Abstract
Isoniazid is a first-line antibiotic used in the treatment of infections caused by Mycobacterium tuberculosis. Isoniazid is a prodrug requiring oxidative activation by the catalase-peroxidase hemoprotein, KatG. Resistance to isoniazid can be obtained by point mutations in the katG gene, with one of the most common being a threonine-for-serine substitution at position 315 (S315T). The S315T mutation is found in more than 50% of isoniazid-resistant clinical isolates and results in an ≈200-fold increase in the MIC of isoniazid compared to that for M. tuberculosis H37Rv. In the present study we investigated the hypothesis that superoxide plays a role in KatG-mediated isoniazid activation. Plumbagin and clofazimine, compounds capable of generating superoxide anion, resulted in a lower MIC of isoniazid for M. tuberculosis H37Rv and a strain carrying the S315T mutation. These agents did not cause as great of an increase in isoniazid susceptibility in the mutant strain when the susceptibilities were assessed by using the inhibitory concentration that causes a 50% decrease in growth. These results provide evidence that superoxide can play a role in isoniazid activation. Since clofazimine alone has antitubercular activity, the observation of synergism between clofazimine and isoniazid raises the interesting possibility of using both drugs in combination to treat M. tuberculosis infections.
Entering the 21st century, Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), remains a major cause of morbidity and mortality worldwide. TB causes 1.9 million deaths annually among a pool of infected individuals close to 2 billion people (47).
Isoniazid (INH) is one of the most effective and widely used therapeutic agents for the treatment of TB. M. tuberculosis shows exceptional sensitivity to INH, being several orders of magnitude more sensitive than most other bacterial species. Much is known about the mechanism of INH activation, including that fact that it is a prodrug requiring activation by the catalase-peroxidase hemoprotein, KatG (13, 18, 19), in a process that requires molecular oxygen (49). It has been further shown that the activated form of INH forms a covalent adduct with NAD+ to generate a potent inhibitor of the InhA protein of M. smegmatis, an enoyl-acyl carrier protein reductase important in mycolic acid biosynthesis (26, 30, 40). Recent investigations have led to the hypothesis that the sensitivity of M. tuberculosis to INH may be governed by the level of expression of the alkyl hydroperoxide reductase, AhpC, since M. tuberculosis does not express the ahpC gene due to inactivation of the oxidative stress regulatory gene oxyR (5, 6, 32, 38, 52).
Nevertheless, specific details regarding the mechanism of action of INH, such as the chemical nature of the activated form of INH, have yet to be determined. For example, InhA as the primary target for INH in M. tuberculosis has been questioned following the discovery of a covalent complex of INH, acyl carrier protein (AcpM), and the β-ketoacyl acyl carrier protein synthase, KasA (21, 22).
Rates of resistance to INH and other antibiotics have been increasing such that now approximately 13% of all TB cases in the United States are resistant to at least one first-line drug (INH, rifampin, pyrazinamide, ethambutol, and streptomycin) (23). INH-resistant M. tuberculosis has been associated with deletions or point mutations in the katG gene (1, 12, 21, 24, 37, 45, 51, 53), with the threonine-for-serine substitution at position 315 (S315T) in katG being one of the most common mutations found in clinical isolates. A comparison of M. tuberculosis KatG with cytochrome c peroxidase, a member of the same catalase-peroxidase superfamily, suggests that S315 occupies a position near the active site of KatG, and therefore, the S315T mutation could affect the enzymatic activity of KatG (12).
Besides exhibiting catalase and peroxidase activities, several other enzymatic activities have been associated with M. tuberculosis KatG, including Mn(II)-dependent peroxidase (19, 50), peroxynitritase (46), and cytochrome P450-like monooxygenase (18) activities. The KatG protein and the KatG protein with the S315T mutation [KatG(S315T)] have comparable catalase and peroxidase activities, and KatG(S315T) is able to oxidize INH at equivalent rates using a hydroperoxide as oxidant, suggesting that the peroxidase activity of KatG may not be relevant for the in vivo activation of INH (31, 44, 45). In the presence of dioxygen but the absence of a hydroperoxide, INH is oxidized more slowly by KatG(S315T) than by KatG, a phenomenon that has been shown to involve superoxide and that may reflect why the S315T mutation confers INH resistance (44). Superoxide is formed during INH oxidation and is thought to be involved in the activation process (33-35). INH oxidation via this route is thought to involve a monooxygenase pathway in which an oxyferrous KatG intermediate is formed by either dioxygen binding to ferrous KatG (18) or superoxide binding to ferric KatG (44). These biochemical observations support the hypothesis that a mechanism other than the catalase-peroxidase route plays an important role in INH resistance. Direct evidence for this was provided by Drlica and colleagues (41), who showed that the superoxide generator plumbagin resulted in an increase in the bacteriostatic activity of INH against M. smegmatis, a strain normally resistant to INH.
In the study described in this report we tested the hypothesis that superoxide plays a role in KatG-mediated INH oxidation in M. tuberculosis. Radiometric analyses were conducted to determine MICs and the inhibitory concentrations of drug that cause a 50% decrease in strain growth (IC50s) of INH alone and INH in combination with superoxide-generating substances, plumbagin or clofazimine, for an INH-sensitive M. tuberculosis strain and an INH-resistant strain containing the katG S315T mutation.
MATERIALS AND METHODS
Bacterial strains.
The M. tuberculosis strains used in this study, strains H37Rv and TBC3, were grown on Middlebrook 7H10 slants at 37°C. TBC3 is an INH-resistant clinical isolate.
Drugs and reagents.
Dimethyl sulfoxide (DMSO), INH, clofazimine, and plumbagin (technical grade) were purchased from Sigma Chemical Co. (St. Louis, Mo.); shrimp alkaline phosphatase and exonuclease I were provided by U.S. Biochemicals (Cleveland, Ohio). INH was recrystallized from boiling methanol by using activated charcoal decolorization and hot filtration through Whatman no. 1 filter paper (Whatman International Ltd., Maidstone, United Kingdom). The crystals were vacuum filtered, washed with ice-cold methanol, and air dried, yielding white needles with a melting point of 172°C. INH was dissolved in water and filter sterilized through a 0.22-μm-pore-size filter. For stock solutions of clofazimine (16.9 μM) and plumbagin (1.6 mM), 4% DMSO was used as the solvent. Appropriate controls were included to ensure that the inhibitory effects of the drugs were not due to the addition of DMSO, although there are data indicating that this solvent enhances the potencies of the drugs (3).
DNA sequencing and sequence analysis.
The presence of a wild-type katG gene in strain H37Rv and a katG gene harboring the S315T mutation in strain TBC3 was confirmed by restriction fragment length polymorphism analysis (4). Sequencing of the DNA of the entire katG gene from TBC3 was also carried out to confirm the presence of the S315T mutation and the absence of secondary mutations. Briefly, the katG gene was amplified by PCR, and the genomic DNA was isolated from TBC3. Genomic DNA was isolated by inoculating a 10-μl loopful of a culture into a tube containing 100 μl of water. Approximately 2 ml of alkaline wash solution (0.05 M sodium citrate, 0.5 M sodium hydroxide) was added. After centrifugation (at 28,800 × g for 2 min) the supernatant was discarded. Approximately 500 μl of 0.5 M Tris HCl (pH 8.0) buffer was added. After centrifugation the supernatant was discarded and 100 μl of sterile distilled water was added. The tube was incubated at 95°C for 15 min. The following oligonucleotide primers whose sequences are specific for sequences flanking the katG gene (GenBank database accession number X68081) were used as primers in the PCR: KatG 5′ (5′-CCGACACTTCGCGATCACATCCGTGATCACAGCCC-3′) and KatG 3′ (5-GGTGCTGCGGCGGGTTGTGGTTGATCGG-3′). The PCR was carried out with a 50-μl reaction volume containing PCR buffer, genomic DNA (2 μl), 1.1 mM magnesium diacetate, 200 μM deoxynucleoside triphosphates, each primer at a concentration of 0.2 μM, and 1 U of rTth DNA polymerase (GeneAmp XL PCR kit; Applied Biosystems, Foster City, Calif.). The mixture was overlaid with AmpliWax PCR Gem (Applied Biosystems) and heated for 1 min at 94°C, followed by 35 cycles of 1 min at 94°C and 2.5 min at 72°C. A single 2,200-bp band corresponding to the amplified product was detected by ethidium bromide staining after electrophoresis on 0.9% (wt/vol) agarose. The PCR product was prepared for sequencing by treatment with shrimp alkaline phosphatase to dephosphorylate all remaining deoxynucleoside triphosphates and with exonuclease I to degrade all residual single-stranded DNA present in the PCR mixture. The entire katG gene was sequenced with the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase on an ABI PRISM 377 DNA sequencer with XL Upgrade and 96-well Upgrade (Perkin-Elmer Applied Biosystems, Foster City, Calif.) by using eight primers whose sequences were specific for sequences that spanned the length of the gene (the primers and their sequences are presented in the Appendix). The sequence data assembled with the Sequencher program (Gene Codes Inc.) confirmed the presence of the S315T mutation and the absence of secondary mutations.
Susceptibility testing.
The susceptibilities of M. tuberculosis H37Rv and TBC3 to INH alone and INH in combination with plumbagin and clofazimine were determined by using the radiometric criteria of the BACTEC 460 system (Becton Dickinson, Sparks, Md.) (2, 25, 27, 36). All procedures were carried out in a biological safety cabinet inside a biosafety level 3 biocontainment facility. Studies involving combinations of drugs were carried out with weakly inhibitory concentrations of clofazimine and plumbagin, as determined by use of the radiometric criteria.
According to the instructions of the manufacturer (S. H. Siddiqi, BACTEC 460TB system, product and procedure manual, 1995, Becton Dickinson, Sparks, Md.), a suspension of an actively growing culture was made in water, and 0.1 ml was transferred to a BACTEC 12B bottle. These bottles were incubated until the appropriate growth index (GI) was obtained, after which 0.1-ml aliquots were removed and transferred to fresh BACTEC 12B bottles containing drugs. The bottles were incubated at 37°C and analyzed daily until the GI of the growth control diluted 1:100 was ≥30. Resistance was determined by comparing the change in GI over that of the previous day (ΔGI) between the control vial (GIcontrol) and the vials containing drugs (GIdrugs). The results are interpreted as follows: if ΔGIcontrol is >ΔGIdrug, the strain is referred to as “susceptible”; if ΔGIcontrol is <ΔGIdrug, the strain is referred to as “resistant”; and if ΔGIcontrol is equal to ΔGIdrug, the strain is referred to as “intermediate.”
The MIC, determined by the procedure recommended by the manufacturers of the BACTEC 460 radiometric system, was defined as the lowest concentration of drug for which the GI of the drug-containing vial was less than the GI of the control diluted 1:100, obtained from measurements on the day when the GI of the control vial was ≥30, and corresponds to the daily concentration that resulted in >99% inhibition of the bacterial population growth. The IC50 corresponds to the concentration of drug that causes a 50% decrease in the GI. The IC50s of INH were obtained by least-squares fitting of the data to the Langmuir isotherm equation: B = 1/[1 + (IC50/[INH])], where B is equal to percent GI/100%.
The percent reduction of the IC50 of INH in the presence of clofazimine and plumbagin is determined by the following equation: {[IC50(INH) − IC50(X)]/IC50(INH)} × 100, where IC50(INH) and IC50(X) represent the IC50s of INH in the absence and presence of plumbagin or clofazimine, respectively.
The relative error was calculated by applying the basic theory of error: [IC50(X)/IC50(INH)] × {[ΔIC50(INH)/IC50(INH)] + [ΔIC50(X)/IC50(X)]}, where ΔIC50(INH) represents the error of determination of the IC50 of INH, and ΔIC50(X) is the error of determination of the IC50 of INH in the presence of plumbagin or clofazimine.
RESULTS
The effect of the superoxide-generating compounds plumbagin and clofazimine on the activity of INH against M. tuberculosis strains H37Rv and TBC3 was assessed by use of the BACTEC radiometric criteria. ΔGI values for cultures of these strains in the presence of INH alone were determined. The range of INH concentrations chosen allows accurate determination of the MIC of INH for a strain with wild-type katG (strain H37Rv) and a strain with katG harboring the S315T mutation (strain TBC3) (Fig. 1). The MIC of INH for TBC3 was 5 μg/ml, 200-fold higher than the MIC for H37Rv (0.025 μg/ml), confirming the efficacy of the S315T mutation in conferring resistance to INH (12, 13). It should be noted that TBC3 is not an isogenic mutant of H37Rv. Therefore, genotypic differences other than the S315T mutation that could contribute to the increased MIC cannot be excluded.
FIG. 1.
INH susceptibilities of M. tuberculosis (Mtb) strains H37Rv (A) and TBC3 (B) using BACTEC radiometric analysis. The control reaction represents that in a vial containing a starter culture diluted 1:100 relative to the inoculum in the vials containing INH. The asterisks indicate the MICs of INH for each strain, as defined in Materials and Methods. GI data were obtained on a daily basis following inoculation of BACTEC vials, but data for only selective days are shown for clarity.
The susceptibilities of H37Rv and TBC3 to clofazimine and plumbagin alone were determined as well (Fig. 2). For both strains, the MIC of clofazimine was >0.1 μg/ml but ≤0.2 μg/ml, but a decrease in GI on day 7 was observed with clofazimine at concentrations ≥0.1 μg/ml. This is in agreement with previous studies, which showed a MIC of 0.12 μg/ml (15, 28, 39). A concentration of 0.1 μg of clofazimine per ml was therefore chosen as the subinhibitory concentration to be used in combination with INH (see below). Plumbagin also caused a decline in the GI values for both strains, with noticeable decreases in the GI values at 10 to 20 μM. TBC3 appeared to be slightly more sensitive to plumbagin, with a slight (≈30%) decline in the GI value by day 7 in the presence of 10 μM plumbagin, whereas for H37Rv, 20 μM plumbagin led to a similar, modest decrease in the GI value (Fig. 2). As a result, the subinhibitory concentrations of plumbagin were chosen to be 20 μM for H37Rv and 10 μM for TBC3.
FIG. 2.
Susceptibilities of M. tuberculosis (Mtb) H37Rv (A) and TBC3 (B) to plumbagin and clofazimine.
The influences of subinhibitory concentrations of clofazimine and plumbagin on susceptibility to INH were assessed by analysis with the BACTEC system. Both compounds led to significant increases in the susceptibility of wild-type strain H37Rv to INH (Fig. 3; Table 1). The MIC of INH decreased from 0.025 μg/ml (with INH alone) to 0.012 μg/ml in the presence of 0.1 μg of clofazimine per ml and to 0.008 μg/ml in the presence of 20 μM plumbagin (Table 1). Susceptibility was also assessed by estimating the IC50 of INH (Fig. 3A). The IC50, being the concentration of drug that causes a 50% decrease in GI, is a more sensitive measure of the effects of these agents than the MIC, which is the lowest concentration of drug that results in a >99% decrease in cell growth. IC50s were determined by a least-squares fit of the data presented in Fig. 3, as described in Materials and Methods. The IC50 of INH was found to be 0.015 ± 0.007 μg/ml (Fig. 3A, top), decreasing twofold (to 0.0068 ± 0.0015 μg/ml) in the presence of 20 μM plumbagin (Fig. 3A, middle) and decreasing about 30% (to 0.0099 ± 0.0026 μg/ml) in the presence of 0.1 μg of clofazimine per ml (Fig. 3A, bottom).
FIG. 3.
Effects of plumbagin and clofazimine on INH susceptibility. The percent GI is plotted versus the log[INH] (p[INH]), expressed in units of nanograms per milliliter, alone (top) or in the presence of plumbagin (middle) or clofazimine (bottom), for M. tuberculosis H37Rv (A) and TBC3 (B). Weakly inhibitory concentration of clofazimine (0.1 μg/ml) and plumbagin (20 μM in panels A, 10 μM in panels B) were included in each vial. IC50s were calculated for each graph as the concentration of INH resulting in a 50% decrease in the GI value relative to that for the control vials, as described in Materials and Methods. The vertical dotted line in each set of panels represents the IC50 of INH alone (0.015 μg/ml in panels A, 2.1 μg/ml in panels B). (C) Reduction of IC50 of INH for H37Rv and TBC3 by plumbagin and clofazimine. The percent GI is expressed as the average value ± the standard error for three experiments.
TABLE 1.
MICs of INH used alone and in combination with plumbagin or clofazimine for M. tuberculosis strains H37Rv and TBC3
| Drug | INH MIC (μg/ml)
|
|
|---|---|---|
| H37Rv (wild-type KatG) | TBC3 [KatG(S315T)] | |
| Isoniazid alone | 0.025a | 5a |
| Isoniazid + plumbaginb | 0.008 | 2.5 |
| Isoniazid + clofazimine (0.1 μg/ml) | 0.012 | 2.5 |
Prior studies have shown that the MIC for INH-resistant M. tuberculosis strains is >1 μg/ml (low-level resistance); wild-type isolates are inhibited by INH, with MICs of 0.05 to 0.2 μg/ml (14).
The weakly inhibitory concentrations of plumbagin used were 20 μM for H37Rv and 10 μM for TBC3.
The activity of INH alone or in combination with plumbagin (10 μM) or clofazimine (0.1 μg/ml) against TBC3 was also investigated (Table 1; Fig. 3B). Both the MIC and the IC50 of INH also appeared to decrease in the presence of these compounds. With INH alone, the IC50 was 2.1 ± 0.7 μg/ml, whereas in the presence of plumbagin or clofazimine, the IC50s were 1.3 ± 0.5 and 1.5 ± 1.2 μg/ml, respectively. Compared to the reductions in the IC50s of INH in combination with plumbagin or clofazimine for strain H37Rv (55 and 34%, respectively), the reductions in the IC50s for TBC3 were lower. For TBC3, the corresponding decreases were 38 and 28% (Fig. 3C).
DISCUSSION
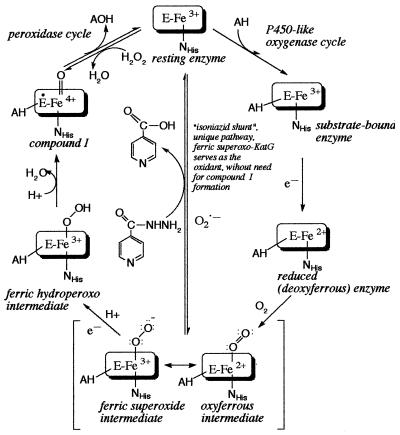
Although a role for KatG in mediating INH activation is firmly established, the mechanism of activation and the chemical nature of the activated product are still unresolved. Initially described as a catalase-peroxidase on the basis of the homology of its amino acid sequence to that of the Escherichia coli hydroperoxidase I enzyme (51), the enzyme has subsequently been shown to exhibit a number of different catalytic activities. Three of these, the catalase, peroxidase, and Mn2+-dependent peroxidase activities, share a common mechanism in which a hydroperoxide substrate (e.g., H2O2 and tert-butylhydroperoxide) oxidizes the Fe3+ form of the enzyme and forms an intermediate, compound I, which is 2 equivalents more oxidized than the resting enzyme. Compound I, in turn, oxidizes a second substrate (e.g., INH), returning the enzyme to the resting ferric state. The initial hypothesis that the S315T mutation might confer resistance to M. tuberculosis by affecting the catalase-peroxidase mechanism was tested by comparing these activities for recombinant forms of the wild-type and S315T enzymes (31, 44, 45). These studies demonstrated that the catalytic efficiency of KatG(S315T) is comparable to or only slightly lower than the catalytic efficiency of the wild-type enzyme for all three activities and does not correlate with the ca. 200-fold difference in the MICs of INH for strains carrying wild-type versus S315T katG alleles, as measured in this and other studies (12, 13, 51). It has been shown that KatG-mediated INH activation is probably not by direct peroxidation, as the enzyme catalyzes INH oxidation in the absence of peroxide (17). An alternative mechanism for KatG-dependent INH turnover, in which dioxygen is used as the oxidant, has gained attention in recent years (16-18, 44). The mechanism for this reaction is not completely understood but is thought to proceed in a manner analogous to those for cytochromes P450. In this reaction, dioxygen binds to the Fe2+ form of KatG to form an oxyferrous intermediate, which goes on to form a compound I-type species after an additional redox reaction (16-20, 43). The oxyferrous enzyme is a resonance form of the ferric-superoxo enzyme. Consequently, the oxyferrous intermediate can also form by reaction of superoxide anion with the Fe3+ form of KatG (Fig. 4) (41). On the basis of the observation that KatG-dependent activation of INH generates reactive oxygen species (16, 33-35), it has been hypothesized that superoxide, formed via a one-electron reduction of dioxygen concomitant with INH oxidation, can bind to the ferric heme to form oxyferrous KatG and bring about further INH oxidation and activation. Support for the physiological relevance of this mechanism was provided by Wang et al. (41), who showed that the superoxide generator plumbagin resulted in an increase in the bacteriostatic activity of INH against M. smegmatis, a strain normally resistant to INH. Other supporting experiments have shown that KatG(S315T) has a reduced ability to oxidize INH to isonicotinic acid when superoxide is used as the oxidant (44).
FIG. 4.
The INH shunt, a proposed mechanism for INH oxidation by ferric superoxo-KatG.
In this work, the role of superoxide anion in INH oxidation in vivo was investigated by radiometric analysis of INH susceptibility in the presence versus the absence of plumbagin and clofazimine. Plumbagin is a known redox cycling agent expected to increase intracellular superoxide concentrations (8, 10, 41). The precise mechanism of action of clofazimine is unknown, but it has been suggested that it generates intracellular hydrogen peroxide and superoxide (15, 28, 29, 39). Clofazimine has been used for the treatment of leprosy and has excellent in vitro inhibitory activity against M. avium-M. intracellulare complex strains, with MICs that range from 0.1 to 5 μg/ml. The drug also has potent in vitro activity against M. tuberculosis, but there is little or no information on its in vivo activity (11, 14, 42).
The M. tuberculosis strain carrying the wild-type katG allele, strain H37Rv, exhibited increased susceptibility to INH when INH was used in combination with either plumbagin or clofazimine, as evidenced by a decrease in both the IC50s and the MICs of INH in the presence of these reagents. Hence, a superoxide-dependent mechanism appears to be important for KatG-mediated INH activation in this strain. KatG(S315T) also appears to utilize superoxide to activate INH, as evidenced by the fact that INH turnover by the purified enzyme is completely abolished in the presence of a catalytic quantity of superoxide dismutase (SOD) (44). Indeed, although the potency of INH was increased by a putative increase in superoxide production by plumbagin and clofazimine in INH-resistant strain TBC3, our data indicate that the katG S315T mutation attenuates this activation. Thus, KatG-mediated INH oxidative activation is superoxide dependent in M. tuberculosis and the S315T mutation may confer an INH resistance phenotype through a reduced activity toward superoxide. Although the results implicate superoxide potentiation of INH toxicity in both strains, H37Rv appeared to be more sensitive to increased levels of superoxide than TBC3. The hypothesis that superoxide participates in the KatG-mediated INH oxidation is intriguing because M. tuberculosis contains both ferric and Cu,Zn SODs. Superoxide-dependent activation may be possible only because all of the Cu,Zn SOD and 76% of the ferric SOD are exported from the cell, presumably to act as extracellular defenses against the host respiratory burst (7, 9, 48).
The potential chemical implications of these findings are intriguing. It is possible that clofazimine in combination with INH may result in synergistic activity in vitro against M. tuberculosis. This synergistic activity may be more for the wild-type M. tuberculosis strain than for strains harboring the katG S315T mutation. Further animal and human studies are required to validate these assumptions.
The following primers were used for sequencing: KatG 5′, CCG ACA CTT CGC GAT CAC ATC CGT GAT CAC AGC CC; KatG 2, ATG ACC ACC TCG CAG CCG; KatG 3, GGC TTC GGC CGG GTC GAC; KatG 4, GTC GGC CCC GAA CCC GAG; KatG 5, CTG CGG GTG GAT CCG ATC; KatG 6, CGT GGT AGC GAC AAG CGC; KatG 7, TTT GCC GTG CTG GAG CCC; KatG 8, AGT GGC AAG GTG AAG TGG; and KatG 2′ reverse primer, CCG TAG TCG GCG GGC CAC CAC GGC T.
Acknowledgments
This work was supported by NIH grant AI47142.
We thank Slobodan Macura for comment on statistical interpretation of the data.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Bosne-David, S., V. Barros, S. Cabo Verde, C. Portugal, and H. L. David. 2000. Intrisinic resistance of Mycobacterium tuberculosis to clarithromycin is effectively reversed by subinhibitory concentrations of cell wall inhibitors. J. Antimicrob. Chemother. 46:391-395. [DOI] [PubMed] [Google Scholar]
- 3.Chinnaswamy, J., V. M. Reddy, and R. J. Gangadharam. 1995. Enhancement of drug susceptibility of multi-drug resistant strains of Mycobacterium tuberculosis by ethambutol and dimethyl sulfoxide. J. Antimicrob. Chemother. 55:381-390. [DOI] [PubMed] [Google Scholar]
- 4.Cockerill, F. R., III, J. R. Uhl, Z. Temesgen, Y. Zhang, L. Stockman, G. D. Roberts, D. L. Williams, and B. C. Kline. 1995. Rapid identification of a point mutation of the Mycobacterium tuberculosis catalase-peroxidase (katG) gene associated with isoniazid-resistance. J. Infect. Dis. 171:240-245. [DOI] [PubMed] [Google Scholar]
- 5.Deretic, V., E. Pogan-Ramos, Y. Zhang, S. Dhandayuthapani, and L. Via. 1996. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat. Biotechnol. 14:1557-1561. [DOI] [PubMed] [Google Scholar]
- 6.Dhandayuthapani, S., Y. Zhang, M. H. Mudd, and V. Deretic. 1996. Oxidative stress response and its role in sensitivity to isoniazid in mycobacteria: characterization and inducibility of ahpC by peroxides in Mycobacterium smegmatis and lack of expression in M. aurum and M. tuberculosis. J. Bacteriol. 178:3641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dussurget, O., G. Stewart, O. Neyrolles, P. Pescher, D. Young, and G. Marchal. 2001. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect. Immun. 69:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floreani, M., A. Forlin, L. Pandolfo, M. Petrone, and S. Bellin. 1996. Mechanisms of plumbagin action on guinea pig isolated atria. J. Pharmacol. Exp. Ther. 278:763-770. [PubMed] [Google Scholar]
- 9.Harth, G., and M. A. Horowitz. 1999. Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery. J. Biol. Chem. 274:4281-4292. [DOI] [PubMed] [Google Scholar]
- 10.Hassan, H. M., and I. Fridovich. 1979. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 196:385-395. [DOI] [PubMed] [Google Scholar]
- 11.Heifets, L. B. 1991. Antituberculosis drugs: antimicrobial activity in vitro, p. 40-41, 80, 164-165. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infections. CRC Press, Inc., Boca Raton, Fla.
- 12.Heym, B., P. M. Alzari, N. Honoré, and S. T. Cole. 1995. Missense mutations in the catalase-peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol. Microbiol. 15:235-245. [DOI] [PubMed] [Google Scholar]
- 13.Heym, B., Y. Zhang, S. Poulet, D. Young, and S. T. Cole. 1993. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 175:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inderlied, C. B., and M. Salfinger. 1999. Antimicrobial agents and susceptibility tests, p. 1606-1623. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 15.Jagannath, C., M. V. Reddy, S. Kailasam, J. F. O'Sullivan, and P. R. J. Gangadharam. 1995. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 151:1083-1086. [DOI] [PubMed] [Google Scholar]
- 16.Johnsson, K., and P. Shultz. 1994. Mechanistic studies of the oxidation of isoniazid by the catalase-peroxidase from Mycobacterium tuberculosis. J. Am. Chem. Soc. 116:7425-7426. [Google Scholar]
- 17.Johnsson, K., D. King, and P. Shultz. 1995. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J. Am. Chem. Soc. 117:5009-5010. [Google Scholar]
- 18.Magliozzo, R. S., and J. A. Marcinkeviciene. 1996. Evidence for isonaizid oxidation by oxyferrous mycobacterial catalase-peroxidase. J. Am. Chem. Soc. 118:11303-11304. [Google Scholar]
- 19.Magliozzo, R. S., and J. A. Marcinkeviciene. 1997. The role of Mn(II)-peroxidase activity of mycobacterial catalase-peroxidase in the antibiotic isoniazid. J. Biol. Chem. 272:8867-8870. [DOI] [PubMed] [Google Scholar]
- 20.Marcinkeviciene, J. A., R. S. Magliozzo, and J. S. Blanchard. 1995. Purification and characterization of the Mycobacterium smegmatis catalase-peroxidase involved in isoniazid activation. J. Biol. Chem. 270:22290-22295. [DOI] [PubMed] [Google Scholar]
- 21.Mdluli, K., D. R. Sherman., M. J. Hickey, B. N. Kreiswirth, S. Morris, C. K. Stover, and C. E. Barry III. 1996. Biochemical and genetic data suggest that inhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J. Infect. Dis. 174:1085-1090. [DOI] [PubMed] [Google Scholar]
- 22.Mdluli, K., R. A. Slayden, Y. Q. Zhu, S. Ramaswamy, X. Pan, D. Mead, D. D. Crane, J. M. Musser, and C. E. Barry III. 1998. Inhibition of a Mycobacterium tuberculosis β-ketoacyl ACP synthase by isoniazid. Science 280:1607-1610. [DOI] [PubMed] [Google Scholar]
- 23.Moore, M., I. M. Onorato, E. McCray, and K. G. Castro. 1997. Trends in drug-resistant tuberculosis in the United States, 1993-1996. JAMA 278:833-837. [PubMed] [Google Scholar]
- 24.Musser, J. M., V. Kapur, D. L. Williams, B. N. Kreiswirth, D. van Soolingen, and J. D. A. van Embden. 1996. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J. Infect. Dis. 173:196-202. [DOI] [PubMed] [Google Scholar]
- 25.Pfyffer, G. E., D. A. Bonato, A. Ebrahimzadeh, W. Gross, J. Hotaling, J. Kornblum, A. Laszlo, G. Roberts, M. Salfinger, F. Wittwer, and S. Siddiqi. 1999. Multicenter laboratory validation of susceptibility testing of Mycobacterium tuberculosis against classical second-line and newer antimicrobial drugs by using the radiometric BACTEC 460 technique and the proportion method with solid media. J. Clin. Microbiol. 37:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quemard, A., J. C. Sacchettini, A. Dessen, C. Vilchèze, R. Bittman, W. R. Jacobs, Jr., and J. S. Blanchard. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235-8241. [DOI] [PubMed] [Google Scholar]
- 27.Rastogi, N., K. S. Goh, E. L. Wright, and W. W. Barrow. 1994. Potential drug targets for Mycobacterium avium defined by radiometric drug-inhibitor combination techniques. Antimicrob. Agents Chemother. 38:2287-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy, V. M., G. Nadadhur, D. Daneluzzi, J. F. O'Sullivan, and P. R. J. Gangadharam. 1996. Antituberculosis activities of clofazimine and its new analogs B4154 and B4157. Antimicrob. Agents Chemother. 40:633-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy, V. M., J. F. O'Sullivan, and P. R. J. Gangadharam. 1999. Antimycobacterial activities of riminophenazines. J. Antimicrob. Chemother. 43:615-623. [DOI] [PubMed] [Google Scholar]
- 30.Rozwarski, D. A., G. A. Grant, D. H. R. Barton, W. R. Jacobs, Jr., and J. C. Sacchettini. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98-102. [DOI] [PubMed] [Google Scholar]
- 31.Saint-Joanis, B., H. Souchon, M. Wilming, K. Johnsson, P. M. Alzari, and S. T. Cole. 1999. Use of site-directed mutagenesis to probe the structure, function and isoniazid activation of the catalase/peroxidase, KatG, from Mycobacterium tuberculosis. Biochem. J. 338:753-760. [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman, D. R., K. Mdluli, M. J. Hickey, T. M. Arian, S. L. Morris, C. E. Barry III, and C. K. Stover. 1996. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272:1641-1643. [DOI] [PubMed] [Google Scholar]
- 33.Shoeb, H. A., B. U. Bowman, Jr., A. C. Ottolenghi, and A. J. Merola. 1985. Enzymatic and nonenzymatic superoxide-generating reactions of isoniazid. Antimicrob. Agents Chemother. 27:408-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoeb, H. A., B. U. Bowman, Jr., A. C. Ottolenghi, and A. J. Merola. 1985. Evidence for the generation of active oxygen by isoniazid treatment of extracts of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 27:404-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoeb, H. A., B. U. Bowman, Jr., A. C. Ottolenghi, and A. J. Merola. 1985. Peroxidase-mediated oxidation of isoniazid. Antimicrob. Agents Chemother. 27:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snider, D. E., Jr., M. Raviglione, and A. Kochi. 1994. Global burden of tuberculosis, p. 3-11. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 38.Sreevatsan, S., X. Pan, Y. Zhang, V. Deretic, and J. M. Musser. 1997. Analysis of the oxyR-ahpC region in isoniazid-resistant and -susceptible Mycobacterium tuberculosis complex organisms recovered from diseased humans and animals in diverse localities. Antimicrob. Agents Chemother. 41:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Rensburg, C. E. J., G. K. Jooné, F. A. Sirgel, N. M. Matiola, and J. F. O'Sulivan. 2000. In vitro investigation of the antimicrobial activities of novel tetramethylpiperidine-substituted phenazines against Mycobacterium tuberculosis. Chemotherapy (Basel) 46:43-48. [DOI] [PubMed] [Google Scholar]
- 40.Vilchèze, C., H. R. Morbidoni, T. R. Weisbrod, H. Iwamoto, M. Kuo, J. C. Sacchettini, and W. R. Jacobs, Jr. 2000. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J. Bacteriol. 182:4059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, J.-Y., R. M. Burger, and K. Drlica. 1998. Role of superoxide in catalase-peroxidase-mediated isoniazid action against mycobacteria. Antimicrob. Agents Chemother 42:709-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warek, U., and J. O. Falkinham III. 1996. Action of clofazimine on the Mycobacterium avium complex. Res. Microbiol. 147:43-48. [DOI] [PubMed] [Google Scholar]
- 43.Wengenack, N. L. 2000. Ph.D. thesis. Department of Biochemistry and Molecular Biology, Mayo Graduate School, Rochester, Minn.
- 44.Wengenack, N. L., H. M. Hoard, and F. Rusnak. 1999. Isoniazid oxidation by Mycobacterium tuberculosis KatG: a role for superoxide which correlates with isoniazid susceptibility. J. Am. Chem. Soc. 121:9748-9749. [Google Scholar]
- 45.Wengenack, N. L., J. R. Uhl, A. L. St. Amand, A. J. Tomlinson, L. M. Benson, S. Naylor, B. C. Kline, F. R. Cockerill III, and F. Rusnak. 1997. Recombinant Mycobacterium tuberculosis KatG(S315T) is a competent catalase-peroxidase with reduced activity towards isoniazid. J. Infect. Dis. 176:722-777. [DOI] [PubMed] [Google Scholar]
- 46.Wengenack, N. L., M. P. Jensen, F. Rusnak, and M. K. Stern. 1999. Mycobacterium tuberculosis KatG is a peroxynitritase. Biochem. Biophys. Res. Commun. 256:485-487. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. 2002. Report on infectious diseases. World Health Organization, Geneva, Switzerland.
- 48.Wu, H. C. H., J.-J. Tsai-Wu, Y.-T. Huang, C.-Y. Lin, G.-G. Lioua, and F.-J. S. Lee. 1998. Identification and subcellular localization of a novel Cu,Zn superoxide dismutase of Mycobacterium tuberculosis. FEBS Lett. 439:192-196. [DOI] [PubMed] [Google Scholar]
- 49.Youatt, J. 1960. The uptake of isoniazid and related compounds by mycobacteria. Aust. J. Exp. Biol. 38:331-338. [DOI] [PubMed] [Google Scholar]
- 50.Zabinski, R. F., and J. S. Blanchard. 1997. The requirement for manganese and oxygen in the isoniazid-dependent inactivation of Mycobacterium tuberculosis enoyl reductase. J. Am. Chem. Soc. 119:2331-2332. [Google Scholar]
- 51.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Y., S. Dhandayuthapani, and V. Deretic. 1996. Molecular basis for exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl. Acad. Sci. USA 93:13212-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Y., T. Garbe, and D. Young. 1993. Transformation with katG restores isoniazid sensitivity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol. Microbiol. 8:521-524. [DOI] [PubMed] [Google Scholar]