Abstract
Susceptibilities to 16 antimicrobial agents were determined by measurement of MICs for 344 isolates of anaerobic bacteria recovered from patients with significant infections. Resistance rates varied among antimicrobial agents and the species tested. The β-lactams were more active in gram-positive than in gram-negative anaerobes. Resistance to meropenem was low (<1%). For β-lactam-β-lactamase inhibitors, piperacillin-tazobactam was most active for all species (resistance, <6%). The rates of resistance to cefoxitin (31 to 65%) and clindamycin (50 to 70%) for non-Bacteroides fragilis species of the B. fragilis group were higher than those for B. fragilis (4% resistant to cefoxitin and 33% resistant to clindamycin). Among members of B. fragilis group, Bacteroides thetaiotaomicron was the most resistant to clindamycin (70%) and cefoxitin (65%). Rates of susceptibility to imipenem and metronidazole for B. fragilis continue to be high compared to those from a previous study 10 years ago. However, resistance to metronidazole was found recently in five strains of B. fragilis. We analyzed the genetic relationships among the metronidazole-resistant B. fragilis strains by pulsed-field gel electrophoresis. The metronidazole-resistant B. fragilis strains showed genotypic heterogeneity, excluding the dissemination of a single clone.
Over the past 10 years, there has been a significant problem with increasing resistance to antimicrobial agents among anaerobic bacteria (1-3, 7-9). Antimicrobial resistance is becoming less predictable and may fluctuate from one medical center to another, as well as from one geographic region to another (1-4, 7-9). The increasing resistance among several species emphasizes the need to survey the susceptibility patterns of these organisms. Data on susceptibilities of anaerobes are very limited in Taiwan except those from a previous report of 10 years ago (16). The objective of this study was to determine the susceptibility profiles of clinical isolates of anaerobes in Taiwan and to monitor susceptibility changes over time.
Organisms tested included gram-positive and gram-negative anaerobes which were clinically commonly encountered. The test antimicrobial agents included old and new agents. Among the older agents, clindamycin, cefoxitin, and piperacillin are commonly used as the initial empirical treatment for B. fragilis group infections. However, resistance to these agents has been shown to increase in North America, Europe, and other countries during the past decades (1-4, 6-9, 14). The 5-nitroimidazole molecules are very potent anaerobicidal agents commonly used to treat or prevent Bacteroides infections. Although resistance to metronidazole in Bacteroides fragilis strains has been reported in several countries (12), resistance to metronidazole in B. fragilis strains has not been reported in Taiwan before. The emergence of metronidazole-resistant B. fragilis strains (MIC, >32 μg/ml) in Taiwan is reported in this study.
MATERIALS AND METHODS
Bacterial strains.
A total of 344 clinical isolates of anaerobes were collected between 1998 and 2000 from the Bacteriology Laboratory, National Taiwan University Hospital, a 2,000-bed teaching hospital in northern Taiwan. These isolates were recovered from blood, pus from the intra-abdominal cavity, abscesses, soft tissue, head or neck wounds, and others. Only one isolate per patient was included.
Antimicrobial susceptibility testing.
The antimicrobial agents used for susceptibility testing were as follows: penicillin, ampicillin, piperacillin, sulbactam, tazobactam, cefoperazone, clindamycin, chloramphenicol, and metronidazole (Sigma Chemical Co., St. Louis, Mo.); ticarcillin and clavulanic acid (SmithKline Beecham, Philadelphia, Pa.); cefoxitin and imipenem (Merck Sharp & Dohme, West Point, Pa.); meropenem (Sumitomo Pharmaceuticals, Osaka, Japan); cefmetazole (Sankyo, Tokyo, Japan); and moxifloxacin (Bayer Corporation, West Haven, Conn.).
Antimicrobial susceptibility was tested by an agar dilution method in accordance with guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (10). An inoculum of 105 CFU per well was applied with a Steers replicator onto brucella agar supplemented with vitamin K1 and 5% pooled sheep blood. Plates were incubated in an anaerobic chamber for 48 h at 35°C. The MIC was defined as the concentration at which there was a marked change in the appearance of growth, compared with that in the control plate. Reference strains of B. fragilis ATCC 25285 and Bacteroides thetaiotaomicron ATCC 29741 were used for quality control of the susceptibility tests.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed to genotype five metronidazole-resistant B. fragilis strains. Each strain was grown overnight at 37°C in 10 ml of brain heart infusion broth in an anaerobic chamber. The preparation of DNA was performed as described previously (5). After appropriate preparation, the DNAs in each plug were digested with 20 U of XbaI (New England Biolabs, Hitchin, United Kingdom) at 37°C for 4 h. The plugs were applied to a 1% agarose gel. Electrophoresis was performed in 0.5× Tris-borate-EDTA buffer at 14oC by using a CHEF-DR III apparatus (Bio-Rad Laboratories, Hercules, Calif.), with pulse times ranging from an initial value of 4 s to a final value of 30 s, for 16 h at 200 V.
RESULTS
Antimicrobial susceptibilities.
The MIC ranges, MICs at which 50% of the isolates were inhibited (MIC50s), MICs at which 90% of the isolates were inhibited (MIC90s), and the percentages of 344 clinical isolates of anaerobes that were susceptible, intermediate, or resistant to various antimicrobial agents are summarized in Table 1 The rates of susceptible, intermediate, and resistant isolates were determined by using the NCCLS breakpoints (10).
TABLE 1.
In vitro susceptibilities of clinical isolates of anaerobes
| Bacterium (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
%a
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | I | R | |
| B. fragilis (100) | ||||||
| Penicillin | 2->128 | 64 | >128 | 0 | 0 | 100 |
| Ampicillin | 32->128 | >128 | >128 | 0 | 0 | 100 |
| Piperacillin | 0.5->128 | 32 | >128 | 55 | 5 | 40 |
| Ticarcillin | 1->128 | 64 | >128 | 46 | 10 | 44 |
| AMP-sulbactamb | 1-64 | 16 | 32 | 50 | 33 | 17 |
| Piperacillin-TAZc | <0.03-16 | 0.5 | 8 | 100 | 0 | 0 |
| Ticarcillin-CVAd | 0.12-4 | 0.5 | 2 | 100 | 0 | 0 |
| Cefoxitin | 4-64 | 8 | 32 | 74 | 22 | 4 |
| Cefmetazole | 4-32 | 8 | 16 | 93 | 7 | 0 |
| Cefoperazone | 4->128 | 128 | >128 | 16 | 9 | 75 |
| Imipenem | 0.25-8 | 0.5 | 4 | 99 | 1 | 0 |
| Meropenem | <0.03-16 | 0.25 | 8 | 94 | 5 | 1 |
| Moxifloxacin | 0.06-4 | 0.25 | 2 | |||
| Clindamycin | ≤0.03->128 | 0.25 | >128 | 67 | 0 | 33 |
| Chloramphenicol | 1-2 | 2 | 2 | 100 | 0 | 0 |
| Metronidazole | 1-64 | 1 | 2 | 95 | 2 | 3 |
| B. thetaiotaomicron (40) | ||||||
| Penicillin | 16->128 | 32 | >128 | 0 | 0 | 100 |
| Ampicillin | 32->128 | 64 | >128 | 0 | 0 | 100 |
| Piperacillin | 8->128 | 128 | >128 | 28 | 18 | 55 |
| Ticarcillin | 32->128 | 8 | 16 | 5 | 35 | 60 |
| AMP-sulbactam | 2-64 | 16 | 32 | 53 | 13 | 35 |
| Piperacillin-TAZ | 1-64 | 16 | 32 | 90 | 10 | 0 |
| Ticarcillin-CVA | 0.06-64 | 8 | 16 | 95 | 5 | 0 |
| Cefoxitin | 16->128 | 64 | >128 | 10 | 25 | 65 |
| Cefmetazole | 8->128 | 64 | >128 | 10 | 10 | 80 |
| Cefoperazone | 64->128 | 64 | >128 | 0 | 0 | 100 |
| Imipenem | 0.12-16 | 0.25 | 4 | 93 | 0 | 8 |
| Meropenem | 0.12-2 | 0.25 | 2 | 100 | 0 | 0 |
| Moxifloxacin | 0.06-4 | 2 | 4 | |||
| Clindamycin | 0.5->128 | 32 | >128 | 25 | 5 | 70 |
| Chloramphenicol | 4-8 | 4 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.5-2 | 1 | 2 | 100 | 0 | 0 |
| B. caccae (18) | ||||||
| Penicillin | 16->128 | 128 | >128 | 0 | 0 | 100 |
| Ampicillin | 8->128 | 128 | >128 | 0 | 0 | 100 |
| Piperacillin | 32->128 | 128 | >128 | 28 | 17 | 55 |
| Ticarcillin | 8->128 | >128 | >128 | 11 | 0 | 89 |
| AMP-sulbactam | 8-32 | 16 | 32 | 11 | 39 | 50 |
| Piperacillin-TAZ | ≤0.03->32 | 8 | 32 | 100 | 0 | 0 |
| Ticarcillin-CVA | 0.12-32 | 2 | 16 | 100 | 0 | 0 |
| Cefoxitin | 8->128 | 32 | >128 | 28 | 22 | 50 |
| Cefmetazole | 32->128 | 64 | >128 | 0 | 17 | 83 |
| Cefoperazone | 64->128 | 128 | >128 | 0 | 0 | 100 |
| Imipenem | 0.12-4 | 0.5 | 2 | 100 | 0 | 0 |
| Meropenem | 0.25-8 | 0.5 | 2 | 89 | 11 | 0 |
| Moxifloxacin | 0.25-32 | 1 | 4 | |||
| Clindamycin | 0.25->128 | 8 | >128 | 33 | 0 | 67 |
| Chloramphenicol | 1-4 | 4 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.5-16 | 1 | 2 | 89 | 11 | 0 |
| B. uniformis (32) | ||||||
| Penicillin | 8->128 | 128 | >128 | 0 | 0 | 100 |
| Ampicillin | 32->128 | 128 | >128 | 0 | 0 | 100 |
| Piperacillin | 64->128 | 128 | >128 | 25 | 13 | 63 |
| Ticarcillin | 64->128 | >128 | >128 | 6 | 0 | 94 |
| AMP-sulbactam | 2-32 | 16 | 32 | 25 | 12 | 62 |
| Piperacillin-TAZ | 0.5->128 | 8 | 32 | 94 | 0 | 6 |
| Ticarcillin-CVA | 0.5->128 | 2 | 16 | 88 | 6 | 6 |
| Cefoxitin | 2->128 | 32 | >128 | 38 | 31 | 31 |
| Cefmetazole | 32->128 | 64 | >128 | 0 | 13 | 88 |
| Cefoperazone | 32->128 | 128 | >128 | 0 | 6 | 94 |
| Imipenem | 0.25-4 | 0.5 | 2 | 100 | 0 | 0 |
| Meropenem | 0.25-8 | 0.5 | 2 | 94 | 6 | 0 |
| Moxifloxacin | 0.5-8 | 2 | 8 | |||
| Clindamycin | 0.5->128 | 4 | >128 | 38 | 6 | 56 |
| Chloramphenicol | 2-32 | 4 | 8 | 94 | 0 | 6 |
| Metronidazole | 0.25-1 | 0.5 | 1 | 100 | 0 | 0 |
| Bacteroides vulgatus (12) | ||||||
| Penicillin | ≤0.03->128 | 128 | >128 | 0 | 0 | 100 |
| Ampicillin | 0.06->128 | 128 | >128 | 0 | 0 | 100 |
| Piperacillin | 1->128 | 128 | >128 | 25 | 25 | 50 |
| Ticarcillin | ≤0.03->128 | >128 | >128 | 33 | 8 | 58 |
| AMP-sulbactam | 0.12-64 | 16 | 32 | 33 | 67 | 0 |
| Piperacillin-TAZ | ≤0.03->128 | 8 | 32 | 92 | 8 | 0 |
| Ticarcillin-CVA | ≤0.03->128 | 2 | 16 | 100 | 0 | 0 |
| Cefoxitin | 1->128 | 8 | >128 | 50 | 17 | 33 |
| Cefmetazole | 0.12->128 | 64 | >128 | 8 | 25 | 67 |
| Cefoperazone | 0.12->128 | 128 | >128 | 42 | 33 | 25 |
| Imipenem | 0.12-16 | 0.5 | 2 | 100 | 0 | 0 |
| Meropenem | ≤0.03-8 | 0.5 | 2 | 100 | 0 | 0 |
| Moxifloxacin | 0.06-32 | 1 | 4 | |||
| Clindamycin | 0.06->128 | 8 | >128 | 50 | 0 | 50 |
| Chloramphenicol | 0.5-32 | 4 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.12-16 | 1 | 2 | 100 | 0 | 0 |
| B. fragilis group, other species (20) | ||||||
| Penicillin | ≤0.03->128 | 128 | >128 | 0 | 0 | 100 |
| Ampicillin | 0.06->128 | 128 | >128 | 0 | 0 | 100 |
| Piperacillin | 1->128 | 128 | >128 | 25 | 15 | 60 |
| Ticarcillin | ≤0.03->128 | >128 | >128 | 20 | 10 | 70 |
| AMP-sulbactam | 0.12-64 | 16 | 32 | 45 | 20 | 35 |
| Piperacillin-TAZ | ≤0.03->128 | 8 | 32 | 95 | 5 | 0 |
| Ticarcillin-CVA | ≤0.03->128 | 2 | 16 | 95 | 0 | 5 |
| Cefoxitin | 1->128 | 32 | >128 | 35 | 25 | 40 |
| Cefmetazole | 0.12->128 | 64 | >128 | 25 | 0 | 75 |
| Cefoperazone | 0.12->128 | 128 | >128 | 15 | 10 | 75 |
| Imipenem | 0.12-16 | 0.5 | 2 | 95 | 5 | 0 |
| Meropenem | ≤0.03-8 | 0.5 | 2 | 95 | 5 | 0 |
| Moxifloxacin | 0.06-32 | 1 | 4 | |||
| Clindamycin | 0.06->128 | 8 | >128 | 35 | 5 | 60 |
| Chloramphenicol | 0.5-32 | 4 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.12-16 | 1 | 2 | 100 | 0 | 0 |
| Fusobacterium speciese (19) | ||||||
| Penicillin | ≤0.03->128 | 0.5 | >128 | 55 | 0 | 45 |
| Ampicillin | ≤0.03->128 | 16 | >128 | 25 | 5 | 70 |
| Piperacillin | 0.12->128 | 16 | 128 | 63 | 21 | 16 |
| Ticarcillin | 0.06->128 | 8 | 64 | 79 | 11 | 11 |
| AMP-sulbactam | ≤0.03-8 | 2 | 8 | 100 | 0 | 0 |
| Piperacillin-TAZ | ≤0.03-64 | 4 | 16 | 95 | 5 | 0 |
| Ticarcillin-CVA | 0.5-32 | 4 | 32 | 100 | 0 | 0 |
| Cefoxitin | 0.25-16 | 4 | 16 | 100 | 0 | 0 |
| Cefmetazole | ≤0.03-32 | 4 | 32 | 95 | 5 | 0 |
| Cefoperazone | ≤0.03-64 | 8 | 64 | 68 | 5 | 26 |
| Imipenem | 0.12-1 | 0.25 | 1 | 100 | 0 | 0 |
| Meropenem | ≤0.03-8 | 0.12 | 5 | 95 | 5 | 0 |
| Moxifloxacin | 0.06-4 | 0.25 | 4 | |||
| Clindamycin | 0.25->128 | 4 | >128 | 45 | 20 | 35 |
| Chloramphenicol | 0.5-32 | 2 | 8 | 95 | 0 | 5 |
| Metronidazole | 0.06->128 | 0.5 | >128 | 75 | 0 | 25 |
| Prevotella speciesf (16) | ||||||
| Penicillin | ≤0.03-128 | 4 | 32 | 38 | 0 | 62 |
| Ampicillin | ≤0.03->128 | 64 | >128 | 31 | 6 | 63 |
| Piperacillin | 0.25-128 | 64 | 64 | 31 | 56 | 13 |
| Ticarcillin | 0.06-64 | 16 | 32 | 88 | 12 | 0 |
| AMP-sulbactam | 0.06->128 | 4 | 16 | 88 | 12 | 0 |
| Piperacillin-TAZ | ≤0.03-64 | 0.06 | 64 | 75 | 25 | 0 |
| Ticarcillin-CVA | 0.06->128 | 0.5 | 32 | 94 | 0 | 6 |
| Cefoxitin | 0.5->128 | 8 | 32 | 88 | 6 | 6 |
| Cefmetazole | 0.12->128 | 2 | 64 | 88 | 0 | 12 |
| Cefoperazone | 0.5->128 | 128 | 128 | 13 | 19 | 68 |
| Imipenem | 0.06-4 | 0.25 | 2 | 100 | 0 | 0 |
| Meropenem | 0.06-1 | 0.12 | 0.5 | 100 | 0 | 0 |
| Moxifloxacin | 0.06-4 | 0.5 | 2 | |||
| Clindamycin | ≤0.03->128 | 0.25 | >128 | 69 | 0 | 31 |
| Chloramphenicol | 1-4 | 2 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.12->128 | 2 | 32 | 81 | 6 | 13 |
| Veillonella speciesg (20) | ||||||
| Penicillin | ≤0.03-16 | 2 | 16 | 25 | 5 | 70 |
| Ampicillin | 0.25-128 | 4 | 16 | 25 | 5 | 70 |
| Piperacillin | ≤0.03-128 | 16 | 64 | 85 | 10 | 5 |
| Ticarcillin | 0.06-128 | 32 | 32 | 90 | 5 | 5 |
| AMP-sulbactam | 0.12-4 | 2 | 4 | 100 | 0 | 0 |
| Piperacillin-TAZ | 0.12->128 | 16 | 128 | 85 | 10 | 5 |
| Ticarcillin-CVA | ≤0.03->128 | 32 | 128 | 90 | 5 | 5 |
| Cefoxitin | 0.06-128 | 16 | 32 | 65 | 25 | 10 |
| Cefmetazole | ≤0.03-32 | 1 | 8 | 95 | 5 | 0 |
| Cefoperazone | 0.06-128 | 16 | 64 | 60 | 20 | 20 |
| Imipenem | ≤0.03-4 | 0.5 | 2 | 100 | 0 | 0 |
| Meropenem | ≤0.03-0.5 | 0.12 | 0.5 | 100 | 0 | 0 |
| Moxifloxacin | 0.06-2 | 0.12 | 1 | |||
| Clindamycin | ≤0.03->128 | 8 | >128 | 40 | 5 | 55 |
| Chloramphenicol | 0.5-8 | 1 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.12->128 | 4 | >128 | 75 | 5 | 20 |
| Peptostreptococcus speciesh (31) | ||||||
| Penicillin | ≤0.03-128 | 0.25 | 8 | 84 | 0 | 16 |
| Ampicillin | ≤0.03-128 | 0.5 | 64 | 87 | 0 | 13 |
| Piperacillin | ≤0.03-64 | 0.12 | 16 | 97 | 3 | 0 |
| Ticarcillin | 0.06-128 | 0.5 | 2 | 87 | 3 | 10 |
| AMP-sulbactam | ≤0.03-32 | 0.12 | 0.5 | 90 | 0 | 10 |
| Piperacillin-TAZ | ≤0.03-32 | 0.06 | 8 | 100 | 0 | 0 |
| Ticarcillin-CVA | ≤0.03-128 | 1 | 64 | 84 | 16 | 0 |
| Cefoxitin | 0.06->128 | 2 | 32 | 87 | 10 | 3 |
| Cefmetazole | ≤0.03-128 | 2 | 32 | 81 | 16 | 3 |
| Cefoperazone | ≤0.03->128 | 1 | 128 | 84 | 3 | 13 |
| Imipenem | ≤0.03-32 | 0.12 | 4 | 94 | 3 | 3 |
| Meropenem | ≤0.03-4 | 0.12 | 2 | 100 | 0 | 0 |
| Moxifloxacin | ≤0.03-32 | 0.25 | 4 | |||
| Clindamycin | 0.06->128 | 8 | 128 | 45 | 0 | 55 |
| Chloramphenicol | 1-32 | 2 | 8 | 94 | 3 | 3 |
| Metronidazole | 0.06->128 | 1 | >128 | 68 | 0 | 32 |
| C. perfringens (20) | ||||||
| Penicillin | ≤0.03-0.12 | ≤0.03 | 0.12 | 100 | 0 | 0 |
| Ampicillin | ≤0.03-0.12 | ≤0.03 | 0.12 | 100 | 0 | 0 |
| Piperacillin | ≤0.03-≤0.03 | ≤0.03 | ≤0.03 | 100 | 0 | 0 |
| Ticarcillin | 0.06-0.5 | 0.25 | 0.5 | 100 | 0 | 0 |
| AMP-sulbactam | ≤0.03-0.12 | ≤0.03 | 0.12 | 100 | 0 | 0 |
| Piperacillin-TAZ | ≤0.03-0.25 | 0.06 | 0.12 | 100 | 0 | 0 |
| Ticarcillin-CVA | 0.12-1 | 0.5 | 1 | 100 | 0 | 0 |
| Cefoxitin | 0.5-2 | 0.5 | 1 | 100 | 0 | 0 |
| Cefmetazole | ≤0.03-0.25 | 0.06 | 0.12 | 100 | 0 | 0 |
| Cefoperazone | ≤0.03-2 | ≤0.03 | 1 | 100 | 0 | 0 |
| Imipenem | ≤0.03-0.06 | 0.06 | 0.06 | 100 | 0 | 0 |
| Meropenem | ≤0.03-≤0.03 | ≤0.03 | ≤0.03 | 100 | 0 | 0 |
| Moxifloxacin | 0.12-0.25 | 0.25 | 0.25 | |||
| Clindamycin | 0.06-2 | 1 | 2 | 100 | 0 | 0 |
| Chloramphenicol | 0.5-2 | 2 | 2 | 100 | 0 | 0 |
| Metronidazole | 0.5-2 | 1 | 2 | 100 | 0 | 0 |
| Clostridium speciesi (16) | ||||||
| Penicillin | ≤0.03-32 | 0.25 | 16 | 88 | 0 | 12 |
| Ampicillin | ≤0.03-64 | 0.12 | 32 | 69 | 6 | 25 |
| Piperacillin | ≤0.03-2 | 0.5 | 2 | 100 | 0 | 0 |
| Ticarcillin | 0.25-128 | 1 | 4 | 88 | 0 | 12 |
| AMP-sulbactam | ≤0.03-16 | 0.25 | 16 | 88 | 12 | 0 |
| Piperacillin-TAZ | ≤0.03-8 | 1 | 4 | 100 | 0 | 0 |
| Ticarcillin-CVA | ≤0.03-128 | 1 | 128 | 88 | 12 | 0 |
| Cefoxitin | 0.06-16 | 2 | 16 | 100 | 0 | 0 |
| Cefmetazole | 0.06-32 | 2 | 32 | 88 | 12 | 0 |
| Cefoperazone | 0.06-8 | 0.5 | 8 | 100 | 0 | 0 |
| Imipenem | ≤0.03-1 | 0.06 | 0.5 | 100 | 0 | 0 |
| Meropenem | ≤0.03-1 | 0.12 | 1 | 100 | 0 | 0 |
| Moxifloxacin | 0.12-2 | 0.25 | 0.5 | |||
| Clindamycin | ≤0.03-4 | 0.5 | 4 | 88 | 12 | 0 |
| Chloramphenicol | 0.5-4 | 2 | 4 | 100 | 0 | 0 |
| Metronidazole | 0.12->128 | 1 | >128 | 69 | 0 | 31 |
S, susceptible; I, intermediate; R, resistant.
AMP, ampicillin.
TAZ, tazobactam.
CVA, clavulanic acid.
Fusobacterium species included F. mortiferum (3), F. necrophorum (3), F. nucleatum (6), F. varium (6), and an unidentified Fusobacterium sp. (1).
Prevotella species included P. buccae (4), P. intermedia (3), P. melaninogenicus (2), P. oralis (2), and unidentified Prevotella spp. (5).
B. fragilis group isolates were the most encountered clinically significant isolates among the gram-negative anaerobes. The B. fragilis group isolates were uniformly resistant to penicillin and ampicillin. Most isolates were susceptible to imipenem or meropenem. Only one isolate of B. fragilis was found to have intermediate resistance to imipenem. Comparison of the susceptibilities of the individual species of the B. fragilis group showed that 40% of B. fragilis isolates were resistant to piperacillin, 44% were resistant to ticarcillin, and 17% were resistant to ampicillin-sulbactam. Four percent of B. fragilis isolates were resistant to cefoxitin; however, 22% had intermediate susceptibility. Other members of the B. fragilis group were more resistant to cefoxitin, with resistance rates between 31 and 65%. B. thetaiotaomicron was the species with the greatest resistance to cefoxitin (65%) and cefmetazole (80%) among the B. fragilis group. Resistance to clindamycin varied among the species from 33 to 70%, with the highest resistance rate occurring in B. thetaiotaomicron (70%), followed by Bacteroides caccae (67%). Chloramphenicol and metronidazole were active against >90% of isolates of the B. fragilis group. Only 3% of B. fragilis isolates were resistant to metronidazole and 2% were intermediately resistant to metronidazole.
All Fusobacterium isolates were susceptible to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefoxitin, and imipenem. The majority of Fusobacterium isolates (95%) were susceptible to cefmetazole. Thirty-five percent of Fusobacterium isolates were resistant to clindamycin.
More than half of Prevotella species isolates (62%) were resistant to penicillin and ampicillin. Rates of resistance to cefoxitin, cefmetazole, and clindamycin were 6, 12, and 31%, respectively. All of the Prevotella isolates tested were susceptible to imipenem, meropenem, and chloramphenicol.
Among Veillonella isolates, 70% were resistant to penicillin and ampicillin. Five percent of Veillonella isolates were resistant to piperacillin, ticarcillin, piperacillin-tazobactam, and ticarcillin-clavulanic acid. Ten percent were resistant to cefoxitin, 55% were resistant to clindamycin, and 20% were resistant to metronidazole. All isolates were susceptible to ampicillin-sulbactam, imipenem, meropenem, and chloramphenicol.
Of the gram-positive isolates, the 20 Clostridium perfringens isolates were susceptible to all of the agents tested. Other gram-positive anaerobes showed various degrees of resistance to penicillin (12 to 16%) and ampicillin (13 to 25%). Among Peptostreptococcus species isolates, 16% were resistant to penicillin. All isolates were susceptible to piperacillin-tazobactam and meropenem. Of Peptostreptococcus species isolates, 3% were resistant to cefoxitin and imipenem but 55% were resistant to clindamycin. Clostridium species other than C. perfringens were more resistant than C. perfringens, with 12% of the isolates resistant to penicillin and ticarcillin, 25% resistant to ampicillin, and 31% resistant to metronidazole.
The MIC50s and MIC90s of moxifloxacin for all species ranged from 0.12 to 2 μg/ml (MIC50s) and from 0.25 to 8 μg/ml (MIC90s).
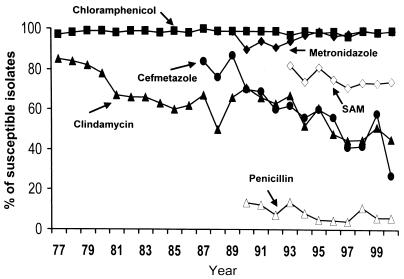
Trend of cefoxitin and clindamycin resistance in B. thetaiotaomicron.
Figure 1 shows the annual rates of susceptibility to six routinely tested agents for all of the Bacteroides species isolates at National Taiwan University Hospital from 1977 to 2000. The rates of susceptibility to cefmetazole and clindamycin decreased. Susceptibility testing was performed by the disk diffusion method before 1990 and by the breakpoint agar dilution method after 1991. A stepwise increase in the rates of resistance to cefoxitin and clindamycin resistance was noted.
FIG. 1.
Rates of susceptibility to six routinely tested agents in all of the Bacteroides species isolates at National Taiwan University Hospital from 1977 to 2000. Susceptibility testing was performed by the disk diffusion method before 1990 and by the breakpoint agar dilution method after 1991. SAM, ampicillin- sulbactam.
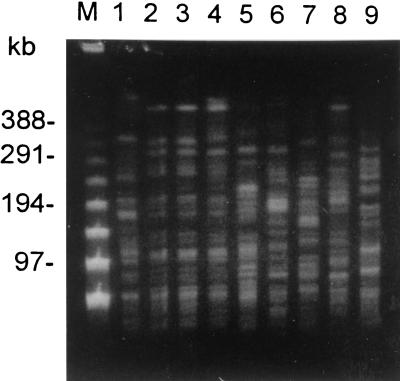
PFGE of metronidazole-resistant B. fragilis isolates.
Five metronidazole-resistant strains of B. fragilis were found in this study. Since no metronidazole-resistant strains of B. fragilis were reported before in Taiwan. We analyzed the genetic relationships among these strains by PFGE. The results are shown in Fig. 2. Lanes 1 to 6 show metronidazole-nonsusceptible B. fragilis isolates; lanes 2 and 3 show isolates from the same individual. Lanes 7 to 9 show metronidazole-susceptible B. fragilis isolates. Two strains (lanes 2 or 3 and 4) were similar. Other strains have distinct patterns. These isolates showed genotypic heterogeneity, suggesting that they correspond to a highly heterogeneous population rather than to the dissemination of a single clone.
FIG. 2.
PFGE analysis of XbaI-digested genomic DNA from B. fragilis isolates. Lane M, lambda ladder molecular size markers; lanes 1 to 6, metronidazole-nonsusceptible B. fragilis isolates (lanes 2 and 3, isolates from the same individual); lanes 7 to 9, metronidazole-susceptible B. fragilis isolates.
DISCUSSION
In this study the antimicrobial susceptibilities of 344 isolates of anaerobes to various agents were determined. In agreement with other reports, the susceptibility results varied among genera and species. Compared to a previous study by our group of 100 B. fragilis isolates done 10 years ago (16), the increased resistance of the B. fragilis group to cefoxitin and clindamycin is noted. Rates of resistance to cefoxitin for B. fragilis increased from 1 to 4% and rates of resistance to clindamycin increased from 29 to 33% over the period from 1991 to 2000. The level of chloramphenicol susceptibility remained unchanged.
As expected, the β-lactams were more active in gram-positive than in gram-negative anaerobes. According to NCCLS guidelines, members of the B. fragilis group are presumed to be resistant to ampicillin. Peptostreptococcus species have been considered fully susceptible to several β-lactam drugs, including penicillin G. In the present study, isolates resistant to penicillin within Peptostreptococcus species were mostly Peptostreptococcus anaerobius. In Korea, the rate of resistance to penicillin of P. anaerobius was also high, while those of other species were lower (7). Addition of a β-lactamase inhibitor generally reversed the resistance. However, Veillonella displayed similar susceptibilities to piperacillin and piperacillin-tazobactam and ticarcillin and ticarcillin-clavulanic acid. The rate of resistance to ampicillin-sulbactam in B. fragilis isolates increased from 8 (1991) to 17% (this study). Resistance to piperacillin-tazobactam was low (<6%).
Data collected from our hospital's clinical microbiology laboratory reveal the decrease in susceptibility to cefmetazole in Bacteroides species from 1987 to 2000. Cefoxitin was more active than cefmetazole against most species. Rates of resistance to cefoxitin varied greatly with species and country. In our institution, the percentage of resistance to cefoxitin rose from 1% in 1991 to 4% for B. fragilis and from 10 to 70% for B. thetaiotaomicron. The rate of resistance to cefoxitin among the B. fragilis group was 12.8% in Spain (4), 11.3% in Canada (1997) (7), and 1.7 to 14.2% depending on the species in the United States (1995 and 1996) (14), 2.9 to 34.5% in Japan (1990 to 1992) (15), and 5% in South Africa (6). MICs for many isolates were 32 μg/ml, which was considered by the NCCLS as intermediate in susceptibility. Fusobacterium isolates remained susceptible to cefoxitin. The high rates of resistance to cefoxitin in non-B. fragilis Bacteroides species were unusual. The use of cefoxitin and cefmetazole has not increased in the past 10 years. Therefore the reason for the high incidence of resistance to cefoxitin is unclear.
For all species, high rates of susceptibility to imipenem and meropenem were observed. Resistance to meropenem was low (<1%). One isolate of B. fragilis and one isolate of Peptostreptococcus displayed intermediate susceptibility, and another Peptostreptococcus isolate displayed resistance, to imipenem. In general, The MIC90s of imipenem and meropenem were similar except that some isolates showed discordant susceptibilities to imipenem and meropenem. For example, six isolates of B. fragilis (five intermediate and one resistant) were not susceptible to meropenem, but only one isolate was not susceptible to imipenem. Eight percent of B. thetaiotaomicron isolates were resistant to imipenem but susceptible to meropenem. Compared to the data of our previous report, the rate of resistance to imipenem for B. fragilis has not increased, but the MIC50 (from 0.12 to 0.5 μg/ml) and MIC90 (from 1 to 4 μg/ml) have increased slightly. Resistance to the carbapenems has been occasionally and infrequently recorded (13).
Clindamycin has long been considered the drug of choice for treatment of anaerobes. However, over the past 20 years, there has been a significant increase in the rate of resistance to clindamycin among isolates of the B. fragilis group in many areas (1, 8, 11, 13-15). In our institution, the overall activities of clindamycin against the B. fragilis group were poor (33 to 70%). The high prevalence of resistance to clindamycin in B. fragilis group isolates has been described previously in several reports. For example, a high prevalence of resistance to clindamycin (49%) in the B. fragilis group was observed by Betriu et al. in Spain (4). In Korea, in 1994, the rates of resistance to clindamycin for B. fragilis, B. thetaiotaomicron, and other Bacteroides spp. were 38, 45.5, and 69%, respectively (8). However, in several areas the rate of resistance to clindamycin for the B. fragilis group remained low. In South Africa, only 5% of isolates were resistant to clindamycin in the B. fragilis group (6). It was also reported that clindamycin resistance is associated with hospital-acquired infections (11). Rates of resistance for isolates varied greatly with species and country. In the present study, high rates of resistance to clindamycin were found for the following organisms: B. thetaiotaomicron (70%), B. caccae (67%), Bacteroides uniformis (56%), Veillonella and Peptostreptococcus spp. (55%), Fusobacterium spp. (35%), and B. fragilis and Prevotella spp. (31%). B. thetaiotaomicron isolates were also more resistant to cefoxitin than other species. In agreement with other reports, the resistance rates for non-B. fragilis species of the B. fragilis group were found higher than that for B. fragilis (33%). Aldridge et al. reported that Bacteroides distasonis and Bacteroides ovatus were more resistant to clindamycin than other species (1). Since B. thetaiotaomicron is usually the second most frequently encountered Bacteroides species, rapid detection and identification are important. We recently described a PCR assay which provided a rapid and accurate method for identification of B. thetaiotaomicron (17).
Among gram-positive anaerobes, C. perfringens was the most susceptible. Other Clostridium species were less susceptible to penicillin, ampicillin, ticarcillin, and metronidazole. This result is similar to the finding of a study done in Korea (8) but is different from that of a study done in South Africa (6).
In the present study, the MIC results for moxifloxacin confirm the broad spectrum of its activity against gram-positive and gram-negative anaerobic bacteria. Although no interpretation standard is available for anaerobes, for the anaerobic bacteria tested, the moxifloxacin MIC50 varied from 0.12 to 2 μg/ml and MIC90 varied from 0.25 to 8 μg/ml. Many new fluoroquinolones have been tested for in vitro activities against gram-positive, gram-negative, and anaerobic bacteria previously (4, 9). Similarly, other studies have demonstrated the good activity of new fluoroquinolones against various anaerobic species. Moxifloxacin is, therefore, a potentially useful antibiotic against anaerobes.
The rates of resistance to metronidazole for several gram-positive anaerobes, Peptostreptococcus (32%) and Clostridium species (31%) other than C. perfringens, were higher than those for gram-negative anaerobes Fusobacterium (25%), Veillonella (20%), Prevotella (13%), and Bacteroides species (<3%). Previous reports also showed that peptostreptococci are generally less susceptible to metronidazole than gram-negative anaerobes. A similar percentage of resistance to metronidazole for peptostreptococci in Korea was described by Lee et al. (8). Resistance to metronidazole among B. fragilis isolates in Taiwan is first documented in this report. By PFGE analysis, five strains (four patterns) showed genotypic heterogeneity, suggesting that they correspond to a heterogeneous population rather than to the dissemination of a single clone. The results suggest that the emergence of these resistant strains may be sporadic. The development of antibiotic resistance in anaerobic bacteria has a tremendous impact on the selection of antimicrobial agents for empirical therapy. It suggests the need to monitor antibiotic susceptibility patterns of anaerobes related to geographic regions periodically.
REFERENCES
- 1.Aldridge, K. E., D. Ashcraft, K. Cambre, C. L. Pierson, S. G. Jenkins, and J. E. Rosenblatt. 2001. Multicenter survey of the changing in vitro antimicrobial susceptibilities of clinical isolates of Bacteroides fragilis group, Prevotella, Fusobacterium, Porphyromonas, and Peptostreptococcus species. Antimicrob. Agents Chemother. 45:1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrés, M. T., W. O. Chung, M. C. Roberts, and J. F. Fierro. 1998. Antimicrobial susceptibilities of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens spp. isolated in Spain. Antimicrob. Agents Chemother. 42:3022-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleman, M. D., P. N. R. Heseltine, and C. E. Cherubin. 1991. Epidemiology, antimicrobial susceptibility, pathology, and significance of Bacteroides fragilis group organisms isolated at Los Angeles County-University of Southern California Medical Center. Clin. Infect. Dis. 13:12-18. [DOI] [PubMed] [Google Scholar]
- 4.Betriu, C., M. Gomez, M. L. Palau, A. Sanchez, and J. J. Picazo. 1999. Activities of new antimicrobial agents (trovafloxacin, moxifloxacin, sanfetrinem, and quinupristin-dalfopristin) against Bacteroides fragilis group: comparison with the activities of 14 other agents. Antimicrob. Agents Chemother. 43:2320-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohm, H., and H. Karch. 1992. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 30:2169-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch, C. L., P. Derby, and V. R. Abratt. 1998. In-vitro antibiotic susceptibility and molecular analysis of anaerobic bacteria isolated in Cape Town, South Africa. J. Antimicrob. Chemother. 42:245-248. [DOI] [PubMed] [Google Scholar]
- 7.Labbe, A. C., A. M. Bourgault, J. Vincelette, P. L. Turgeon, and F. Lamothe. 1999. Trends in antimicrobial resistance among clinical isolates of the Bacteroides fragilis group from 1992 to 1997 in Montreal, Canada. Antimicrob. Agents Chemother. 43:2517-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, K., Y. Chong, S. H. Jeong, X. S. Xu, and O. H. Kwon. 1996. Emerging resistance of anaerobic bacteria to antimicrobial agents in South Korea. Clin. Infect. Dis. 23(Suppl.):S73-S77. [DOI] [PubMed] [Google Scholar]
- 9.Milatovic, D., F. J. Schmitz, S. Brisse, J. Verhoef, and A. C. Fluit. 2000. In vitro activities of sitafloxacin (DU-6859a) and six other fluoroquinolones against 8,796 clinical bacterial isolates. Antimicrob. Agents Chemother. 44:1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard M11-A4, 4th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Redondo, M. C., M. D. J. Arbo, J. Grindlinger, and D. R. Snydman. 1995. Attributable mortality of bacteremia associated with the Bacteroides fragilis group. Clin. Infect. Dis. 20:1492-1496. [DOI] [PubMed] [Google Scholar]
- 12.Rotimi, V. O., M. Khoursheed, J. S. Brazier, W. Y. Jamal, and F. B. Khodakhast. 1999. Bacteroides species highly resistant to metronidazole: an emerging problem? Clin. Microbiol. Infect. 5:166-169. [DOI] [PubMed] [Google Scholar]
- 13.Snydman, D. R., L. McDermott, G. J. Cuchural, Jr., D. W. Hecht, P. B. Iannini, L. J. Harrell, S. G. Jenkins, J. P. O'Keefe, C. L. Pierson, J. D. Rihs, V. L. Yu, S. M. Finegold, and S. L. Gorbach. 1996. Analysis of trends in antimicrobial resistance patterns among clinical isolates of Bacteroides fragilis group species from 1990 to 1994. Clin. Infect. Dis. 23(Suppl. 1):S54-S65. [DOI] [PubMed] [Google Scholar]
- 14.Snydman, D. R., N. V. Jacobus, L. A. McDermott, S. Supran, G. J. Cuchural, Jr., S. Finegold, L. Harrell, D. W. Hecht, P. Iannini, S. Jenkins, C. Pierson, J. Rihs, and S. L. Gorbach. 1999. Multicenter study of in vitro susceptibility of the Bacteroides fragilis group, 1995 to 1996, with comparison of resistance trends from 1990 to 1996. Antimicrob. Agents Chemother. 43:2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka-Bandoh, K., N. Kato, K. Watanabe, and K. Ueno. 1995. Antibiotic susceptibility profiles of Bacteroides fragilis and Bacteroides thetaiotaomicron in Japan from 1990 to 1992. Clin. Infect. Dis. 20(Suppl. 2):S352-S355. [DOI] [PubMed] [Google Scholar]
- 16.Teng, L. J., S. W. Ho, S. C. Chang, K. T. Luh, and W. C. Hsieh. 1991. In vitro activities of 36 antimicrobial agents against clinically isolated Bacteroides fragilis. J. Formos. Med. Assoc. 90:796-799. [PubMed] [Google Scholar]
- 17.Teng, L. J., P. R. Hsueh, J. C. Tsai, F. L. Chiang, C. Y. Chen, S. W. Ho, and K. T. Luh. 2000. PCR assay for species-specific identification of Bacteroides thetaiotaomicron. J. Clin. Microbiol. 38:1672-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]