently closed circular DNA is translocated into the nucleus and serves as the template for several viral RNAs (4, 33). A hepadnavirus polymerase, which is encoded by a gene in the longest 3.5-kb pregenome RNA, transcribes full-length negative-strand HBV genome from a pregenome RNA within nucleocapsids (3, 30, 35). Sequentially, the polymerase exerts its DNA-dependent DNA polymerase activity to synthesize the positive-strand HBV genome (28). Since the replication cycle of hepadnavirus genome is dependent upon the action of its own polymerase, HBV polymerase could be a good target for an antiviral therapy.
Recently, lamivudine was approved to treat chronic HBV infection (11, 16, 18). Lamivudine is a dideoxycytidine analogue that is active against human immunodeficiency virus (HIV) and HBV (7, 12). It is also shown that lamivudine triphosphate acts as a chain terminator against viral DNA synthesis (5, 37). However, prolonged lamivudine treatment results in the emergence of lamivudine-resistant HBV mutants in 17 to 46% of patients treated for 1 year and more than 50% of patients within 2 years of treatment (1, 11, 16, 20). The emergence of drug-resistant HBV emphasizes the need to develop other antiviral agents and therapeutic strategies. One of the candidates, adefovir dipivoxil, which is an oral prodrug of adefovir (PMEA) and is active against HIV and HBV replication (15, 27, 36), is now in phase III clinical trials to treat HBV infection (31). Although adefovir dipivoxil is suggested to be active against lamivudine-resistant HBV strains (9, 10, 22, 24), nephrotoxicity, characterized by changes in renal function laboratory markers, is observed among HIV patients treated long-term with adefovir dipivoxil (21).
We synthesized more than 100 derivatives of phosphonomethoxyethylpurine and evaluated anti-HBV activity. The absorbability of test compounds was also examined using ex vivo samples from mice and rats given the drug orally (34). Finally, we found 2-amino-6-arylthio-9-phosphonomethoxyethylpurine bis(2,2,2-trifluoroethyl) ester derivatives that showed high antiviral activity and apparent absorption in the animals. In this report, we evaluated the in vitro antiviral properties of MCC-478, 2-amino-6-(4-methoxyphenylthio)-9-[2-(phosphonomethoxy)ethyl]purine bis(2,2,2-trifluoroethyl) ester. Since the bis(2,2,2-trifluoroethyl) ester group was used to improve the oral bioavailability of the phosphonomethoxyethylpurine derivatives and might be hydrolyzed in vivo to give its monoester and free phosphonic acid, the monoester and free phosphonic acid derivatives of MCC-478 were also tested. In order to investigate the toxicological profile of MCC-478, cytotoxicity and reduction of the mitochondrial DNA content was evaluated. It was shown that MCC-478 is a potent inhibitor of HBV replication, and its antiviral profile is specific to HBV.
MATERIALS AND METHODS
Compounds.
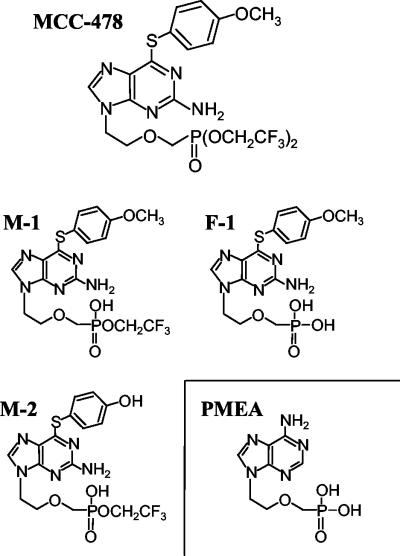
The following test compounds were chemically synthesized at Mitsubishi Pharma Corporation: MCC-478 (2-amino-6-(4-methoxyphenylthio)-9-[2-(phosphonomethoxy)ethyl]purine bis(2,2,2-trifluoroethyl) ester), monoester derivative 1 (M-1) (2-amino-6-(4-methoxyphenylthio)-9-[2-(phosphonomethoxy)ethyl]purine 2,2,2-trifluoroethyl ester), free phosphonic acid derivative 1 (F-1) (2-amino-6-(4-methoxyphenylthio)-9-[2-(phosphonomethoxy)ethyl]purine), and monoester derivative 2 (M-2) (2-amino-6-(4-hydroxyphenylthio)-9-[2-(phosphonomethoxy)ethyl]purine 2,2,2-trifluoroethyl ester). PMEA (9-[2-(phosphonomethoxy)ethyl]adenine) was synthesized by the method of Holy and Rosenberg (15). Lamivudine was extracted and purified from tablets of Epivir (GlaxoWellcome). Epivir tablets (60 150-mg tablets) were ground and extracted with hot methanol. Silica gel was added to this methanol solution, and the resultant slurry was stirred for 30 min. Methanol was removed by evaporation, and the residue was transferred to a silica gel column. The desired material was eluted with a 9:1 mixture of chloroform-methanol. The eluate was concentrated and dissolved in methanol-toluene to produce crystals of lamivudine. The yield was 8.14 g. The structure was identified with proton nuclear magnetic resonance, and the purity (>99%) was checked by thin-layer chromatography, high-pressure liquid chromatography, and measurement of optical rotation. The structures of MCC-478, M-1, M-2, F-1, and PMEA are shown in Fig. 1.
FIG. 1.
Chemical structures of MCC-478 and its metabolites in vivo.
Cell culture.
HB611 (32), a stably transfected cell line which contains three copies of the HBV genome in tandem in its chromosome, and HuH-6, a parent cell line, were kindly provided by K. Matsubara (Institute for Molecular and Cellular Biology, Osaka University, Osaka, Japan; now at the Department of Molecular Biology, Nara Institute of Science and Technology, Nara, Japan). HB611 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 0.2 mg of Geneticin (GibcoBRL) per ml and 10% fetal calf serum (FCS). HuH-6 cells were maintained in DMEM supplemented with 10% FCS. HepG2 cells were obtained from Dainippon Pharmaceuticals (Osaka, Japan) and maintained in MEM supplemented with nonessential amino acids, 1 mM sodium pyruvate, and 10% FCS. All cell lines were cultured at 37°C with 5% CO2.
Analysis of anti-HBV activities.
Antiviral activities of the test compounds were analyzed by a modified version of the method of Yokota et al. (36). HB611 cells were plated at a density of 2 × 104 cells per well on 24-well plates. When the cells had grown to confluence, the medium was changed to medium containing a test compound. Test compounds and the final concentrations in the experiments were as follows: experiment 1, MCC-478 at 0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 μM and PMEA and lamivudine at 0.1, 0.3, 1.0, 3.0, and 10 μM; experiment 2, MCC-478, M-1, and F-1 at 0.01, 0.03, 0.1, 0.3, and 1.0 μM; experiment 3, M-1 and M-2 at 0.03, 0.1, 0.3, 1.0, and 3.0 μM. The cells were cultured for 8 or 9 days with the drug-containing medium changed every 3 or 4 days. On the last day of cultivation, the monolayers were washed with phosphate-buffered saline, and the cells were lysed at 37°C for 1 h with 0.5 ml of lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% sodium dodecyl sulfate [SDS], 0.1 mg of proteinase K [Boehringer Mannheim] per ml). The lysates were collected and placed in 1.5-ml Eppendorf tubes and were further digested by proteinase K for 2 days with gentle agitation. After digestion with 0.1 mg of RNase A per ml at 65°C for 15 min, the lysate was extracted by phenol-chloroform-isoamyl alcohol. The total cellular DNA was precipitated by ethanol and was digested with HindIII (Boehringer Mannheim) at 37°C overnight. The digested DNA was electrophoresed on a 0.9% agarose-1× Tris-borate-EDTA gel and stained with ethidium bromide. The separated DNA samples were treated with 0.25 N hydrochloric acid for 15 min, denatured in 0.5 N NaOH for 30 min, and neutralized with 0.5 M Tris-HCl [pH 7.5] containing 1.5 M NaCl and 1 mM EDTA for 1 h. DNA was transferred to a nylon membrane (Hybond N+; Amersham) using a VacuGene vacuum blotting system (Amersham). The membranes were dried, linked by using a UV illuminator, and hybridized to a digoxigenin (DIG)-labeled HBV DNA at 42°C overnight in hybridization buffer (DIG Easy Hyb; Boehringer Mannheim). The hybridized membranes were washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS at room temperature for 5 min and twice with 0.1× SSC containing 1% SDS at 65°C for 30 min. The HBV-specific bands were detected by anti-DIG-AP Fab fragments (Boehringer Mannheim) and CDP-star (New England Biolabs). After exposure to X-ray film (XJB; Kodak), the developed film was scanned in a quantitative densitometric manner using ImageQuant image analysis software on a Personal Densitometer (Molecular Dynamics). The densities of the signals derived from integrated HBV and free HBV DNA were measured. HBV DNA content of each sample was determined by the ratio of HBV DNA replication intermediates to integrated HBV DNA.
Cytotoxicity.
HuH-6 cells were plated at a density of 105 cells per ml on 96-well plates and cultured at 37°C with 5% CO2 for 3 days in DMEM supplemented with 10% FCS and each test compound at final concentrations of 10, 30, 100, 300, and 1,000 μM. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) (CellTiter96 aqueous nonradioactive cell proliferation assay; Promega) was added at the end of the culture period, and the plate was incubated at 37°C for 2 h. The absorbance at 490 nm of each well was measured with a microplate reader (NJ-2000; Intermed).
Anti-HIV activity.
Determination of antiviral activity against HIV type 1 (HIV-1) replication was based on inhibition of the HIV-induced cytopathic effect in MT-4 cells, as described previously (23). MT-4 cells (105/ml) were infected with HIV-1 (HTLV-IIIB strain) at a multiplicity of infection of 0.02. The cells were cultured in the presence of 0.032, 0.16, 0.8, 4, and 20 μg of the test compounds per ml at 37°C with 5% CO2. In order to assess the viability of both HIV- and mock-infected MT-4 cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added on day 4 after virus infection and incubated at 37°C for 2 h. The absorbance at 540 nm was measured, and the percent inhibition value was calculated.
Mitochondrial DNA content.
HepG2 cells (2.5 × 104 cells/well) were plated in a 12-well plate and treated with each test compound for 8 days. On the last day of culture, the cells were washed with phosphate-buffered saline, harvested, placed in a 1.5-ml Eppendorf tube, and stored at −80°C until use. Cells were lysed and digested by proteinase K at 37°C for one night with lysis buffer. The lysate was digested with RNase A (0.1 mg/ml) at 65°C for 10 min and extracted by phenol-chloroform-isoamyl alcohol. The total cellular DNA was precipitated by ethanol and dissolved in distilled water. DNA (2.5 μg) was denatured in 0.5 N NaOH and 1.5 M sodium chloride for 10 min and dot blotted onto a nylon membrane (Hybond N+; Amersham). The membrane was dried, linked by using a UV illuminator, and hybridized to a DIG-labeled mitochondrion-specific oligonucleotide DNA probe (5′-DIG-CTTATATGAT ATGTCTCCAT ACCCATTACA ATCTCCAGCA-3′), which extends from nucleotides 4207 to 4246 of mitochondrial DNA (2), in the hybridization buffer (DIG Easy Hyb; Boehringer Mannheim) at 42°C overnight.
RESULTS
Antiviral activities of MCC-478 and other nucleoside or nucleotide analogues.
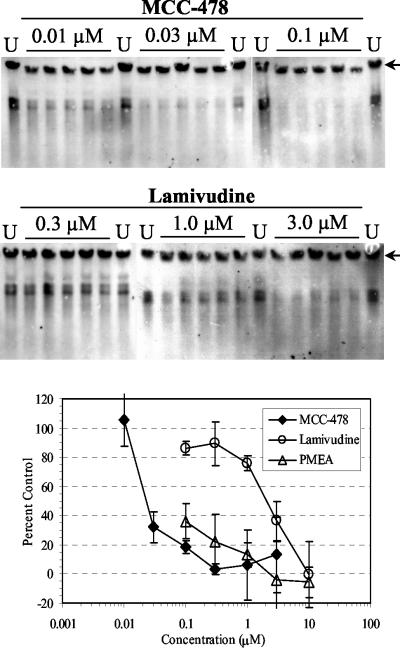
We first attempted to evaluate anti-HBV activities of MCC-478, lamivudine, and PMEA in HB611 cells, which is a stable transfected cell line producing HBV. HB611 cells were treated with various concentrations of MCC-478, lamivudine, or PMEA (both lamivudine and PMEA are used as reference drugs in many studies for anti-HBV agents). The cells in five wells on a 24-well plate were treated independently at each concentration for 8 days. The extent of HBV DNA replication in cells was evaluated from the ratio of the replicative intermediates to the integrated HBV genome. Antiviral effects were reported as percent control values (untreated cells). Figure 2 shows the average percent control values of HBV DNA replication in cells treated with drugs at each concentration. Also, WinNonlin version 1.1 (Scientific Consulting Inc.) was used to calculate the 50% and 90% effective concentrations (EC50 and EC90, respectively) of MCC-478 and lamivudine. The EC50 and EC90 of MCC-478 were 0.027 and 0.24 μM, respectively, while those of lamivudine were 2.2 and 7.8 μM, respectively. The EC50 of PMEA could not be calculated, since PMEA gave percent control values below 50 even at the lowest concentration (0.1 μM). These results clearly suggested that MCC-478 was a potent inhibitor of HBV DNA replication.
FIG. 2.
Inhibitory effects of MCC-478, PMEA, and lamivudine against HBV replication in HB611 cells. Cells were treated with the indicated concentrations of test compound for 8 days and harvested on the last day of culture. After total cellular DNA was prepared, HBV DNAs derived from both replication intermediates and integrated HBV genome were detected and analyzed by Southern blotting. Percent control values were calculated from the untreated controls (U). The bands derived from integrated HBV are indicated by the arrows. The mean value at each concentration is plotted on the graph, and the standard deviations are indicated by the error bars.
Comparison of the anti-HBV activities of the hydrolyzed derivatives of MCC-478.
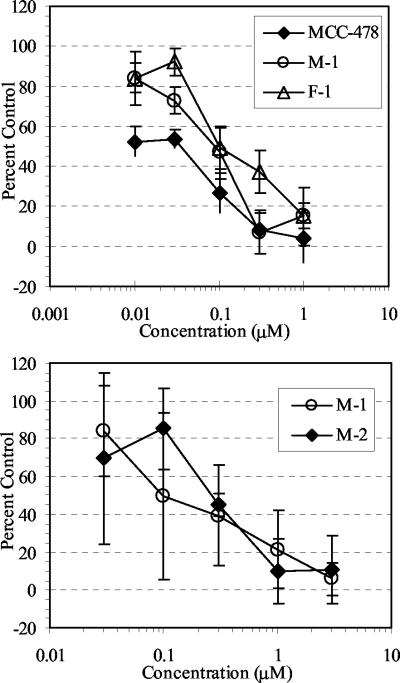
Anti-HBV activity was also observed in sera from mice given MCC-478 orally (data not shown). Since some of the bisalkyl ester prodrugs of PMEA were not cleaved completely to a free phosphonic acid form (29), two monoester derivatives (M-1 and M-2) and one phosphonic acid derivative (F-1) of MCC-478 (Fig. 1) were putative metabolites in serum. In order to understand their contribution to efficacy in vivo, they were synthesized and tested in HB611 cells. As shown in Fig. 3, the anti-HBV activities of M-1 and F-1 were similar to that of MCC-478, and the anti-HBV activity of M-2 was similar to that of M-1.
FIG. 3.
Comparison of the inhibitory effects of MCC-478, M-1, M-2, and F-1 against HBV replication in HB611 cells. Cells were treated with the indicated concentrations of test compound for 8 days. Anti-HBV activity was evaluated as indicated in the legend to Fig. 2. The mean value at each concentration is plotted on the graph, and the standard deviations are indicated by the error bars.
Cytotoxicity and anti-HIV activity of MCC-478.
The 50% cytotoxic concentration (CC50) of each test compound was determined in the HuH-6 cell line, the parent cell line of HB611. MCC-478 and its derivatives were well tolerated (Table 1). To evaluate the specificity for antiviral activity, anti-HIV activities of MCC-478, PMEA, and lamivudine were determined by examining the inhibition of the HIV-induced cytopathic effect in the MT-4 cell line. Treatment with MCC-478 showed no significant selectivity between the cytotoxicity against mock-infected MT-4 cells and the inhibition of HIV-induced cytopathic effect, e.g., 16 and 11%, respectively, even at the highest concentration. The EC50s and CC50s of the test compounds in MT-4 cells are summarized in Table 1. These results suggested that anti-HIV activity of MCC-478 was much weaker than anti-HBV activity, while PMEA retained anti-HBV and anti-HIV activity.
TABLE 1.
Summary of the antiviral and cytotoxic effects of the test compounds
| Test compound | HBV in HB611 cells
|
HIV-1 in MT-4 cells
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM)a
|
EC90 (μM)a
|
CC50 (μM) | EC50 (μM)b | CC50 (μM) | |||||
| Expt 1 | Expt 2 | Expt 3 | Expt 1 | Expt 2 | Expt 3 | ||||
| MCC-478 | 0.027 | 0.026 | 0.24 | 0.23 | <1,000 | >34 | >34 | ||
| M-1 | 0.072 | 0.15 | 0.64 | 1.37 | 548 | NTc | NT | ||
| F-1 | 0.15 | 1.36 | <1,000 | NT | NT | ||||
| M-2 | 0.29 | 2.43 | <1,000 | NT | NT | ||||
| PMEA | <0.1 | <1,000 | 31 | >70 | |||||
| Lamivudine | 2.2 | 7.76 | <1,000 | 0.9 | >100 | ||||
Effect of MCC-478 on mitochondrial DNA content.
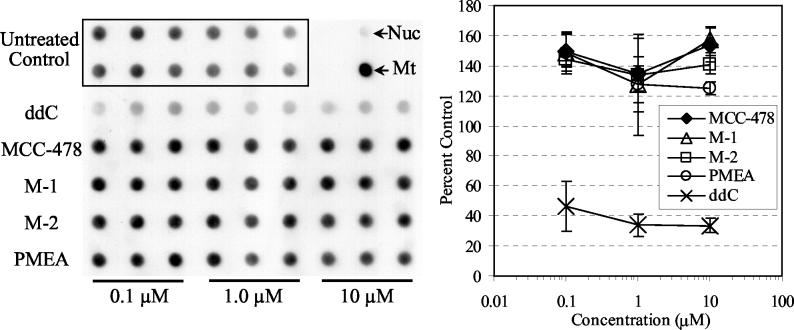
Therapies with nucleoside analogues sometimes cause mitochondrial dysfunction, which could be derived from incorporation of the nucleoside analogue into mitochondrial DNA (8) or reduction in mitochondrial DNA content (6). In this study, the effects of MCC-478 and its hydrolyzed derivatives on mitochondrial DNA content were examined, using 2′,3′-dideoxycytidine (ddC) as a positive control. HepG2 cells were treated with each test compound at a final concentration of 0.1, 1.0, or 10 μM for 8 days. Mitochondrial DNA content was examined by dot blot hybridization. Treatment with MCC-478, M-1, M-2, or PMEA showed no reduction in mitochondrial DNA content, even though treatment with ddC at every concentration reduced mitochondrial DNA content to nearly half that of the untreated control (Fig. 4).
FIG. 4.
Effects of MCC-478 and its derivatives on mitochondrial DNA content in HepG2 cells. Cells were treated with the indicated concentrations of test compound for 8 days and harvested on the last day of culture. After total cellular DNA was prepared, mitochondrial DNA content in 2.5 μg of total cellular DNA was evaluated by dot blot analysis. The DNAs from nuclei (Nuc) and mitochondrial (Mt) fractions of HepG2 cells are indicated by the arrows. Percent control values were calculated from the untreated controls. The mean value at each concentration is plotted on the graph, and the standard deviations are indicated by the error bars.
DISCUSSION
MCC-478 is a novel phosphonomethoxyethylpurine derivative which has an arylthio group at position 6 of the purine base. Also, MCC-478 has 2-aminopurine nucleobase structure, which indicates that it is more closely related to 9-[2-(phosphonomethoxy)ethyl]guanine (PMEG) than to PMEA. However, introduction of the arylthio group at position 6 of the 2-aminopurine base has been demonstrated to endow the purine base with a higher specificity against HBV and low cytotoxicity comparable to that of PMEA (Table 1), while PMEG is known to have higher cytotoxicity than PMEA (17). These results suggest that substitution at position 6 of the purine base may alter the biological and toxicological profiles of the phosphonomethoxyethyl purine derivatives.
It was reported that adefovir dipivoxil is primarily hydrolyzed to the monoester form and is further metabolized to adefovir (PMEA) in the presence of either cells or serum (27). This finding may mean that there was an esterase to hydrolyze both bis(pivaloyloxymethyl) and mono(pivaloyloxymethyl) ester forms in both serum and cells. In the case of MCC-478, which is a bis(2,2,2-trifluroroethyl) ester, it was hydrolyzed to only the monoester form, even in the presence of serum from humans or rats (data not shown). In addition, major metabolites in rats and monkeys were found to be the monoester forms of MCC-478 (M-1 and M-2), rather than the free phosphonic acid form (F-1) (Y. Yamaguchi, personal communication). On the other hand, the free phosphonic acid form, PMEA, was identified as the major metabolite in sera from the animals given adefovir dipivoxil orally. This speculated difference of metabolites between MCC-478 and adefovir dipivoxil in vivo suggested the necessity of examining the antiviral activities of these metabolites in order to estimate their efficacy in vivo. Here, not only the monoester forms but also the free phosphonic acid form were recognized to have antiviral activities similar to that of MCC-478 in HB611 cells (Fig. 3). The anti-HBV activities of these MCC-478 derivatives were high enough to suggest that they could contribute to in vivo efficacy of MCC-478. These results also indicated that the mono(2,2,2-trifluoroethyl) ester forms of MCC-478 may be hydrolyzed only within the cell and further metabolized to an active form of MCC-478.
In addition to the antiviral profile of MCC-478, it was also shown that the cytotoxic effects of MCC-478 and its hydrolyzed derivatives were quite low (Table 1). It may also be important to evaluate mitochondrial toxicity to understand the biological profile of a nucleotide analogue, since long-term treatment with antiviral nucleoside analogues could give rise to delayed and possibly severe mitochondrial toxicity (19). Nucleoside and nucleotide analogs can be classified into two types on the basis of their mechanism of mitochondrial toxicity. The first type of nucleoside analogue, such as fialuridine, has a 3′-hydroxyl group (3′-OH), and mitochondrial toxicity is associated with nucleoside incorporation into the mitochondrial DNA (8). The second type of nucleoside analogue, such as ddC, does not have a 3′-OH group, and mitochondrial toxicity is associated with reduced mitochondrial DNA content by chain termination (6). Since MCC-478 and its hydrolyzed derivatives do not have 3′-OH, they may belong to the latter type of nucleoside analogues. However, as shown in Fig. 4, there was no evidence of inhibition of mitochondrial DNA replication. This lack of evidence of inhibition may mean that MCC-478 and its metabolites did not have inhibitory activity against DNA polymerase γ. A chain-terminating analysis using DNA polymerase α also revealed that neither F-1 nor its diphosphate showed chain-terminating activity against DNA polymerase α; however, PMEA diphosphate was thought to have chain-terminating activity at the same concentrations (data not shown). These results emphasize the specific antiviral profiles of MCC-478 and its derivatives. Interestingly, the diphosphates derived from MCC-478 were not found intracellularly, so far as we tried. While there is a possibility that the diphosphates might be a very potent and selective inhibitor of hepadnavirus polymerase, MCC-478 and its derivatives might not require phosphorylation for biological activity. More studies of intracellular metabolism and active metabolites would be needed to elucidate the mechanisms of MCC-478 antiviral activity.
Lamivudine is the first approved nucleoside analogue for treatment of HBV infection. It is known to have potent activity against both HBV and HIV replication (16). However, the rapid emergence of viruses resistant to lamivudine in HIV-infected patients is also known, even with short-term therapy, and recently a similar phenomenon was observed with lamivudine monotherapy (1, 11, 20). Most lamivudine-resistant virus strains have methionine (M) substituted for isoleucine (I) or valine (V) on the YMDD (tyrosine-methionine-aspartate-aspartate) motif in catalytic domains of HIV polymerase (14) and hepadnavirus polymerase (1). Another drug-resistant substitution of leucine (L) to M in B domain (L528M) is reported from a clinical trial of famciclovir in HBV-infected patients (25). The L528M mutation frequently accompanies the YMDD mutation, and HBV carrying these two mutations is cross-resistant to lamivudine and famciclovir (10). In the case of HIV, a steric hindrance between the mutant amino acid side chain and lamivudine triphosphate is suggested from the molecular model analysis of comparisons of crystal structures of the YMDD mutant and wild-type HIV polymerases with or without double-strand DNA (26). Moreover, this molecular model suggested that a steric hindrance between the mutant amino acid side chain and the lamivudine sugar ring might be expected in nucleoside analogues with β-l- ring configurations. In the case of HBV, a recent study reporting molecular modeling analysis using the HBV polymerase homology model (9) also supports this concept. Ono et al. (22) evaluated the inhibitory effects of a panel of 11 nucleoside and nucleotide analogues on HBVs with one or two mutations by in vitro full-length HBV DNA transfection and found that only a few nucleoside analogues, including PMEA, are active against lamivudine-resistant HBVs. Since MCC-478 was shown to be a novel nucleotide analogue having potent and selective anti-HBV activity, even if it was a derivative of phosphonomethoxyethylpurine, like PMEA, MCC-478 should be further examined for an inhibitory profile against drug-resistant HBVs, including lamivudine-resistant HBVs.
The aim of this work was to evaluate MCC-478 and its derivatives as potent and specific agents for the treatment of HBV infections. Although many studies have to be done to understand the mechanism of action, toxicological pharmocokinetic profiles, and so on, MCC-478 could be a promising new anti-HBV agent.
Acknowledgments
We thank Masanori Baba (Kagoshima University, Kagoshima, Japan) for measuring the inhibitory activity of MCC-478 against HIV.
REFERENCES
- 1.Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K. A. Walters, D. L. J. Tyrrell, N. Brown for the Lamivudine Clinical Investigation Group, and L. D. Condreay. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology 27:1670-1677. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S., A. T. Bankier, B. G. Barrell, M. H. L. de Bruijn, A. R. Coulson, J. Drouin, I. C. Eperon, D. P. Nierlich, B. A. Roe, F. Sanger, P. H. Schreier, A. J. H. Smith, R. Staden, and I. G. Young. 1981. Sequence and organization of the human mitochondrial genome. Nature 290:457-465. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager, R., and H. Schaller. 1992. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 11:3413-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscher, M., W. Resiser, H. Will, and H. Schaller. 1985. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell 40:717-724. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. N., V. Skalski, J. H. Zhou, and Y. C. Cheng. 1992. Biochemical pharmacology of (+)- and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J. Biol. Chem. 267:22414-22420. [PubMed] [Google Scholar]
- 6.Chen, C. H., and Y. C. Cheng. 1989. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J. Biol. Chem. 264:11934-11937. [PubMed] [Google Scholar]
- 7.Coates, J. A., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui, L., S. Yoon, R. F. Schinazi, and J. P. Sommadossi. 1995. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-beta-d-arabinofuranosyl)-5-iodouracil in human liver cells. J. Clin. Investig. 95:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, K., X. Xiong, H. Yang, C. E. Westland, C. S. Gibbs, S. G. Sarafianos, and E. Arnold. 2001. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine and emitricitabine. J. Virol. 75:4771-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney, W. E., IV, R. Edwards, D. Colledge, T. Shaw, J. Torresi, T. G. Miller, H. C. Isom, C. T. Bock, M. P. Manns, C. Trautwein, and S. Locarnini. 2001. Cross-resistance testing of antihepadnaviral compounds using novel recombinant baculoviruses which encode drug-resistant strains of hepatitis B virus. Antimicrob. Agents Chemother. 45:1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dienstag, J. L., E. R. Schiff, T. L. Wright, R. P. Perrillo, H. W. Hann, Z. Goodman, L. Crowther, L. D. Condreay, M. Woessner, M. Rubin, and N. A. Brown. 1999. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 341:1256-1263. [DOI] [PubMed] [Google Scholar]
- 12.Doong, S. L., C. H. Tsai, R. F. Schinazi, D. C. Liotta, and Y. C. Cheng. 1991. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc. Natl. Acad. Sci. USA 88:8495-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell, G. C. 2000. Clinical potential of emerging new agents in hepatitis B. Drugs 60:701-710. [DOI] [PubMed] [Google Scholar]
- 14.Gao, Q., Z. Gu, M. A. Parniak, J. Cameron, N. Cammack, C. Boucher, and M. A. Wainberg. 1993. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 37:1390-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holy, A., and I. Rosenberg. 1987. Synthesis of 9-(2-phosphonylmethoxyethyl) adenine and related compounds. Collect. Czech. Chem. Commun. 52:2801-2809. [Google Scholar]
- 16.Jarvis, B., and D. Faulds. 1999. Lamivudine: a review of its therapeutic potential in chronic hepatitis B. Drugs 58:101-141. [DOI] [PubMed] [Google Scholar]
- 17.Kramata, P., and K. M. Downey. 1999. 9-(2-Phosphonomethoxyethyl) derivatives of purine nucleotide analogues: a comparison of their metabolism and interaction with cellular DNA synthesis. Mol. Pharmacol. 56:1262-1270. [DOI] [PubMed] [Google Scholar]
- 18.Lai, C. L., R. N. Chien, N. W. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and D. F. Gray. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 19.Lewis, W., and M. C. Dalakas. 1995. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1:417-422. [DOI] [PubMed] [Google Scholar]
- 20.Liaw, Y. F., R. N. Chien, C. T. Yeh, S. L. Tsai, and C. M. Chu. 1999. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology 30:567-572. [DOI] [PubMed] [Google Scholar]
- 21.Noble, S., and K. L. Goa. 1999. Adefovir dipivoxil. Drugs 58:479-487. [DOI] [PubMed] [Google Scholar]
- 22.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide-binding site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels, R., J. Balzarin, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 24.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 25.Pichoud, C., B. Seignères, Z. Wang, C. Trèpo, and F. Zoulim. 1999. Transient selection of a hepatitis B virus polymerase gene mutant associated with a decreased replication capacity and famciclovir resistance. Hepatology 29:230-237. [DOI] [PubMed] [Google Scholar]
- 26.Sarafianos, S. G., K. Das, A. D. Clark, Jr., J. Ding, P. L. Boyer, S. H. Hughes, and E. Arnold. 1999. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with β-branched amino acids. Proc. Natl. Acad. Sci. USA 96:10027-10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas, R. V., B. L. Robbins, M. C. Connelly, Y. F. Gong, N. Bischofberger, and A. Fridland. 1993. Metabolism and in vitro antiretroviral activities of bis(pivaloyloxymethyl) prodrugs of acyclic nucleoside phosphonates. Antimicrob. Agents Chemother. 37:2247-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staprans, S., D. D. Loeb, and D. Ganem. 1991. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of liver viral DNA. J. Virol. 65:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starret, J. E., Jr., D. R. Tortolani, J. Russell, M. J. M. Hitchcock, V. Whiterock, J. C. Martin, and M. M. Mansuri. 1994. Synthesis, oral bioavailability determination and in vitro evaluation of prodrugs of the antiviral agent 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). J. Med. Chem. 37:1857-1864. [DOI] [PubMed] [Google Scholar]
- 30.Summers, J., and W. S. Mason. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403-415. [DOI] [PubMed] [Google Scholar]
- 31.Tsiang, M., J. F. Rooney, J. J. Toole, and C. S. Gibbs. 1999. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29:1863-1869. [DOI] [PubMed] [Google Scholar]
- 32.Tsurimoto, T., A. Fujiyama, and K. Matsubara. 1987. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc. Natl. Acad. Sci. USA 84:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuttleman, J. S., C. Pourcel, and J. Summers. 1986. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 47:451-460. [DOI] [PubMed] [Google Scholar]
- 34.Ubasawa, M., H. Takashima, K. Sekiya, N. Inoue, S. Yuasa, and N. Kamiya. November 1998. Preparation of acyclic nucleotide phosphonates as virucides. U.S. patent 5,840,716.
- 35.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell 71:663-670. [DOI] [PubMed] [Google Scholar]
- 36.Yokota, T., K. Konno, S. Shigeta, A. Holy, J. Balzarini, and E. De Clercq. 1994. Inhibitory effects of acyclic nucleoside phosphonate analogues on hepatitis B virus DNA synthesis in HB611 cells. Antivir. Chem. Chemother. 5:57-63. [Google Scholar]
- 37.Zhu, Y.-L., G. E. Dutschman, S.-H. Liu, E. G. Bridges, and Y.-C. Cheng. 1998. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine. Antimicrob. Agents Chemother. 42:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]