Abstract
Most Helicobacter pylori strains are susceptible to tetracycline, an antibiotic commonly used for the eradication of H. pylori. However, an increase in incidence of tetracycline resistance in H. pylori has recently been reported. Here the mechanism of tetracycline resistance of the first Dutch tetracycline-resistant (Tetr) H. pylori isolate (strain 181) is investigated. Twelve genes were selected from the genome sequences of H. pylori strains 26695 and J99 as potential candidate genes, based on their homology with tetracycline resistance genes in other bacteria. With the exception of the two 16S rRNA genes, none of the other putative tetracycline resistance genes was able to transfer tetracycline resistance. Genetic transformation of the Tets strain 26695 with smaller overlapping PCR fragments of the 16S rRNA genes of strain 181, revealed that a 361-bp fragment that spanned nucleotides 711 to 1071 was sufficient to transfer resistance. Sequence analysis of the 16S rRNA genes of the Tetr strain 181, the Tets strain 26695, and four Tetr 26695 transformants showed that a single triple-base-pair substitution, AGA926-928→TTC, was present within this 361-bp fragment. This triple-base-pair substitution, present in both copies of the 16S rRNA gene of all our Tetr H. pylori transformants, resulted in an increased MIC of tetracycline that was identical to that for the Tetr strain 181.
Helicobacter pylori is a spiral-shaped, gram-negative bacterium that causes chronic infections in the gastric mucosa (6). This infection will persist for life, unless treated with antibiotics. Cure of H. pylori infection results in ulcer healing and may reduce the risk of gastric cancer and gastric lymphoma (22, 28). The highest cure rates have been obtained with antimicrobial treatments that include two or more antimicrobial drugs, a bismuth component, and/or a proton pump inhibitor (14, 25). For the treatment of H. pylori infections, tetracycline-based triple or quadruple therapies are often used as a second-line treatment (7, 9, 17). Until the end of the last century only a few reports were published on spontaneous tetracycline resistance (18; P. D. Midolo, M. G. Korman, J. D. Turnidge, and J. R. Lambert, Letter, Lancet 347:1194-1195, 1996), and it was generally accepted that tetracycline resistance (MIC ≥ 4 μg/ml) in H. pylori is very rare (5, 12). However, in the last 2 years an increase in the incidence of tetracycline resistance in H. pylori has been reported (2, 11, 13, 20, 30).
Tetracycline inhibits the protein synthesis by binding to the 30S ribosomal subunit (3, 19). In most bacteria resistance to tetracycline is due to an energy-dependent efflux of tetracycline-cation complexes across the cell membrane by membrane-associated efflux proteins. Export of tetracycline complexes out of the cell reduces the intracellular drug concentration and protects the ribosomes from tetracycline (4). Overexpression of the efflux genes confers tetracycline resistance, while the sensitivity to tetracycline increases by deletions in these genes. The second common mechanism of resistance is mediated through ribosomal protection proteins. These cytoplasmic proteins confer tetracycline resistance either by a reduction of the affinity of ribosomes for tetracycline or by releasing the bound antibiotic from the ribosome. The ribosomal protection proteins, such as TetM, TetO, and TetS, show homology with the elongation factors EF-G and EF-Tu (Table 1) (4). Beside these two most common tetracycline resistance mechanisms, two other mechanisms have been described. One is based on enzymatic inactivation of tetracycline by the product of TetX in the presence of oxygen and NADPH, and the other originates from mutations in the 16S rRNA genes that affect the binding site of tetracycline (4, 21, 24).
TABLE 1.
H. pylori genes potentially involved in tetracycline resistancea
| Putative function of selected gene product (gene name) | Gene no. (aa in ORF)
|
Primer sequence (5′-3′)b
|
Expected product size (bp)c | PCR programd | ||
|---|---|---|---|---|---|---|
| TIGR 26695 | J99 | Forward | Reverse | |||
| Membrane proteins | ||||||
| GTP-binding membrane protein (lepA) | HP0355 (602) | JHP0329 (604) | AGAGTTTGACTGACGCTATT | TTTGCCATAGAAGCTAAACG | 1,874 | 95°C for 30 s, 50°C for 30 s, 72°C for 2 min 30 s |
| GTP-binding membrane protein, fusA homolog (yihK) | HP0480 (599) | JHP0432 (599) | CGCCATTTGGGGCTATTAT | CCTACAGCTAAAGACTTGCC | 2,018 | 95°C for 30 s, 50°C for 30 s, 72°C for 2 min 30 s |
| α-Ketoglutarate permease (kgtP) | HP1091 (426) | JHP0334 (437) | TCCCTTTTAGCCGCTAGTTC | ATGACATAGCCCACAAACCC | 1,181 | 95°C for 30 s, 52°C for 30 s, 72°C for 2 min |
| Tetracycline resistance protein (tetA) | HP1165 (386) | JHP1092 (386) | GCAGTCATTCGCTAATTCAA | AACGGCTTAGCCTTATACAA | 1,418 | 95°C for 30 s, 55°C for 30 s, 72°C for 2 min |
| Multidrug-efflux transporter | HP1181 (443) | JHP1107 (443) | TTTCCATTAGCGTTAGTGTC | CTAAAGTTTTGCGCTAAGTG | 1,310 | 95°C for 30 s, 55°C for 30 s, 72°C for 2 min |
| Conserved hypothetical integral membrane protein | HP1185 (391) | JHP1111 (391) | CCAAAAGAGCGCCAACAAAC | CTTGCGTGTGGTAGTAATGC | 1,601 | 95°C for 30 s, 55°C for 30 s, 72°C for 2 min |
| Protein export membrane protein (secD) | HP1550 (503) | JHP1449 (526) | CACCCCATAATTGGAATAAC | CTAGAAACTAAAGGCCCTAA | 1,568 | 95°C for 30 s, 50°C for 30 s, 72°C for 2 min 30 s |
| Cytoplasmic proteins | ||||||
| Translation initiation factor IF-2 (infB) | HP1048 (944) | JHP0377 (949) | CGCTAAAGCCTCTTGCAGTA | TGATTGGCAAAGGCCGTAGTT | 3,041 | 95°C for 30 s, 50°C for 30 s, 72°C for 3 min 45 s |
| Translation elongation factor EF-G (fusA) | HP1195 (692) | JHP1118 (692) | TTGCTAGGCACTTCGCCATA | ATGGATGCGGCTAGCGATAA | 2,152 | 95°C for 30 s, 55°C for 30 s, 72°C for 2 min 30 s |
| Translation elongation factor EF-Tu (tufB) | HP1205 (399) | JHP1128 (399) | TCAGAACACTTCAACCCTA | GTTTCCCGCTCCATTTTTA | 1,511 | 95°C for 30 s, 55°C for 30 s, 72°C for 2 min |
| 16S rRNA (rrnA and rrnB)e | TTTATGGAGAGTTTGATCCT | AGGAGGTGATCCAACCGCA | 1,494 | 95°C for 30 s, 55°C for 1 min, 72°C for 2 min | ||
Genes were selected from the published H. pylori genomes as potential Tetr candidate genes, based on their homology with tetracycline resistance genes in other bacteria.
Primers used for amplification were based on the published genome sequences of H. pylori strains 26695 (23) and J99 (1).
Fragment length is based on the genome sequence of H. pylori strain 26695 (23).
The PCR amplification cycle was repeated 35 times and followed by a 10-min extension step at 72°C.
The primers used for amplification of the 16S rRNA genes did not distinguish between the two copies present on the chromosome.
We recently isolated a tetracycline-resistant (Tetr) H. pylori isolate (strain 181), from a 72-year-old male dyspeptic patient. Here we describe the molecular mechanism of tetracycline-resistance in this strain. To achieve this, 12 genes were selected from the published H. pylori genomes (1, 23) as potential candidates, based on their homology with tetracycline resistance genes in other bacteria (Table 1). These putative tetracycline resistance genes were amplified from the genome of the Tetr strain 181 and used for genetic transformation of the tetracycline-sensitive (Tets) strain 26695 in order to identify the changes responsible for tetracycline resistance.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori strains used in this study were the Tetr strain 181 and the Tets H. pylori strains 26695 (23), J99 (1), SS1 (15), and ATCC 43504 (American Type Culture Collection). Bacteria were routinely grown on Columbia agar plates (Becton Dickinson, Cockeysville, Md.) supplemented with 7% lysed horse blood (BioTrading, Mijdrecht, The Netherlands) and H. pylori Dent selective supplement (Oxoid, Basingstoke, United Kingdom), referred to as Dent plates. Bacteria were inoculated on these plates and incubated for 48 to 72 h at 37°C in a microaerobic atmosphere of 5% O2, 10% CO2, and 85% N2. Bacterial stocks were prepared by suspending bacteria, harvested from culture plates with a sterile cotton swab, in brain heart infusion with 20% glycerol and stored at −80°C.
Determination of MIC.
The MIC was routinely determined with the E-test (AB Biodisk, Solna, Sweden) (8). Inocula were prepared from a fresh H. pylori culture grown routinely for 2 days on Dent plates. Columbia agar plates containing 7% lysed horse blood, but no Dent supplement, were inoculated with approximately 2 × 108 CFU in 20 μl of 0.9% NaCl, the plates were dried for 3 to 4 min, and then the E-test strips were applied to the agar surface. The plates were incubated at 37°C under microaerobic conditions, and 3 days later the MIC was determined by the intercept of the zone of inhibition with the graded E-test strip. By this method the susceptibility was determined for tetracycline, doxycycline, minocycline, amoxicillin, clarithromycin, and metronidazole. The isolates were considered resistant when the MICs of tetracycline, doxycycline, and minocycline were ≥4 μg/ml and when those of amoxicillin, clarithromycin, and metronidazole were ≥8, ≥2, and ≥8μg/ml, respectively (5, 13).
Natural transformation of H. pylori.
Bacteria were transformed with ∼1 μg of genomic DNA or ∼250 ng of PCR-amplified gene products from strain 181, as described previously (27). Tetr transformants were selected on Dent plates containing tetracycline (2 μg/ml; Sigma Aldrich Chemie, Zwijndrecht, The Netherlands). As controls, bacteria were transformed with either genomic DNA of the Tetr strain, TE (1 mM Tris-HCl, 0.1 mM EDTA [pH 8.0]), or DNA from the Tets strains 26695, J99, SS1, and ATCC43504. Individual bacterial colonies present on tetracycline-containing plates (2 μg/ml) were selected, and their MICs of tetracycline were determined.
PCR.
Oligonucleotide primers (Isogen, Maarsen, The Netherlands) used for PCR amplification were based on the genome sequences of H. pylori strains 26695 and J99 (Table 1; Fig. 1 and 2) (1, 23). PCR was performed in an automated thermal cycler (I-Cycler; Bio-Rad), in a final volume of 50 μl, using the PCR-core system I (Promega, Madison, Wis.), with approximately 25 pg of template genomic DNA and 25 pmol of each primer.
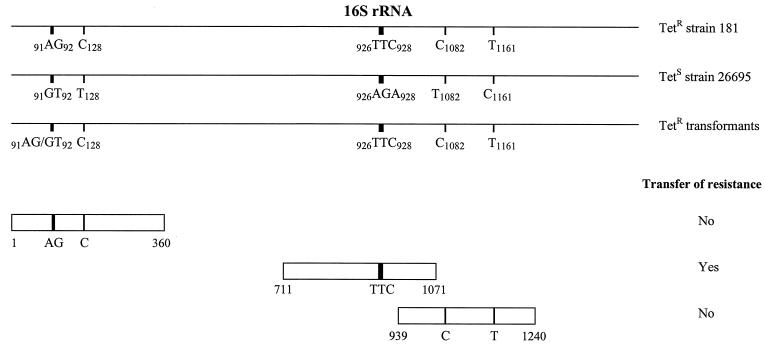
FIG. 1.
Schematic representation of the 16S rRNA genes of H. pylori. Alignment of the 16S rRNA genes (rrnA and rrnB) from the Tetr strain 181, the Tets strain 26695, and four Tetr 26695 transformants is shown. Sequence analysis of the 16S rRNA genes revealed only a few base pair substitutions (numbering according to 16S rrnA of H. pylori strain 26695) in the Tetr strain 181 that did not occur in the Tets strain 26695. For the identification of the 16S rRNA region required for tetracycline resistance, the Tets strain 26695 was transformed with smaller overlapping PCR fragments of the 16S rRNA gene of the Tetr strain 181 (only fragments containing mutations are shown). The transformants were selected on tetracycline (2 μg/ml)-containing Dent plates. Primers used for the amplification of the smaller overlapping PCR fragments started at the outside of the fragment and each had a length of 20 bp.
FIG. 2.
Both 16S rRNA genes are mutated in H. pylori tetracycline resistance. rrnA- and rrnB-specific sequences were amplified using specific primers based on sequences which are found outside the two 16S rRNA genes. The rrnA-specific primers, F1 and F2, are located at position 1207020 and 1207242 (numbers corresponding to the H. pylori 26695 sequence [23]), respectively, and the rrnB-specific primers, F3 and F4, are located at position 1510569 and 1510809, respectively. For amplification, primer R1 (located at position 1208293 and 1511828) was used in combination with one of the other primers. All primers had a length of 20 bp.
Sequence analysis.
Direct sequencing of the obtained PCR products was performed by Baseclear Inc. (Leiden, The Netherlands). Sequence data were analyzed with the help of Lasergene (DNAstar, Madison, Wis.), and Sci Ed Central (Scientific & Educational Software, Durham, N.C.) software.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of Tetr H. pylori strain 181 has been deposited into to the GenBank sequence database, under accession no. AF512997.
RESULTS
Determination of the MICs of various antibiotics.
MICs of the four antibiotics commonly used in anti-H. pylori therapy, as well as those of two antibiotics that belong to the tetracycline family, were determined by E-test for the Tetr H. pylori strain 181 and the H. pylori reference strain 26695 (Table 2). The MIC of tetracycline for strain 181 was 8 μg/ml (susceptibility breakpoint ≥ 4 μg/ml), while the MIC for strain 26695 was 0.19 μg/ml. The MICs of the two other tetracyclines, doxycycline and minocycline, were also significantly higher for strain 181 than for strain 26695. For the three other routinely used antibiotics (amoxicillin, clarithromycin, and metronidazole) the MICs varied between <0.016 and 0.064 μg/ml and did not differ significantly between strains 181 and 26695.
TABLE 2.
MICs for various H. pylori strains as determined by E-test
| Antibiotic | MIC (μg/ml)a for H. pylori
|
||
|---|---|---|---|
| Tetr strain 181 | Reference strain 26695 | Tetr transformant of strain 26695 | |
| Tetracycline | 8 | 0.19 | 8 |
| Doxycycline | 12 | 0.19 | 12 |
| Minocycline | 8 | 0.125 | 6 |
| Amoxicillin | <0.016 | <0.016 | <0.016 |
| Clarithromycin | <0.016 | <0.016 | <0.016 |
| Metronidazole | 0.19 | 0.016 | 0.064 |
Transfer of tetracycline resistance by natural transformation.
Transformation of H. pylori strain 26695 (MIC, 0.19 μg/ml) with genomic DNA of strain 181 (MIC, 8 μg/ml) resulted in Tetr colonies with a transformation frequency of 6 × 10−5. The MIC of tetracycline for the 10 randomly selected Tetr transformants (obtained from three independent transformation experiments), determined by E-test was 8 μg/ml (Table 2), which is identical to that for the Tetr H. pylori strain 181. The Tetr transformants also displayed an increase of MIC of the tetracycline derivatives, doxycycline and minocycline (Table 2).
Transformation with PCR products of putative tetracycline resistance genes.
Based on their homology with tetracycline resistance genes in other bacteria, 12 genes were selected from the published genome sequences of H. pylori strains 26695 (23) and J99 (1) (Table 1). The Tets H. pylori strain 26695 was transformed with the PCR products of the selected tetracycline resistance genes, which were amplified from genomic DNA of the Tetr strain 181. Only transformation with the PCR product of the 16S rRNA genes resulted in Tetr transformants, with a transformation frequency of 4 × 10−5. No Tetr transformants were found after transformation with one of the other selected genes, TE, or DNA from the Tets strain 26695. Similar results were found for the Tets strains J99 (MIC, 0.5 μg/ml), SS1 (MIC, 0.19 μg/ml), and ATCC 43504 (MIC 0.125 μg/ml). For all strains the MIC of tetracycline for 10 randomly selected Tetr transformants (obtained from three independent transformation experiments) determined by E-test was 8 μg/ml, which is identical to that for the Tetr donor strain 181.
Comparison of the 16S rRNA gene sequences of the Tetr strain 181, the Tets strain 26695, and four randomly Tetr 26695 transformants (obtained after transformation with genomic DNA of strain 181) revealed several base pair differences in the Tetr strain 181 as well as in the Tetr transformants that did not occur in the Tets strain 26695 (Fig. 1). Three Tetr 26695 transformants had incorporated the complete 16S rRNA gene of strain 181, while the fourth transformant contained the first part of the 16S rRNA gene of strain 26695 and the second part of strain 181. The DNA crossover in this transformant occurred after nucleotide 93 and before 127 (numbering according to 16S rrnA of H. pylori strain 26695). For each strain or transformant, only one sequence was obtained for the 16S rRNA genes, indicating that these 16S rRNA genes were identical in these strains.
Identification of 16S rRNA mutations involved in tetracycline resistance.
To determine which residues of the 16S rRNA genes were responsible for tetracycline resistance in strain 181, the Tets strain 26695 was genetically transformed with smaller overlapping PCR products of the 16S rRNA gene fragments, amplified from genomic DNA of the Tetr strain 181. Tetr transformants were only observed after transformation with a 361-bp DNA fragment that spanned nucleotides 711 to 1071 (numbering according to 16S rrnA of H. pylori strain 26695), with a transformation frequency of 5 × 10−6. Transformation with the other DNA fragments did not result in transfer of tetracycline resistance. The MIC of tetracycline for 10 randomly selected Tetr transformants determined by E-test was 8 μg/ml, which was identical to that for the Tetr donor strain 181. The only difference found between these Tetr 26695 transformants and the Tets strain was the triple-base-pair substitution AGA926-928→TTC (Fig. 1).
Both copies of 16S rRNA genes are involved in tetracycline resistance.
The primers that were originally used for amplification of the 16S rRNA genes did not distinguish between the two copies present on the H. pylori chromosome (1, 23). To assess the involvement of each copy of the 16S rRNA genes in tetracycline resistance, specific oligonucleotide primers were developed (Fig. 2). These specific primers are based on sequences which are located approximately 350 to 600 bp outside the both 16S rRNA genes, rrnA and rrnB. This allowed amplification of rrnA- and rrnB-specific sequences. rrnA- and rrnB-containing PCR-fragments were obtained for the Tetr strain 181, the eight 26695 transformants, and Tets strain 26695, and their DNA sequences were determined. As expected, the rrnA and rrnB sequences were identical, where as the sequences outside the 16S rRNA genes were different. While the Tets strain-derived fragments contained the AGA sequence in both genes, both for strain 181 and the eight 26695 transformants, the triple-base-pair substitution AGA926-928→TTC was found in both copies of the 16S rRNA genes.
DISCUSSION
Until recently tetracycline resistance in H. pylori was rare (5, 12), but in the last 2 years, several Tetr H. pylori strains have been isolated (2, 13, 30; Midolo et al., letter). These Tetr clinical isolates showed, besides tetracycline resistance, cross-resistance to metronidazole (2, 13, 30; Midolo et al., letter). The tetracycline resistance present in these strains was always transferred together with metronidazole resistance to a Tets strain (13). In these Tetr strains it is not clear whether the tetracycline resistance is caused by a known metronidazole resistance mechanism, a multidrug resistance mechanism, or an unknown tetracycline resistance mechanism (13). In our Tetr H. pylori strain 181, no cross-resistance was found against metronidazole, which indicated that the molecular mechanism of tetracycline resistance in strain 181 could be different from that of these earlier-described Tetr H. pylori strains.
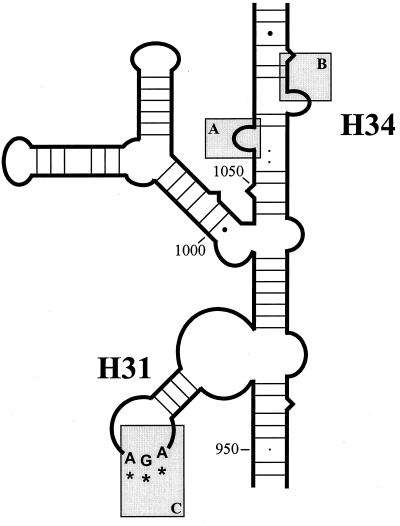
In H. pylori strain 181 resistance to tetracycline is mediated by a single triple-base-pair substitution, AGA926-928 → TTC (corresponding to bp 965 to 967 of Escherichia coli 16S rRNA), present in both copies of the 16S rRNA gene. Tetracycline has one primary and multiple secondary binding sites within the 30S ribosomal subunit (3, 19). In the primary binding site, tetracycline binds exclusively to the 3′-major domain of the 16S rRNA. The primary binding pocket for tetracycline is formed by the 16S rRNA residues 1054 to 1056 and 1196 to 1200 of helix 34 and residues 964 to 967 of helix 31 (numbers corresponding to E. coli 16S rRNA) (3). The residues 1054 and 1196 interact primarily with tetracycline through hydrophobic interactions, but the majority of the interaction with the drug is made through hydrogen bonds and salt bridges between tetracycline and the 16S rRNA residues (Fig. 3) (3). In the Tetr H. pylori strain 181, the triple-base-pair substitution AGA926-928→TTC is located right in the primary binding site of tetracycline. Mutations in this primary binding site are likely to affect the affinity of the drug-ribosome interaction and thus the efficacy of tetracycline as a translational inhibitor.
FIG. 3.
Schematic representation of the primary binding site of tetracycline, based on the 16S rRNA structure of Thermus thermophilus proposed by Wimberly et al. (29). The primary binding pocket for tetracycline is formed by the 16S rRNA residues 1054 to 1056 (box A) and residues 1196 to 1200 (box B) of helix 34 and residues 964 to 967 of helix 31 (box C). The interactions between tetracycline and this pocket are formed by hydrophobic interactions, hydrogen bonds, and salt bridges (3). The triple-base-pair substitution AGA926-928→TTC (corresponding to bp 965 to 967 of E. coli 16S rRNA) is located in box C and is indicated by asterisks.
In E. coli, the nucleotides G966 and C967 are located not only in the primary binding site of tetracycline but also in a functional region of the ribosome, the P site (16, 26). Mutations in this region may affect protein synthesis (10), either by a change in binding of tRNA to the P site itself or by blocking the conformational change needed for the tRNA binding to the A site. In H. pylori strain 181 and the Tetr transformants of strain 26695, the triple-base-pair substitution AGA926-928→TTC had no effect on the growth rate of the bacterium in the presence or absence of tetracycline (data not shown). Similar observations were found with E. coli after the substitution of the nucleotides G966 and C967 (10). This suggests that the triple-base-pair substitution AGA926-928→TTC present in H. pylori strain 181 does not seem to affect protein synthesis of H. pylori.
During revision of this work, Trieber and Taylor (24) reported that an identical AGA→TTC substitution mediates tetracycline resistance in an unrelated H. pylori strain (Midolo et al., letter). None of the other mutations found in their isolates (G332→A, and the deletions G733 and G903 [numbering according to 16S rrnA of H. pylori strain 26695]) play a role in tetracycline resistance of the Tetr strain 181, since these mutations were not present in our Tetr isolate. The differences found in the MIC of tetracycline for the triple-base-pair substitution mutant between the study of Trieber and ours are only due to the methods used for the determination of the MIC (data not shown). The finding that in two unrelated H. pylori strains the exact same mutation is responsible for tetracycline resistance opens possibilities for the development of molecular screening tests for tetracycline resistance in H. pylori.
Acknowledgments
We thank A. H. M. van Vliet for helpful comments and discussions.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori.Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Boyanova, L., I. Stancheva, Z. Spassova, N. Katzarov, I. Mitov, and R. Koumanova. 2000. Primary and combined resistance to four antimicrobial agents in Helicobacter pylori in Sofia, Bulgaria. J. Med. Microbiol. 49:415-418. [DOI] [PubMed] [Google Scholar]
- 3.Brodersen, D. E., W. M. Clemons, Jr., A. P. Carter, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103:1143-1154. [DOI] [PubMed] [Google Scholar]
- 4.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debets-Ossenkopp, Y. J., A. J. Herscheid, R. G. Pot, E. J. Kuipers, J. G. Kusters, and C. M. Vandenbroucke-Grauls. 1999. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin, tetracycline and trovafloxacin in The Netherlands. J. Antimicrob. Chemother. 43:511-515. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisbert, J. P., and J. M. Pajares. 2001. Helicobacter pylori therapy: first-line options and rescue regimen. Dig. Dis. 19:134-143. [DOI] [PubMed] [Google Scholar]
- 8.Glupczynski, Y., M. Labbe, W. Hansen, F. Crokaert, and E. Yourassowsky. 1991. Evaluation of the E-test for quantitative antimicrobial susceptibility testing of Helicobacter pylori. J. Clin. Microbiol. 29:2072-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham, D. Y., M. S. Osato, J. Hoffman, A. R. Opekun, S. Y. Anderson, D. H. Kwon, and H. M. El Zimaity. 2000. Metronidazole containing quadruple therapy for infection with metronidazole resistant Helicobacter pylori: a prospective study. Aliment. Pharmacol. Ther. 14:745-750. [DOI] [PubMed] [Google Scholar]
- 10.Jemiolo, D. K., J. S. Taurence, and S. Giese. 1991. Mutations in 16S rRNA in Escherichia coli at methyl-modified sites: G966, C967, and G1207. Nucleic Acids Res. 19:4259-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J. J., R. Reddy, M. Lee, J. G. Kim, F. A. El Zaatari, M. S. Osato, D. Y. Graham, and D. H. Kwon. 2001. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J. Antimicrob. Chemother. 47:459-461. [DOI] [PubMed] [Google Scholar]
- 12.Kusters, J. G., and E. J. Kuipers. 2001. Antibiotic resistance of Helicobacter pylori. J. Appl. Microbiol. 90:134S-144S. [DOI] [PubMed] [Google Scholar]
- 13.Kwon, D. H., J. J. Kim, M. Lee, Y. Yamaoka, M. Kato, M. S. Osato, F. A. El Zaatari, and D. Y. Graham. 2000. Isolation and characterization of tetracycline-resistant clinical isolates of Helicobacter pylori. Antimicrob. Agents Chemother. 44:3203-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laheij, R. J., L. G. Rossum, J. B. Jansen, H. Straatman, and A. L. Verbeek. 1999. Evaluation of treatment regimens to cure Helicobacter pylori infection: a meta-analysis. Aliment. Pharmacol. Ther. 13:857-864. [DOI] [PubMed] [Google Scholar]
- 15.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 16.Moazed, D., and H. F. Noller. 1990. Binding of tRNA to the ribosomal A- and P-sites protects two distinct sets of nucleotides in 16S rRNA. J. Mol. Biol. 211:135-145. [DOI] [PubMed] [Google Scholar]
- 17.O'Morain, C., and S. Montague. 2000. Challenges to therapy in the future. Helicobacter 5:S23-S26. [DOI] [PubMed] [Google Scholar]
- 18.Piccolomini, R., G. Di Bonaventura, G. Catamo, F. Carbone, and M. Neri. 1997. Comparative evaluation of the E-test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J. Clin. Microbiol. 35:1842-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pioletti, M., F. Schlunzen, J. Harms, R. Zarivach, M. Gluhmann, H. Avila, A. Bashan, H. Bartels, T. Auerbach, C. Jacobi, T. Hartsch, A. Yonath, and F. Franceschi. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 20:1829-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Realdi, G., M. P. Dore, A. Piana, A. Atzei, M. Carta, L. Cugia, A. Manca, B. M. Are, G. Massarelli, I. Mura, A. Maida, and D. Y. Graham. 1999. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter 4:106-112. [DOI] [PubMed] [Google Scholar]
- 21.Ross, J. I., E. A. Eady, J. H. Cove, and W. J. Cunliffe. 1998. 16S rRNA mutation associated with tetracycline resistance in a gram-positive bacterium. Antimicrob. Agents Chemother. 42:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiyama, T., N. Sakaki, H. Kozawa, R. Sato, T. Fujioka, K. Satoh, K. Sugano, H. Sekine, A. Takagi, Y. Ajioka, and T. Takizawa. 2002. Sensitivity of biopsy site in evaluating regression of gastric atrophy after Helicobacter pylori eradication treatment. Aliment. Pharmacol. Ther. 16(Suppl. 2):187-190. [DOI] [PubMed] [Google Scholar]
- 23.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 24.Trieber, C. A., and D. E. Taylor. 2002. Mutations in the 16S rRNA genes of Helicobacter pylori mediate resistance to tetracycline. J. Bacteriol. 184:2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Hulst, R. W., J. J. Keller, E. A. Rauws, and G. N. Tytgat. 1996. Treatment of Helicobacter pylori infection: a review of the world literature. Helicobacter 1:6-19. [DOI] [PubMed] [Google Scholar]
- 26.von Ahsen, U., and H. F. Noller. 1995. Identification of bases in 16S rRNA essential for tRNA binding at the 30S ribosomal P-site. Science 267:234-237. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelmsen, I., and A. Berstad. 1994. Quality of life and relapse of duodenal ulcer before and after eradication of Helicobacter pylori. Scand. J. Gastroenterol. 29:874-879. [DOI] [PubMed] [Google Scholar]
- 29.Wimberly, B. T., D. E. Brodersen, W. M. Clemons, Jr., R. J. Morgan-Warren, A. P. Carter, C. Vonrhein, T. Hartsch, and V. Ramakrishnan. 2000. Structure of the 30S ribosomal subunit. Nature 407:327-339. [DOI] [PubMed] [Google Scholar]
- 30.Wu, H., X. D. Shi, H. T. Wang, and J. X. Liu. 2000. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxycillin. J. Antimicrob. Chemother. 46:121-123. [DOI] [PubMed] [Google Scholar]