Abstract
The activity of AZD2563 against 250 highly resistant pneumococci and 267 drug-susceptible isolates was determined. The AZD2563 MICs for 50 and 90% of the strains tested were 1 and 2 μg/ml and 0.5 and 1 μg/ml, respectively, for the two isolate groups. These MICs were within 1 log2 dilution of those of linezolid.
The incidence of penicillin resistance and multidrug resistance in Streptococcus pneumoniae has continued to increase (3, 8, 13) and has altered empirical choices of therapy for invasive pneumococcal infections (1, 2, 5, 6). Multidrug resistance to three or more drug classes (13) has made therapy for serious infections challenging, especially in severely ill patients. Resistance to traditional choices for therapy of pneumococcal infections (e.g., β-lactams, macrolides) has prompted investigation of the activities of newer agents (e.g., fluoroquinolones, ketolides, carbapenems, and oxazolidinones) for predictable activity against highly resistant strains.
Linezolid was approved by the U.S. Food and Drug Administration in 2000 and represents the first oxazolidinone compound to reach clinical use. It is indicated for therapy of serious infections due to resistant staphylococci or enterococci and also for community-acquired pneumonia due to S. pneumoniae. Oxazolidinones bind to the 50S ribosomal subunit and inhibit protein synthesis by a unique mechanism that does not appear to share cross-resistance with other protein synthesis inhibitors (4). Although resistance to linezolid has been found in Enterococcus isolates (G. E. Zurenko, W. M. Todd, B. Hafkin, B. Meyers, C. Kauffman, J. Bock, J. Slighton, and D. Shinabarger, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 848, 1999) and more recently in Staphylococcus aureus (12), it has not been found in S. pneumoniae or other streptococci. AZD2563 is a newer member of the oxazolidinone class that is currently under development.
Two hundred fifty isolates of S. pneumoniae with previously determined resistance to one or more of the antimicrobial agents currently in use were tested against AZD2563 and selected comparative agents. A group of 267 drug-susceptible, invasive pneumococcal isolates were chosen from among the 1998 to 2000 Centers for Disease Control and Prevention Active Bacterial Core Surveillance/Emerging Infections Program collection. MICs of AZD2563, linezolid, quinupristin-dalfopristin, erythromycin, clindamycin, penicillin, levofloxacin, and vancomycin were determined by the broth microdilution method recommended by the NCCLS (10). This included cation-adjusted Mueller-Hinton broth supplemented with 3% lysed horse blood prepared as frozen microdilution panels (Trek Diagnostic Systems Inc., Westlake, Ohio), an inoculum of approximately 5 × 105 CFU/ml, and incubation at 35°C for 20 to 24 h in ambient air prior to visual MIC determination. S. pneumoniae ATCC 49619 was included with each day's testing as a control organism.
Susceptibility results obtained for the entire collection of 517 S. pneumoniae isolates with AZD2563 and the other comparator agents are depicted in Table 1. Among the drug-susceptible isolates, the MICs of AZD2563 for 50 and 90% of the isolates tested (MIC50 and MIC90, respectively) were 0.5 and 1.0 μg/ml, respectively, versus 1 and 2 μg of linezolid per ml. Among the drug-resistant isolates, the MIC50 and MIC90 were 1 dilution higher and identical to those of linezolid for this group. For both groups of isolates, the MIC90 for AZD2563 was 2 μg/ml.
TABLE 1.
Susceptibilities of selected S. pneumoniae clinical isolates to AZD2563, linezolid, and representative agents of various other antimicrobial classes
| Drug(s) | MICa (μg/ml) for:
|
|||||
|---|---|---|---|---|---|---|
| Drug-susceptibleb isolates (n = 267)
|
Non-drug susceptiblec isolates (n = 250)
|
|||||
| 50% | 90% | Range | 50% | 90% | Range | |
| AZD2563 | 0.5 | 1 | 0.12-2 | 1 | 2 | 0.12-2 |
| Linezolid | 1 | 2 | 0.25-2 | 1 | 2 | 0.25-2 |
| Quinupristin- dalfopristin | 0.25 | 0.5 | 0.12-0.5 | 0.25 | 0.5 | 0.12->8 |
| Erythromycin | 0.03 | 0.06 | 0.008-0.06 | 4 | >16 | 0.015->16 |
| Penicillin | 0.015 | 0.03 | 0.004-0.06 | 2 | 4 | 0.008-8 |
| Levofloxacin | 0.5 | 1 | 0.25-2 | 0.5 | 8 | 0.25->8 |
| Vancomycin | 0.25 | 0.25 | 0.06-0.5 | 0.25 | 0.25 | 0.06-0.5 |
50% and 90%, MICs at which 50 and 90% of the isolates are inhibited, respectively.
Interpretations based upon NCCLS criteria.
Includes isolates with intermediate-susceptibility phenotypes.
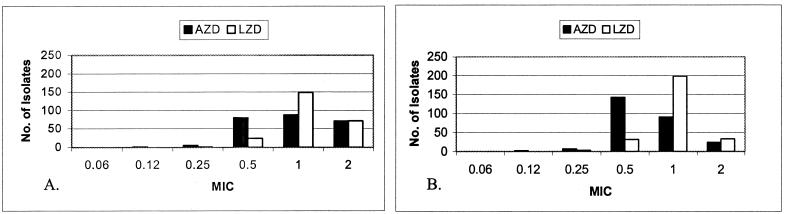
Figure 1 shows the distributions of linezolid and AZD2563 MICs for the drug-resistant and drug-susceptible groups, respectively. The geometric mean MICs of AZD2563 were 0.70 and 0.94 μg/ml for the susceptible and resistant groups, respectively, compared to 0.99 and 1.14 μg/ml, respectively, for linezolid. For 218 isolates, the MICs of linezolid and AZD2563 were different. For 211 (96.8%) of these, the AZD2563 MIC was lower than that of linezolid. Of these, one was lower by 2 dilutions; all others were lower by a single dilution. In seven cases (3.2%), the MIC was lower by a single dilution for linezolid. MICs of linezolid were never lower than those of AZD2563 by more than a single dilution.
FIG. 1.
MICs (micrograms per milliliter) of AZD2563 (AZD) and linezolid (LZD) for 250 drug-resistant S. pneumoniae isolates (A) and 267 drug-susceptible S. pneumoniae isolates (B).
Because of the rising rate of resistance in pneumococci and the potential for development of resistance to antibiotics that have thus far been universally active, new agents are needed to provide predictable activity against multidrug-resistant strains. Linezolid has been shown to have almost universal activity against multiply resistant isolates of enterococci, staphylococci, and S. pneumoniae (7). The newer oxazolidinone AZD2563 has been shown in this study to have activity very similar to that of linezolid against highly resistant, as well as susceptible, recent clinical isolates of S. pneumoniae from geographically diverse regions of the United States. While susceptibility breakpoints have not been determined for AZD2563, the MIC50 and MIC90 of AZD2563 appear to be similar to or slightly lower than those of linezolid. Pharmacokinetic data regarding levels of AZD2563 in blood, cerebrospinal fluid, and other bodily fluid are needed to put into perspective this slightly greater potency of AZD2563. The isolates included in this study were representative of all known resistance phenotypes of S. pneumoniae (including β-lactam, macrolide, lincosamide, fluoroquinolone, and streptogramin resistance), and regardless of the phenotype selected, all isolates were inhibited by AZD2563 at ≤2 μg/ml. Thus, as with linezolid, no cross-resistance to AZD2563 and other classes of antibiotics was observed. This may be a result of the unique mechanism of action of the oxazolidinones, which appears to be inhibition of translation of rRNA (9).
Linezolid has been demonstrated to be effective in the treatment of several serious gram-positive infections, including community-acquired pneumonia (11). Given the similar activities of AZD2563 and linezolid against S. pneumoniae, AZD2563 could also prove to be useful for therapy in patients with severe infections caused by resistant organisms. Additional studies are needed to determine its pharmacokinetics, safety, and in vivo clinical efficacy against invasive pneumococcal infections in humans.
Acknowledgments
This investigation was supported in part by a grant from Astra Zeneca Pharmaceuticals.
REFERENCES
- 1.Appelbaum, P. C. 2000. Microbiological and pharmacodynamic considerations in the treatment of infection due to antimicrobial-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 31(Suppl.):S29-S34. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doern, G. V., A. B. Brueggemann, H. Huynh, E. Wingert, and P. Rhomberg. 1999. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997-1998. Emerg. Infect. Dis. 5:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fines, M., and R. Leclercq. 2000. Activity of linezolid against gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J. Antimicrob. Chemother. 45:797-802. [DOI] [PubMed] [Google Scholar]
- 5.Friedland, I. R., and G. H. McCracken. 1994. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N. Engl. J. Med. 331:377-382. [DOI] [PubMed] [Google Scholar]
- 6.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, C. G. Whitney, and The Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, A. P., M. Warner, and D. M. Livermore. 2000. Activity of linezolid against multi-resistant gram-positive bacteria from diverse hospitals in the United Kingdom. J. Antimicrob. Chemother. 45:225-230. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., S. G. Jenkins, D. J. Hoban, M. N. Pfaller, and R. Ramphal. 2000. In vitro activity of selected cephalosporins and erythromycin against staphylococci and pneumococci isolated at 38 North American medical centers participating in the SENTRY antimicrobial surveillance program, 1997-1998. Diagn. Microbiol. Infect. Dis. 37:93-98. [DOI] [PubMed] [Google Scholar]
- 9.Matassova, N. B., M. V. Rodnina, R. Endermann, H. P. Kroll, U. Pleiss, H. Wild, and W. Wintermeyer. 1999. Ribosomal RNA is the target for oxazolidinones, a novel class of translational inhibitors. RNA 5:939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. NCCLS, Wayne, Pa.
- 11.Plouffe, J. F. 2000. Emerging therapies for serious gram-positive bacterial infections: a focus on linezolid. Clin. Infect. Dis. 31(Suppl. 4):S144-S149. [DOI] [PubMed] [Google Scholar]
- 12.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennerstein, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 13.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]