Abstract
Two putative proteins (NorMI and NorMII) similar to the multidrug efflux protein NorM of Vibrio parahaemolyticus are encoded by the Brucella melitensis 16 M genome. We show that a drug-hypersusceptible Escherichia coli strain overexpressing NorMI displays increased resistance to norfloxacin, ciprofloxacin, gentamicin, tetraphenylphosphonium ion, acriflavine, and berberine. This elevated resistance was proven to be mediated by an energy-dependent efflux mechanism. NorMI belongs to the multidrug and toxic compound extrusion family and is the first multidrug efflux protein identified in Brucella spp.
Although brucellosis is primarily a disease of domestic animals, it is still a common human disease in many developing countries (7). The pathogens responsible for brucellosis, Brucella spp., are facultative intracellular bacteria. Therefore, to be effective, the treatment of acute brucellosis requires drugs that penetrate macrophages. With conventional drugs such as tetracycline, aminoglycosides, or sulfonamides, high relapse rates have been reported (8, 13, 23). More recently, increasing resistance of Brucella spp. to co-trimoxazole was reported (3, 16). This has led to the search for new drugs which penetrate eukaryotic cells more efficiently. The broad-spectrum in vitro activity of fluoroquinolones such as ciprofloxacin, as well as their oral bioavailability and their excellent intracellular penetration, initially made them very promising for treating brucellosis (12). However, a lack of effective bactericidal activity of fluoroquinolones against Brucella spp. (14, 24), an appreciable rate of relapse of patients treated with ciprofloxacin (2), and development of ciprofloxacin resistance associated with cross-resistance to other fluoroquinolones in Brucella melitensis during ciprofloxacin therapy have been also reported (1, 22, 24).
Nothing is known about the mechanism of fluoroquinolone resistance development in B. melitensis. In other bacteria, resistance to fluoroquinolones usually results from multiple mutations in genes encoding their intracellular targets, gyrase and topoisomerase IV (4, 9, 15). In addition to this mechanism, which is antibiotic specific, a more general mechanism involves multidrug efflux pumps which exclude toxic compounds, including antibiotics, from the cells. Some of these pumps exhibit low specificity and confer resistance to several unrelated antibiotics when they are overexpressed.
These bacterial pumps are classified in five large, ubiquitous superfamilies (25). One of these, the multidrug and toxic compound extrusion (MATE) family, was recently identified. It includes several bacterial members but also eukaryotic proteins from fungi and plants (5). Among these, only a few have been functionally characterized. Four bacterial members, NorM and VmrA of Vibrio parahaemolyticus, YdhE of Escherichia coli, and BexA of Bacteroides thetaiotaomicron, have been reported to mediate multidrug resistance (6, 17-19). NorM and VmrA were also reported to function by a drug:Na+ antiport mechanism (6, 19). Among the eukaryotic members, the only two functionally characterized proteins are yeast Erc1, which confers resistance to the methionine analog ethionine, and Arabidopsis Alf5, conferring resistance to toxins (11, 26). Proteins of the MATE family have a common predicted topology with 12 transmembrane helices (5, 11, 18).
A B. melitensis homolog of NorM was recently identified as a potential virulence factor, as strains with mutations in its gene could not be recovered from animals infected with pools of signature tag mutants (M. S. Zygmunt, S. D. Hagius, W. T. Fulton, J. V. Walker, N. J. Booth, and P. H. Elzer, Brucellosis 2000, 53rd Brucellosis Research Conference, abstr. 56, 2000). In the present work, we functionally characterized this transporter and confirmed that it belongs to the MATE family.
Identification of sequences belonging to the MATE family in the genome of B. melitensis 16 M.
By searching for homology with the NorM multidrug efflux protein of V. parahaemolyticus (BlastP) in the B. melitensis 16 M genomic database (accession numbers AE008917 and AE008918), we found that two putative multidrug efflux pumps belonging to the MATE family are encoded by this genome (10). The first one, which we named NorMI, is encoded by gene BMEI1585 and corresponds to the above-mentioned protein that was recently identified as a potential virulence factor. The second, which we named NorMII, is encoded by gene BMEI1612. NorMI and NorMII share 27.7 and 19.9% identical amino acids (in an overlap of 448 amino acids), respectively, with the NorM protein of V. parahaemolyticus. As NorMI is more similar to NorM than NorMII, and consequently is more likely to have a similar function, we focused this work on NorMI. To confirm that NorMI belongs to the MATE family, we searched (with BlastP) the EMBL redundant protein database to determine whether NorMI is also homologous to other proteins of this family. Besides the homology with NorM, NorMI also possesses 22.2 to 28.6% amino acid identity to the E. coli YdhE protein, the B. thetaiotaomicron BexA protein, the V. parahaemolyticus VmrA protein, the Arabidopsis Alf5 protein, and the yeast Erc1 protein. No homology was found with any other functionally characterized bacterial proteins. A prediction of transmembrane helices by using the TMHMM server (version 2.0) from the Center for Biological Sequence Analysis (http://www.cbs.dtu.dk/services/TMHMM-2.0/) revealed that NorMI possesses 12 putative transmembrane helices. These data strongly suggest that NorMI represents a new member of the MATE family.
NorMI confers resistance to multiple drugs.
To study the involvement of NorMI in drug efflux, we overexpressed this protein in the drug-hypersusceptible E. coli strain AG100A, which was deleted of the major multidrug efflux system AcrAB by Okusu et al. (21). The norMI gene was cloned into the pUC19 vector under the control of its natural promoter. In brief, norMI was first amplified by PCR from the chromosomal DNA of B. melitensis 16 M with primers P7 (5′-TCGGATCCGGGACGGAAATTTGCGCTTTC-3′) and P4 (5′-GGAATTCCCTGAAAGGCTTCGGTGCCGC-3′) (1 μM final concentration) and Pfu DNA polymerase (Promega) in an iCycler thermocycler (Bio-Rad) with the following cycling conditions: one cycle of 2 min at 95°C; then 35 cycles of 1 min at 95°C, 30 s at 55°C, and 4 min at 72°C; and finally one cycle of 5 min at 72°C. The amplified DNA fragment was restricted with BamHI and EcoRI and inserted between the BamHI and EcoRI sites of pUC19. The resulting plasmid (pUC19-NorMI) and pUC19 were then electroporated separately in E. coli AG100A.
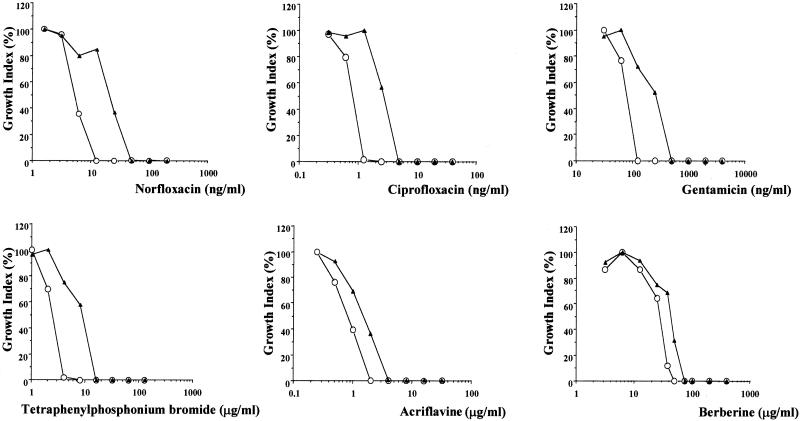
The capacity of the construct to confer drug resistance to E. coli AG100A was evaluated by comparing the susceptibilities of the strains carrying pUC19 and pUC19-NorMI to increasing concentrations of many unrelated drugs (norfloxacin, ciprofloxacin, gentamicin, tetraphenylphosphonium bromide, erythromycin, nalidixic acid, chloramphenicol, ofloxacin, quinacrine, tetracycline, ethidium bromide, carbonyl cyanide m-chlorophenylhydrazone [CCCP], and sulfamethoxazole). Exponentially growing bacteria (105) were inoculated into 5 ml of Mueller-Hinton medium containing twofold-increasing concentrations of each drug. For each drug concentration, growth was monitored by measuring the turbidity of the culture at 600 nm after 20 h of incubation at 37°C under agitation. As shown in Fig. 1, NorMI confers increasing resistance of strain AG100A towards hydrophilic fluoroquinolones such as norfloxacin and ciprofloxacin, to aminoglycosides such as gentamicin, and to the tetraphenylphosphonium cation. A slight increase of resistance to acriflavine and berberine was also observed. On the other hand, AG100A carrying pUC19 and pUC19-NorMI showed indistinguishable susceptibilities to the other antimicrobial agents tested (data not shown). The substrate specificity of NorMI is highly similar to that of YdhE of E. coli (18). Indeed, like NorMI, YdhE confers resistance to norfloxacin, ciprofloxacin, acriflavine, berberine, and tetraphenylphosphonium ion. Although the specificity of YdhE resistance to gentamicin was not tested, this protein confers resistance to similar aminoglycosides such as kanamycin and streptomycin. These results indicated that NorMI mediates multidrug resistance.
FIG. 1.
Effect of drugs on growth of E. coli cells expressing or not expressing the NorMI protein of B. melitensis. E. coli AG100A harboring a vector expressing NorMI from B. melitensis (pUC19-NorMI) (▴) or harboring the vector alone (pUC19) (○) was grown for 20 h at 37°C (with agitation) in Mueller-Hinton medium containing twofold-increasing concentrations of norfloxacin (1.5625 to 200 ng/ml), ciprofloxacin (0.3125 to 40 ng/ml), gentamicin (31.25 to 4,000 ng/ml), tetraphenylphosphonium bromide (1 to 128 μg/ml), acriflavine (0.25 to 32 μg/ml), or berberine (3.125 to 400 μg/ml). Bacterial growth was monitored by turbidimetry (at 600 nm). The growth index, calculated by dividing the OD600 of the culture in the presence of drug by the OD600 of the culture in the absence of drug, is shown.
The contribution of NorMI to the drug resistance of B. melitensis was tentatively approached by disrupting norMI in this strain. Briefly, a kanamycin resistance cassette extracted from pUC-4K (Pharmacia Biotech) by EcoRI restriction was inserted into the unique BssHII site of the pUC19-NorMI construct, a suicide vector for Brucella spp. After introduction of this construct in B. melitensis cells by electroporation, kanamycin-resistant clones were selected and tested by PCR to confirm the correct insertion of the kanamycin cassette and the disruption of norMI. A disruption mutant was selected, and its susceptibility to ciprofloxacin, one of the best substrates of NorMI, was compared with that of the wild-type strain. This was done as described above for E. coli, except that 107 cells inoculated into 5 ml of Trypticase soy broth (Difco Laboratories) supplemented with 0.1% yeast extract were used. The mutant and wild-type strains showed indistinguishable susceptibilities to ciprofloxacin (data not shown). A number of other putative efflux pumps (including seven homologs of the major E. coli AcrAB pump, at least three homologs of the E. coli EmrAB pump, and two ABC transporters similar to those involved in drug efflux) are encoded by the B. melitensis genome (10). Those pumps may well mask the effect of the norMI mutation, and for this reason, we were obliged to analyze the function of NorMI in E. coli cells previously mutated in their major AcrAB pump (21).
Multidrug resistance is mediated by an energy-dependent efflux.
To investigate the mechanism by which NorMI confers multidrug resistance, we compared the norfloxacin accumulation in E. coli AG100A cells harboring the pUC19-NorMI construct with that observed in E. coli AG100A containing pUC19 alone. We also measured the effect of CCCP, a proton motive force uncoupler, on the accumulation of norfloxacin inside the cells. Norfloxacin accumulation was assayed by the method of Mortimer and Piddock (20) with some modifications. Briefly, bacteria were grown in Luria-Bertani broth at 37°C to the mid-log phase of growth (optical density at 600 nm [OD600] = 0.6), harvested by centrifugation, washed in 50 mM sodium phosphate buffer (pH 7.0), and resuspended in the same buffer to an OD600 of 6.0 (corresponding to 10 mg [wet weight] per ml). After incubation for 20 min at 37°C, norfloxacin (final concentration, 100 μM) was added to the bacterial suspension. Fifteen minutes after the addition of norfloxacin, the efflux pump inhibitor CCCP was added to the assay mixture at a final concentration of 100 μM. Samples (1 ml each) were removed at different times after the addition of norfloxacin, centrifuged at 7,000 × g for 30 s at 4°C, washed once with 1 ml of the above-described ice-cold phosphate buffer, and resuspended in 1 ml of 100 mM glycine-HCl (pH 3.0). The suspension was shaken for 20 h at room temperature and then centrifuged at 7,000 × g for 5 min. The fluorescence of the supernatant was measured in a Jasco FP-770 spectrofluorimeter at excitation and emission wavelengths of 277 and 448 nm, respectively. The concentration of norfloxacin in the supernatant was calculated by comparison with the fluorescence of norfloxacin standards (0.161 to 5 nmol/ml) in 100 mM glycine-HCl (pH 3.0).
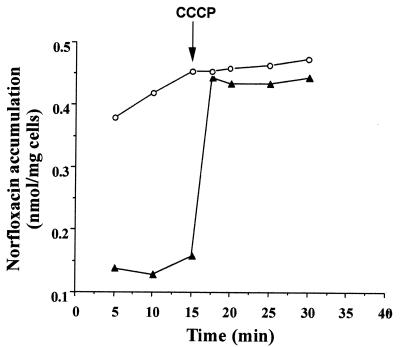
As shown in Fig. 2, at 15 min after addition of norfloxacin, E. coli AG100A cells expressing NorMI accumulated about fourfold less norfloxacin than the control cells. Addition of the protonophore CCCP induced an increase of this accumulation, which reached rapidly a level similar to that observed in control cells. These results indicated that NorMI mediates an active efflux process driven by an electrochemical potential of H+. By comparison with most known bacterial multidrug efflux proteins, it is very likely that NorMI is a drug/ion antiporter.
FIG. 2.
Accumulation of norfloxacin in E. coli cells expressing or not expressing the NorMI protein of B. melitensis. E. coli AG100A harboring a vector expressing NorMI (pUC19-NorMI) (▴) or harboring pUC19 alone (○) was grown in Luria-Bertani broth. Norfloxacin was added to the cell suspensions at a final concentration of 100 μM. After 15 min, CCCP was added to the suspensions at a final concentration of 100 μM. Samples were removed at the indicated times, and the concentration of norfloxacin extracted from the cells was quantified by spectrofluorimetry.
In conclusion, NorMI is the first described protein of B. melitensis that is able to mediate drug resistance by an active efflux mechanism. The existence of such a multidrug transporter indicates that, even if drug resistance is not considered a major problem in treating brucellosis, it is necessary to control the sensitivity patterns of Brucella spp. to ensure appropriate treatment and prevent increasing development of antibiotic resistance in these bacteria.
Acknowledgments
We thank H. Nikaido (University of California, Berkeley) for providing E. coli strain AG100A and P. K. Nandi and H. Ura for the use of a spectrofluorimeter. We are grateful to P. Gilot for critical reading of the manuscript.
REFERENCES
- 1.Al-Sibai, M. B., and S. M. Qadri. 1990. Development of ciprofloxacin resistance in Brucella melitensis. J. Antimicrob. Chemother. 25:302-303. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sibai, M. B., M. A. Halim, M. M. El-Shaker, B. A. Khan, and S. M. Qadri. 1992. Efficacy of ciprofloxacin for treatment of Brucella melitensis infections. Antimicrob. Agents Chemother. 36:150-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannatyne, R. M., M. Rich, and Z. A. Memish. 2001. Co-trimoxazole resistant brucella. J. Trop. Pediatr. 47:60. [DOI] [PubMed] [Google Scholar]
- 4.Belland, R., S. G. Morrison, C. Ison, and W. M. Huang. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol. Microbiol. 14:371-380. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. H., I. T. Paulsen, and R. A. Skurray. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31:393-395. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., Y. Morita, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2002. VmrA, a member of a novel class of Na+-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J. Bacteriol. 184:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbell, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daikos, G. K., N. Papapolyzos, N. Marketos, S. Mochlas, S. Kastanakis, and E. Papasteriadis. 1973. Trimethoprim-sulfamethoxazole in brucellosis. J. Infect. Dis. 128(Suppl.):731-734. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi, T., A. Fukuoka, M. Yasuda, M. Nakano, S. Ozeki, E. Kanematsu, Y. Nishino, S. Ishihara, Y. Ban, and Y. Kawada. 1997. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 41:699-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonsky, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J.-J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diener, A. C., R. A. Gaxiola, and G. R. Fink. 2001. Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13:1625-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easmon, C. S. F., J. P. Crane, and A. Blowers. 1986. Effect of ciprofloxacin on intracellular organisms. In-vitro and in-vivo studies. J. Antimicrob. Chemother. 18(Suppl. D):43-48. [DOI] [PubMed] [Google Scholar]
- 13.Farrell, I. D., P. M. Hinchliffe, and L. Robertson. 1976. Sensitivity of Brucella spp. to tetracycline and its analogues. J. Clin. Pathol. 29:1097-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Rodríguez, J. A., J. E. García-Sánchez, and I. Trujillano. 1991. Lack of effective bactericidal activity of new quinolones against Brucella spp. Antimicrob. Agents Chemother. 35:756-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heisig, P. 1996. Genetic evidence of a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother. 40:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsara, A., A. Al-Mowallad, and A. O. Osoba. 1999. Increasing resistance of brucellae to co-trimoxazole. Antimicrob. Agents Chemother. 43:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamae, S., O. Ueda, F. Yoshimura, J. Hwang, Y. Tanaka, and H. Nikaido. 2001. A MATE family multidrug efflux transporter pumps out fluoroquinolones in Bacteroides thetaiotaomicron. Antimicrob. Agents Chemother. 45:3341-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita, Y., A. Kataoka, S. Shiota, T. Mizushima, and T. Tsuchiya. 2000. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182:6694-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortimer, P. G., and L. J. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa, and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 21.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quadri, S. M. H., M. Aktar, Y. Ueno, and M. B. Al-Sibai. 1989. Susceptibility of Brucella melitensis to fluoroquinolones. Drugs Exp. Clin. Res. 15:483-485. [PubMed] [Google Scholar]
- 23.Rizzo-Naudi, J., N. Griscti-Soler, and W. Ganado. 1967. Human brucellosis: an evaluation of antibiotics in the treatment of brucellosis. Postgrad. Med. J. 43:520-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolain, J. M., M. Maurin, and D. Raoult. 2000. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J. Antimicrob. Chemother. 46:811-814. [DOI] [PubMed] [Google Scholar]
- 25.Saier, M. H., and I. T. Paulsen. 2001. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12:205-213. [DOI] [PubMed] [Google Scholar]
- 26.Shiomi, N., H. Fukuda, Y. Fukuda, K. Murata, and A. Kimura. 1991. Nucleotide sequence and characterization of a gene conferring resistance to ethionine in yeast Saccharomyces cerevisiae. J. Ferment. Bioeng. 71:211-215. [Google Scholar]