Abstract
The present study investigated the role of the Candida dubliniensis CdCDR1 and CdCDR2 genes in the development of fluconazole resistance. The C. dubliniensis CdCDR1 gene was 92% identical at the nucleotide sequence level to the corresponding C. albicans gene. However, 58% (14 of 24) of C. dubliniensis genotype 1 isolates tested harbored a nonsense mutation in the CdCDR1 open reading frame that converted codon 756 (TAT) to a TAG translational stop codon. Analysis of five of these C. dubliniensis isolates by Western immunoblotting showed that they expressed a truncated 85-kDa CdCdr1p compared to the full-length 170-kDa CdCdr1p. Expression of CdCDR1 alleles from six C. dubliniensis isolates in a pdr5 Saccharomyces cerevisiae strain revealed that CdCDR1 alleles from three isolates that encoded truncated proteins were unable to confer resistance to drugs and antifungals. However, reassignment of the TAG sequence at codon 756 to TAT (encoding tyrosine) in an allele from strain CD36 conferred the ability to mediate resistance to multiple drugs. Fluconazole-resistant isolates of C. dubliniensis harboring functional alleles of CdCDR1 were found to exhibit two- to ninefold-higher levels of CdCDR1 mRNA than did matched fluconazole-susceptible isolates. By comparison, levels of CdMDR1 expression ranged from approximately 50- to 100-fold greater in resistant isolates. Fluconazole resistance was also identified in isolates harboring nonfunctional CdCDR1 alleles, but resistance in these isolates was only associated with increased CdMDR1 expression. Targeted disruption of two functional alleles of CdCDR1 in a fluconazole-resistant derivative of C. dubliniensis that overexpressed both CdCDR1 and CdMDR1 revealed that although CdCDR1 was important for mediating reduced susceptibility to itraconazole and ketoconazole, there was no affect on fluconazole susceptibility in the double mutant. Evidence presented in this study reveals that CdCDR1 is not essential for the development of fluconazole resistance in C. dubliniensis.
Resistance to azole antifungal drugs in Candida species is now recognized as a major clinical problem (17, 32). Several studies have shown that a significant proportion of oral Candida albicans isolates recovered from human immunodeficiency virus (HIV)-infected patients are resistant to the azole antifungal drug fluconazole and that some non-C. albicans Candida species, such as Candida krusei, are inherently resistant to this agent (1, 9, 11, 22). Fluconazole resistance has also been described in Candida dubliniensis, a species that was first described in 1995 (14, 30). C. dubliniensis is a significant cause of oral disease in the HIV-infected patient population, who routinely receive fluconazole therapy for the suppression of oral candidiasis (4, 29). Recently, several studies have reported the recovery of C. dubliniensis from the bloodstream, although the true incidence of systemic infection caused by this organism has yet to be determined (2, 3, 12). We and others have previously reported the recovery of C. dubliniensis isolates with reduced susceptibility to fluconazole from the oral cavities of HIV-infected patients (10, 13, 14, 20). In addition, we have shown that fluconazole-susceptible C. dubliniensis clinical isolates can readily develop fluconazole resistance when exposed to this agent in vitro (14). Molecular analysis of fluconazole-resistant C. dubliniensis isolates and in vitro-generated fluconazole-resistant derivatives has shown that in each case, the fluconazole resistance phenotype is associated with increased expression of the CdMDR1 gene encoding the multidrug transporter CdMdr1p (13). Wirsching et al. demonstrated that targeted deletion of both copies of CdMDR1 in a C. dubliniensis clinical isolate with reduced susceptibility to fluconazole was sufficient to render the null mutant susceptible to fluconazole (35).
A second multidrug transporter-encoding gene, termed CdCDR1, has also been identified in C. dubliniensis, and it is homologous to the C. albicans ABC transporter-encoding gene CDR1 (13). Almost all isolates of C. albicans (83%) with reduced susceptibility to azoles analyzed to date exhibit increased expression of CDR1 (16). However, the role of the homologous CdCDR1 gene in C. dubliniensis in determining susceptibility to fluconazole is less clear. Although increased expression of CdMDR1 has been observed in all fluconazole-resistant C. dubliniensis isolates and derivatives analyzed to date, increased CdCDR1 gene expression has only been reported in approximately 50% of fluconazole-resistant C. dubliniensis isolates and derivatives (13). For these reasons, the objectives of the present study were to functionally characterize the C. dubliniensis CdCDR1 gene and to examine its role in determining azole susceptibility in C. dubliniensis.
MATERIALS AND METHODS
Candida strains and culture conditions.
Forty clinical isolates of C. dubliniensis from diverse geographic locations were included in this study (Table 1). Isolates of C. dubliniensis were routinely cultured on potato dextrose agar (Oxoid) medium, pH 5.6, at 37°C (7). For liquid culture, cells were grown in yeast extract-peptone-dextrose (YEPD) broth, also at 37°C.
TABLE 1.
Candida strains and derivatives used in this study
| Strain or isolate | Source and/or notesa | Country of origin | TAGc | Reference(s) |
|---|---|---|---|---|
| C. albicans CA132A | Oral reference isolate | Ireland | NAb | 7 |
| C. dubliniensis | ||||
| Genotype I | ||||
| CD36 | Type strain, CBS 7987 | Ireland | + | 30 |
| CD36R1 | Derivative of CD36, FLUDD | NA | + | 8 |
| CD36R2 | Derivative of CD36, Flur | NA | + | 8 |
| CM1 | Oral isolate | Australia | − | 14, 30 |
| CM2 | Oral isolate, FLUDD | Australia | − | 14, 30 |
| CD57 | Vaginal isolate | Ireland | − | 14 |
| CD57R | Derivative of CD57, FLUDD | NA | − | 14 |
| 57RM1 | CdCDR1/cdcdr1Δ::MPAR-FLIP | NA | − | This study |
| 57RM2 | CdCDR1/cdcdr1Δ::FRT | NA | − | This study |
| 57RM3 | cdcdr1Δ::MPAR-FLIP/cdcdr1Δ::FRT | NA | NA | This study |
| 57RM4 | cdcdr1Δ::FRT/cdcdr1Δ::FRT | NA | NA | This study |
| CD51-II | Oral isolate | Ireland | + | 14 |
| CD51-IIR | Derivative of CD51-II, Flur | NA | + | 14 |
| CD47-I | Oral isolate | Ireland | + | 14 |
| CD47-IIa | Oral isolate | Ireland | + | 14 |
| CD47-IIb | Oral isolate, FLUDD | Ireland | + | 14 |
| CD33 | Oral isolate | Ireland | − | 30 |
| CD38 | Oral isolate | Ireland | + | 30 |
| CD71 | Oral isolate | Argentina | − | 8 |
| CD72 | Oral isolate, Flur | Ireland | − | 14 |
| NCPF3108 | Postmortem lung isolate | United Kingdom | + | 8, 30 |
| CBS8500 | Blood culture isolate | The Netherlands | − | 12 |
| CM5 | Oral isolate | Australia | − | 30 |
| CO4 | Oral isolate | Switzerland | + | 8 |
| P30 | Oral isolate | Switzerland | + | 8 |
| CD96.34 | Oral isolate | Germany | ± | 8 |
| CD159 | Oral isolate | Greece | − | 8 |
| CAN1 | Oral isolate | Canada | + | 8 |
| Wü284 | Oral isolate | Germany | + | 27 |
| B341 | Oral isolate | United States | + | This study |
| B484 | Oral isolate | United States | − | This study |
| B651 | Oral isolate | United States | ± | This study |
| Genotype II | ||||
| CBS2747 | Sputum isolate | The Netherlands | − | 8 |
| CAN3 | Oral isolate | Canada | − | 8 |
| CAN4 | Oral isolate | Canada | − | 8 |
| CAN6 | Oral isolate | Canada | − | 8 |
| CD41 | Oral isolate | Ireland | − | 14 |
| m262b | Oral isolate | United Kingdom | − | 8 |
| CD513 | Oral isolate | Ireland | − | 8 |
| CD506 | Oral isolate | Ireland | − | 8 |
| CD539 | Oral isolate | United Kingdom | − | 8 |
| CD541 | Blood culture isolate | United Kingdom | − | 8 |
| Genotype III | ||||
| p7276 | Respiratory tract isolate | Israel | − | 18 |
| p6265 | Sputum isolate | Israel | − | 18 |
| p6785 | Urine isolate | Israel | − | 18 |
| CD514 | Oral isolate | Ireland | − | 8 |
| CD519 | Oral isolate | Ireland | − | 8 |
| Genotype IV | ||||
| p7718 | Wound isolate | Israel | − | 18 |
Isolates are fluconazole susceptible (MIC, ≤8 μg/ml) unless indicated. Flur, fluconazole resistant (MIC, ≥64 μg/ml); FLUDD, dose-dependent fluconazole resistance (MIC, 16 to 32 μg/ml).
NA, not applicable.
Refers to presence (+) or absence (−) of a TAG at codon 756 of the CdCDR1 ORF; ± refers to heterozygosity at this locus.
Transformants of C. dubliniensis and Saccharomyces cerevisiae were selected and maintained on minimal agar medium (6.7 g of yeast nitrogen base without amino acids [Difco], 20 g of glucose, 15 g of Bacto agar [Difco], and 50 mg [each] of uracil, lysine, adenine, tryptophan, and histidine [Sigma-Aldrich] per liter). For induction of the SAP2 promoter and excision of the mycophenolic acid (MPA) resistance flipper cassette (MPAR-flipper), cells were grown in YCB-BSA medium (23.4 g of yeast carbon base [Difco], 4 g of bovine serum albumin [Sigma-Aldrich] per liter [pH 4.0]).
Antifungal drug susceptibility testing.
Susceptibility testing of C. dubliniensis clinical isolates and their derivatives to antifungal drugs and metabolic inhibitors was performed by using a broth microdilution assay based on the approved NCCLS procedure (14). Susceptibility tests were carried out in RPMI 1640 medium (10.4 g of RPMI 1640 [Sigma-Aldrich], 20 g of glucose, 34.5 g of morpholinepropanesulfonic acid [pH 7.0] per liter) as described by Moran et al. (14). Metabolic inhibitors were purchased from Sigma-Aldrich, fluconazole was a gift from Pfizer Central Research (Sandwich, Kent, United Kingdom), and itraconazole and ketoconazole were gifts from Janssen Pharmaceutical (Cork, Republic of Ireland).
The following antifungal drugs and metabolic inhibitors were prepared as stock solutions in water at the concentrations indicated: fluconazole, 5 mg/ml; fluphenazine, 20 mg/ml; and rhodamine 6G, 5 mg/ml. Stock solutions of other drugs were prepared in dimethyl sulfoxide at the concentrations indicated: ketoconazole, 1 mg/ml; itraconazole, 1 mg/ml; cycloheximide, 20 mg/ml; cerulenin, 1 mg/ml; and brefeldin A, 5 mg/ml. Crystal violet (2 mg/ml) was dissolved in methanol. Stock solutions of drugs were diluted in RPMI 1640 medium to the following concentrations, from which serial twofold dilutions were prepared in 96-well microtiter dishes (Corning): fluconazole, 64 μg/ml; itraconazole, 4 μg/ml; ketoconazole, 2 μg/ml; cerulenin, 64 μg/ml; rhodamine 6G, 16 μg/ml; cycloheximide, 512 μg/ml; brefeldin A, 512 μg/ml; and fluphenazine, 128 μg/ml.
PCR amplification of C. dubliniensis CdCDR1 alleles.
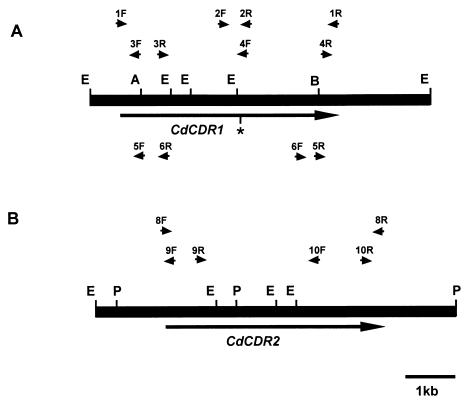
Amplification of CdCDR1 and CdCDR2 genes was carried out by using the Expand high-fidelity PCR system (Roche Molecular Biochemicals). To amplify the entire C. dubliniensis CdCDR1 open reading frame (ORF), the oligonucleotide primer pair 1F-1R (Table 2; Fig. 1) was designed based on the nucleotide sequence of the C. albicans CDR1 gene. Similarly, for amplification of the CdCDR2 ORF, the primer pair 8F-8R was designed based on the nucleotide sequence of the C. albicans CDR2 gene. These primers contained cleavage sites for the restriction endonuclease XbaI (Table 2). Template DNA was isolated from C. dubliniensis isolates as described by Sullivan et al. (28). Sequences flanking the CdCDR1 and CdCDR2 genes were amplified from the C. dubliniensis type strain CD36 by inverse PCR. Briefly, PCR amplification was carried out under the same conditions with self-ligated EcoRI-digested total genomic DNA as template. The 5′ flanking region of the CdCDR1 gene was amplified with the oligonucleotide primer pair 3F-3R, which contained the EcoRI restriction endonuclease recognition sequence (Table 2; Fig. 1). The 3′ region of the CdCDR1 gene was amplified with the oligonucleotide primer pair 4F-4R (Table 2; Fig. 1). Similarly, the 5′ and 3′ flanking sequences of the CdCDR2 gene were amplified with the oligonucleotide primer pairs 9F-9R and 10F-10R, respectively. PCR products were purified from the reaction mixture by using the Wizard PCR system (Promega). PCR primers were designed with restriction endonuclease recognition sequences at their 5′ ends (Table 1) that allowed PCR products to be digested and ligated directly to the plasmid vector pBluescript II KS(−), transformed into Escherichia coli DH5α as described previously, and subjected to DNA sequence analysis (21).
TABLE 2.
Oligonucleotide primers used in PCRs
| Gene and primer | Nucleotide sequence (5′-3′)a | Nucleotide coordinatesb |
|---|---|---|
| CdCDR1 | ||
| 1F | AATCTAGAATTATGTCAGATTCTAAGATGTC | −3 to +20 |
| 1R | ATTCTAGATTATTTCTTATTTTTTTTCTCTCTG | +4482 to +4506 |
| 2F | ATCCTGTTGGTTATGTGTTCG | +2071 to +2091 |
| 2R | GGGAAATCAACACTTCCAGTG | +2557 to +2537 |
| 3F | AGGAATTCCACTTGTGTGGGCATCAAACC | +121 to +101 |
| 3R | AGGAATTCCCAATGTTGGGATAATGCCAC | +969 to +989 |
| 4F | AGGAATTCAAACCTGGGCCACTTGGAAC | +2165 to +2146 |
| 4R | AGGAATTCCATTGTACAGTGAAAGATGGAG | +4373 to +4394 |
| 5F | ACCGCTCGAGCAGGTCTCATGATAGCATCC | +538 to +519 |
| 5R | ATCCCCGCGGAACTTGTTCTAGTTATTTGG | +4248 to +4267 |
| 6F | ATCCCCGCGGCAATACTTGTTGGTCAGTTGG | +3532 to +3552 |
| 6R | ACCGCTCGAGATATGGAGATGCTGGTCTGG | +1467 to +1448 |
| 7Rc | AATCTAGAGTTGATAATCAGAATCATTAGC | +421 to +400 |
| CdCDR2 | ||
| 8F | AATCTAGAAATATGAGTACTGCAAACACGTC | −3 to +20 |
| 8R | ATTCTAGATTATTTTTTCATCTTCTTTTCTCTATT | +4503 to +4467 |
| 9F | GCGAATTCAAACCTTGATATTCTTGAACTG | +97 to +76 |
| 9R | GCGAATTCGTTGGGATAATGCCACTAG | +989 to +971 |
| 10F | GCGAATTCTAATGCTTCTCTAACAGTAAGTGG | +2862 to +2840 |
| 10R | GCGAATTCCTATGGGACAATTGGCAATC | +3986 to +4005 |
| 7Rc | AATCTAGAGTTGATAATCAGAATCATTAGC | +418 to +394 |
| TEF3d | ||
| TEF3F | CGGAATTCCGATTGGTCCAAATGGTGCTGG | +2113 to +2132 |
| TEF3R | CGGAATTCCGATCTTGTTACCCATAGCATCG | +3012 to +3032 |
Restriction endonuclease recognition sequences are underlined. XbaI, 5′-TCTAGA; EcoRI, 5′-GAATTC; XhoI, 5′-CTCGAG; SacII, 5′-CCGCGG.
Nucleotide coordinates for CdCDR1 (accession no. AJ439073) or CdCDR2 (accession no. AJ439075) are as indicated, with the first base of the ATG start codon designated + 1.
The primer 7R is homologous to both CdCDR1 and CdCDR2.
Nucleotide coordinates of the TEF3 primers refer to the C. albicans TEF3 gene (accession no. Z12822).
FIG. 1.
Restriction map of CdCDR1 (A) and CdCDR2 (B) encoding DNA from C. dubliniensis CD36. The black rectangular boxes represent C. dubliniensis genomic DNA which was amplified by PCR from genomic DNA (see Materials and Methods). The large arrows show the positions and directions of transcription of the CdCDR1 and CdCDR2 ORFs. The asterisk in the CdCDR1 ORF shows the position of the polymorphic codon 756. The approximate positions of the CdCDR1- and CdCDR2-specific PCR primers shown in Table 1 are indicated by small arrows. Primer pairs 5F-5R and 6F-6R shown at the bottom of panel A were used in the inverse PCR experiments performed to disrupt the CdCDR1 gene. Restriction endonuclease cleavage sites: A, AccI; B, BamHI; E, EcoRI; P, PvuII.
Heterologous expression of CdCDR genes in S. cerevisiae.
The oligonucleotide primer pairs 1F-1R and 8F-8R (Table 2; Fig. 1) were used to amplify the entire CdCDR1 and CdCDR2 ORFs, respectively. The CdCDR1 and CdCDR2 ORFs were amplified from genomic DNA purified from C. dubliniensis isolates CD36, CD57, CD51-II, CD47-IIb, CM1, and CM2 and C. albicans strain CA132A. These products were cloned by using standard techniques into the XbaI site of the expression plasmid pYES (15) and transformed into the Δpdr5 S. cerevisiae strain YKKB-13 as described by Sanglard et al. (24). Transformants of YKKB-13 were selected on minimal medium without uracil. In order to induce expression from the GAL1 promoter of pYES, transformants were subsequently maintained on minimal medium without uracil containing 2% (wt/vol) galactose as the sole carbon source. S. cerevisiae transformants were tested for susceptibility to antifungal drugs and metabolic inhibitors on minimal medium containing galactose as described by Sanglard et al. (24). Briefly, a suspension (2 × 107 CFU/ml) of each transformant to be tested was prepared in sterile saline. This solution was then serially diluted 10-fold, and 5 μl of each dilution was spotted onto plates containing fixed concentrations of each metabolic inhibitor (see Fig. 3). Susceptibility to each drug was determined based on the highest dilution of each culture which could grow in the presence of the inhibitor as described by Sanglard et al. (24).
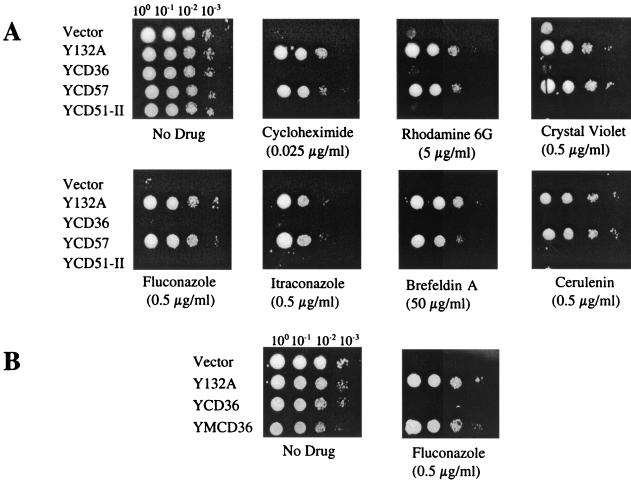
FIG. 3.
Susceptibility of S. cerevisiae YKKB-13 (pdr5) transformants harboring cloned CDR1 genes to antifungal drugs and metabolic inhibitors. (A) CDR1 alleles from C. albicans and C. dubliniensis isolates were amplified by PCR, cloned into the GAL1 expression vector pYES and transformed into the S. cerevisiae strain YKKB-13. The transformants harbored the pYES plasmid (Vector), cloned CDR1 from C. albicans CA132A (Y132A), and cloned CdCDR1 genes from the C. dubliniensis isolates CD36 (YCD36), CD57 (YCD57), and CD51-II (YCD51-II). A suspension (2 × 107 CFU/ml) of each transformant was serially diluted, and 5 μl of each dilution was spotted onto minimal agar medium plates containing fixed concentrations of the antifungal drug or metabolic inhibitor indicated. (B) Fluconazole susceptibility of YKKB-13 transformants harboring the cloned C. albicans CA132A CDR1 gene (Y132A), the CD36 CdCDR1 gene (YCD36), and the mutated CD36 CdCDR1 gene (YMCD36) in which the premature stop codon has been reassigned.
Western immunoblotting.
Crude protein extracts were prepared from C. dubliniensis isolates, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to Western immunoblot analysis with anti-Cdr1p polyclonal sera (a gift from D. Sanglard) as described by Moran et al. (13). Antibody-protein complexes were detected with the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Ill.). Molecular sizes of protein bands were estimated by using the Bio-Rad broad-range SDS-PAGE standards (Bio-Rad) and the GelWorks one-dimensional gel analysis software package (UVP, Irvine, Calif.).
Site-directed mutagenesis.
Site-directed mutagenesis was carried out by using the GeneEditor system (Promega). Alleles of CdCDR1 for mutagenization were subcloned into the plasmid vector pGEM-11Z(f+). Point mutations were introduced by hybridization with the 5′-phosphorylated mutagenic oligonucleotide 5′-GCTTACCAATATTATAATTC, which contained a single G to T substitution (underlined), according to the manufacturer's instructions. Mutant alleles were sequenced on both strands in order to confirm the introduction of the desired mutation.
Isolation of total RNA from C. dubliniensis and Northern blot analysis.
Total RNA was isolated from C. dubliniensis isolates grown to the mid-exponential phase (optical density at 600 nm, 0.6) in YEPD broth at 37°C as described by Moran et al. (13). RNA was fractionated on 1.2% (wt/vol) agarose gels containing 6% (vol/vol) formaldehyde and transferred to nylon membranes (Osmonics, Westborough, Mass.) by capillary transfer. RNA was hybridized at 42°C with DNA probes homologous to CdCDR1, CdMDR1, and CdTEF3 labeled with [α-32P]dATP (6,000 Ci/mmol, 220 TBq/mmol; NEN Life Sciences, Boston, Mass.) by random primer labeling as described by Moran et al. (13). A CdCDR1-specific probe was constructed by PCR amplification of the 5′ region of the CdCDR1 gene (nucleotides +1 to +421) with the primer pair 1F-7R (Table 2). Similarly, a 418-bp CdCDR2-specific probe (nucleotides +1 to +418) was amplified from C. dubliniensis genomic DNA by using the primer pair 8F-7R (Table 2). A CdTEF3-specific probe was created by amplification of an internal fragment of the CdTEF3 gene with the primer pair TEF3F-TEF3R (Table 2). Signals from Northern blot autoradiograms were quantified by scanning densitometry and normalized for loading against the CdTEF3 expression signal with the Gelworks one-dimensional software package (UVP).
Targeted disruption of the CdCDR1 gene.
Targeted disruption of the CdCDR1 gene in C. dubliniensis CD57R was carried out by using the MPAR-flipper technique (34, 35). Two different deletion constructs were made to inactivate both alleles of the CdCDR1 gene. To create the first construct, the entire CdCDR1 ORF was cloned in the XbaI site of pUC19. A deletion was created in this clone by inverse PCR with the primer pair 5F-5R (Table 2; Fig. 1). This allowed amplification of the 5′ and 3′ ends of the CdCDR1 ORF and the entire plasmid vector (21). These primers contained SacII and XhoI restriction endonuclease recognition sequences that enabled the insertion of the SacII/XhoI fragment of pSFI1 (containing the site-specific recombinase FLP and the MPA resistance gene) (34, 35), thereby replacing the central region of the CdCDR1 ORF to create plasmid pCDRΔ1. To create the second construct, the internal AccI/BamHI fragment of CdCDR1 was cloned into pUC19 and subjected to inverse PCR with the primer pair 6F-6R (Table 2; Fig. 1). This product was also ligated with the SacII/XhoI fragment from pSFI1 to create the plasmid pCDRΔ2. C. dubliniensis was sequentially transformed by using approximately 1 μg of the linear SacI/SphI fragments from plasmids pCDRΔ1 and pCDRΔ2 by electroporation as described by Staib et al. (27). MPA-resistant transformants were selected on minimal medium agar plates containing 10, 15, or 20 μg of MPA per ml.
Isolation of genomic DNA and Southern blotting.
Genomic DNA from C. dubliniensis isolates and derivatives for use in PCR and Southern blotting experiments was isolated by using the method of Sullivan et al. (28). For Southern blotting, 10 μg of genomic DNA was digested with the appropriate restriction endonuclease and separated on 0.8% (wt/vol) agarose gels. DNA was transferred to nylon membranes (Osmonics) by capillary transfer overnight. Hybridization was carried out under high-stringency conditions by standard techniques with DNA probes labeled with α-32P by random primer labeling (28).
RESULTS
Isolation and sequence analysis of CdCDR1.
In order to isolate the C. dubliniensis homologue of the C. albicans CDR1 gene, a 6,848-bp region of C. dubliniensis chromosomal DNA was PCR amplified by using a high-fidelity polymerase mixture from genomic DNA isolated from the C. dubliniensis type strain CD36 (CBS 7987). Initially, the primer pair 1F-1R (Table 2; Fig. 1), which was designed to amplify the entire C. albicans CDR1 ORF, was used to amplify a 4,506-bp region of C. dubliniensis genomic DNA corresponding to the putative C. dubliniensis CDR1 homologue. The nucleotide sequences 5′ and 3′ to this region were then amplified from C. dubliniensis CD36 genomic DNA by an inverse PCR method with the primer pairs 3F-3R and 4F-4R (Table 2; Fig. 1), respectively. Using this strategy, a 614-bp region upstream of the putative CdCDR1 ORF and a 1,728-bp downstream region were amplified from C. dubliniensis CD36 chromosomal DNA. The entire amplified chromosomal DNA region from strain CD36 was then sequenced on both strands. The putative CdCDR1 ORF amplified with the primer pair CDR1F-CDR1R was 4,506 bp in length and was 92% identical at the nucleotide sequence level to the C. albicans CDR1 ORF. The 614-bp 5′-flanking region of this sequence shared 46.6% identity at the nucleotide sequence level with the C. albicans CDR1 promoter and contains a putative TATA box at nucleotide position −126 (the first base of the ATG start codon was designated +1). In addition, the C. dubliniensis promoter sequence contains a motif at the nucleotide coordinates −371 to −350 (5′-CGGTTATCGGATATTTTTTTT) matching the drug response element (DRE) in C. albicans (5). The 3′-flanking region of the putative CdCDR1 ORF also contained sequences homologous to the C. albicans SAP3 gene encoding a member of the secreted aspartyl-proteinase family (33), which we have termed CdSAP3. This is the same gene order as observed in the C. albicans genome (26). However, comparison of the C. albicans and C. dubliniensis CDR1 ORFs revealed that the C. dubliniensis CD36 CdCDR1 gene contained a TAG translation stop codon at the nucleotide coordinates +2266 to +2268 (codon 756) which corresponded to a single base difference (G to T) that converted the tyrosine (Y756)-encoding TAT codon found in the C. albicans CDR1 gene to a TAG translation stop signal (Fig. 1). The predicted polypeptide encoded by this shorter ORF was 755 amino acids in length and had a predicted molecular mass of 85 kDa; the C. albicans protein is 1,501 amino acids in length and has a predicted molecular mass of 168 kDa. The truncated CdCdr1p was 96.7% identical to the corresponding amino acid sequences of CaCdr1p. In order to confirm that this substitution was not a PCR artifact, this region was sequenced in six clones generated in six separate PCR amplifications and an identical nucleotide sequence was found in each clone. These data strongly suggested that the substitution was present in both alleles of CdCDR1.
Identification of polymorphic alleles of CdCDR1.
In order to determine whether other strains of C. dubliniensis harbored the TAG nonsense codon, the region of the CdCDR1 ORF containing codon 756 was sequenced in C. dubliniensis isolates CM1, CM2, CD57, CD51-II, and CD47-IIb (Table 1). In order to achieve this, a 490-bp region of the CdCDR1 ORF was amplified by PCR with the primer pair 2F-2R (Table 2; Fig. 1). This amplified product encompassed the nucleotide region +2071 to +2557 of the CdCDR1 ORF, including the TAG codon at the nucleotide coordinates +2266 to +2268 in C. dubliniensis strain CD36. Nucleotide sequence analysis of this region demonstrated that C. dubliniensis isolates CD51-II and CD47-IIb harbor a nonsense mutation identical to that found in the CdCDR1 ORF of isolate CD36. However, isolates CD57, CM1, and CM2 contained a tyrosine codon identical to that found in the C. albicans CDR1 ORF.
The entire nucleotide sequence of the CdCDR1 gene from C. dubliniensis CD57 was determined in order to compare the sequence of a gene without the nonsense mutation with that from strain CD36. The nucleotide sequence of the C. dubliniensis CD57 CdCDR1 ORF was 99.7% identical (containing 9 base differences) to the CdCDR1 ORF from strain CD36. The predicted amino acid sequence of the CD57 CdCdr1p contained three amino acid substitutions (H60R, V173M, and S1264L) compared to the CD36 sequence. However, the predicted CdCDR1 ORF from strain CD57 was not interrupted by a premature stop codon at nucleotide positions +2266 to +2268 and was identical in size (4,506 bp) to the homologous C. albicans CDR1 gene (19). The nucleotide sequence of the CdCDR1 ORF from strain CD57 was 92% identical to the corresponding sequence of the C. albicans CDR1 gene. The CD57 CdCDR1 gene encodes a predicted polypeptide of 169.6 kDa that is 96.5% identical at the amino acid sequence level to the corresponding C. albicans sequence. This highly homologous sequence contained the typical features described in C. albicans Cdr1p, namely the conserved Walker A and Walker B motifs and an ATP binding motif in the N-terminal hydrophilic domain (19).
The tyrosine-encoding TAT at codon position 756 in isolates CM1, CM2, and CD57 was found to be located within the recognition sequence for the restriction endonuclease SspI (5′-AATATT). This SspI recognition sequence was absent in alleles from CD36, CD51-II, and CD47-IIb containing the TAG stop codon (5′-AATAGT). PCR products amplified from isolates CM1, CM2, and CD57 with the primer pair 2F-2R could be digested with the restriction endonuclease SspI to yield two distinct fragments of 200 and 290 bp, respectively, in agarose gels. However, SspI digestion of the amplified product from isolates CD36, CD51-II, and CD47-IIb, which contained the TAG nonsense codon, yielded a single band of approximately 500 bp in agarose gels, indicating that the SspI recognition sequence was absent. We utilized this restriction fragment length polymorphism as the basis of a screening assay to analyze a larger group of C. dubliniensis isolates for the presence or absence of the nonsense mutation. A group of 40 C. dubliniensis isolates (all isolates in Table 1, excluding derivatives of CD36, CD57, and CD51-II), including representative isolates from the four C. dubliniensis genotypes recently described by Gee et al. (8), were analyzed for the presence of the TAT codon by SspI digestion of PCR products generated from these isolates with the primer pair 2F-2R (Table 1). This analysis revealed that all of the isolates tested (n = 16) belonging to C. dubliniensis genotypes 2, 3, and 4 harbored CdCDR1 alleles that contained the TAT tyrosine codon, as SspI digestion of the 2F-2R-amplified region yielded two fragments in agarose gels (Table 1). However, analysis of 24 genotype 1 C. dubliniensis isolates revealed that 14 (58%) harbored CdCDR1 alleles that could not be digested by SspI at this region. DNA sequence analysis of the 2F-2R-amplified region from these 14 isolates revealed the presence of a TAG stop codon that disrupted the SspI recognition sequence (Table 1). These data confirm that the premature stop codon is present in both alleles of CdCDR1 in these isolates. Two of these isolates were found to harbor CdCDR1 alleles containing both the TAT and TAG codon sequences, indicating heterozygosity at this locus. In total, 35% (14 of 40) of the C. dubliniensis isolates tested were found to contain CdCDR1 alleles containing the TAG stop codon.
Isolation and sequence analysis of CdCDR2.
In order to isolate the C. dubliniensis homologue of CDR2, the primer pair 8F-8R was designed based on the nucleotide sequence of the C. albicans CDR2 gene (24). This primer pair was used to amplify the complete C. dubliniensis CdCDR2 gene from the genomic DNA of strain CD36. The amplified CdCDR2 ORF was 4,503 bp in length, which is 3 bp longer than the C. albicans CDR2 ORF due to the presence of an additional codon. The C. dubliniensis CdCDR2 gene shared 91% identity with the C. albicans CDR2 ORF at the nucleotide sequence level. This ORF was preceded at the 5′ end by a TATA box at position −99 (the first base of the ATG start codon was designated +1). Like CdCDR1, the C. dubliniensis CdCDR2 gene was preceded by a DRE motif at nucleotide coordinates −210 to −189. The C. dubliniensis CdCDR2 ORF encodes a protein with a predicted molecular mass of 168.9 kDa that is 94.4% identical at the amino acid sequence level to the corresponding C. albicans protein. This sequence contained identical Walker A and Walker B motifs and an identical ATP binding motif to that described by Sanglard et al. in the C. albicans Cdr2p (24).
Western immunoblot analysis of CdCdr1p.
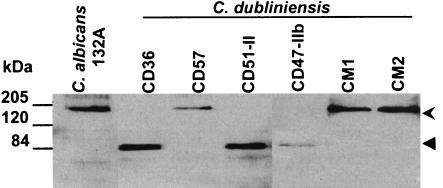
Crude protein extracts of C. dubliniensis were analyzed by Western immunoblotting with polyclonal sera raised against the N-terminal region of the C. albicans Cdr1p. In protein extracts from C. albicans CA132A and C. dubliniensis isolates CD41, CD57, CD72, CM1, and CM2 (Table 1), a protein of approximately 170 kDa was detected, matching the predicted size of C. albicans Cdr1p and the protein encoded by the full-length CdCDR1 ORF (Fig. 2). However, in protein extracts from C. dubliniensis isolates CD36, CD51-II, CD47-IIb, CO4, and P30, whose CdCDR1 ORFs were found by sequence analysis to contain a TAG translational stop signal at codon 756, anti-Cdr1p sera reacted with a smaller polypeptide of 85 kDa (Fig. 2). This smaller protein is identical in size to the truncated protein predicted from the CdCDR1 nucleotide sequence obtained from C. dubliniensis strain CD36.
FIG. 2.
Western immunoblot analysis of crude protein extracts from C. albicans CA132A and several C. dubliniensis isolates following SDS-PAGE. Western blots were screened with an anti-Cdr1p polyclonal serum (14). The uppermost arrow at the right of the figure indicates the position of full-length (170 kDa) Cdr1p, which is expressed by C. albicans CA132A and by C. dubliniensis CD57, CM1, and CM2, respectively. The lower arrow indicates the position of truncated (85 kDa) CdCdr1p, which is expressed by CD36, CD51-II, and CD47-IIb, respectively.
Functional analysis of the CdCDR1 and CdCDR2 genes.
The CdCDR1 and CdCDR2 ORFs were amplified from genomic DNA recovered from C. dubliniensis isolates CD36, CD57, CD51-II, CD47-IIb, CM1, and CM2 and C. albicans strain CA132A (Table 1). Amplicons were cloned in the S. cerevisiae GAL1 expression vector pYES (15) and were transformed into the azole-susceptible Δpdr5 S. cerevisiae strain YKKB-13 (24). Transformants were cultured on minimal agar medium containing 2% (wt/vol) galactose as the sole carbon source in order to induce expression of the cloned CDR genes. Northern blot analysis of total RNA from galactose-grown transformants revealed high levels of expression of the mRNAs for CdCDR1 and CdCDR2 in the respective transformants (data not shown). Transformants were tested for susceptibility to azole antifungal drugs and metabolic inhibitors known to be substrates for C. albicans Cdr1p and Cdr2p (19, 24) (Fig. 3). Cloned CDR1 amplicons from C. albicans CA132A and from the C. dubliniensis isolates CM1, CM2, and CD57 were found to confer resistance to fluconazole and itraconazole and to the metabolic inhibitors rhodamine 6G, cycloheximide, brefeldin A, crystal violet, and cerulenin in S. cerevisiae YKKB-13 (Table 3; Fig. 3A). However, CdCDR1 amplicons from C. dubliniensis isolates CD36, CD51-II, and CD47-IIb, which encoded the truncated CdCdr1p, did not confer resistance to any of these compounds in S. cerevisiae YKKB-13 (Fig. 3A), despite the fact that high levels of CdCDR1 mRNA could be detected in these transformants by Northern analysis (data not shown). For each of these isolates, at least six separate CdCDR1-encoding amplicons cloned from separate PCRs were analyzed and identical phenotypes were obtained from each clone.
TABLE 3.
Susceptibility of S. cerevisiae YKKB-13 CdCDR1 transformants to antifungal drugs and metabolic inhibitors determined by broth microdilution
| Transformanta | MIC (μg/ml) ofb:
|
||||||
|---|---|---|---|---|---|---|---|
| FLU | ITRA | CYCL | CRYV | BREF | CER | R6G | |
| Vector (pYES) | 0.5 | <0.03 | 0.015 | 0.25 | 64 | 0.06 | 2 |
| Y132A | 64 | 1 | 0.5 | 16 | 256 | 2 | 32 |
| YCD36c | 0.5 | <0.03 | 0.015 | 0.25 | 64 | 0.06 | 2 |
| YCD51-IIc | 0.5 | <0.03 | 0.015 | 0.25 | 64 | 0.06 | 2 |
| YCD57 | 64 | 1 | 0.5 | 16 | 256 | 2 | 32 |
| YCM1 | 64 | 1 | 0.5 | 16 | 256 | 2 | 32 |
Transformants harbor cloned CDR1 from C. albicans CA132A (Y132A), and cloned CdCDR1 genes from the C. dubliniensis isolates CD36 (YCD36), CD51-II (YCD51-II), CD57 (YCD57), and CM1 (YCM1).
FLU, fluconazole; ITRA, itraconazole; CYCL, cycloheximide; CRYV, crystal violet; BRET, brefeldin A; CER, cerulenin; R6G, rhodamine 6G.
The CdCDR1 genes from C. dubliniensis isolates CD36 and CD51-II harbored by these transformants contain a TAG at codon 756.
In order to unequivocally determine if the premature translational stop signal at codon 756 of the CdCDR1 gene was responsible for the loss of function of the heterologously expressed protein in S. cerevisiae, we carried out site-directed mutagenesis to restore the tyrosine-encoding TAT codon at this position in the cloned C. dubliniensis CD36 CdCDR1 gene. Reassignment of this codon fully restored the ability of the expressed protein to impart a drug resistance phenotype in S. cerevisiae YKKB-13 compared to that of the truncated C. dubliniensis CdCdr1p (Fig. 3B).
The susceptibility of the CdCDR2-harboring transformants to fluconazole and itraconazole was also determined. When compared to S. cerevisiae harboring the plasmid vector alone (fluconazole MIC, 0.5 μg/ml; itraconazole MIC, <0.03 μg/ml), transformants harboring the C. albicans CDR2 gene or the C. dubliniensis CdCDR2 gene were resistant to fluconazole (MIC, 32 to 64 μg/ml) and itraconazole (MIC, 0.5 to 1 μg/ml).
Analysis of CdCDR1 and CdCDR2 mRNA expression levels in C. dubliniensis.
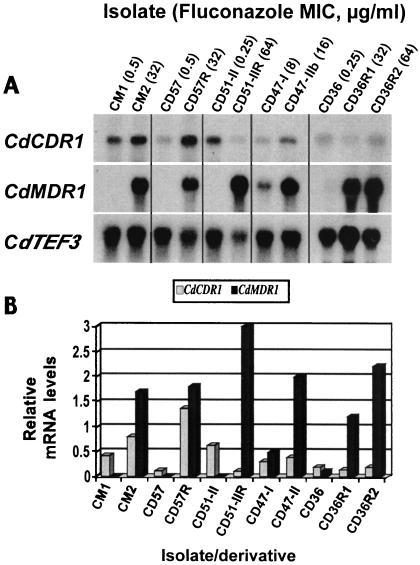
Total RNA was isolated from C. dubliniensis isolates and in vitro-generated derivatives with reduced susceptibility to fluconazole in order to ascertain the expression levels of CdCDR1 and CdCDR2 mRNA in these organisms (Fig. 4). As reported by Moran et al. (13), all clinical isolates and in vitro-generated derivatives of C. dubliniensis with reduced susceptibility to fluconazole displayed high levels of CdMDR1 mRNA compared to matched fluconazole-susceptible isolates (Fig. 4A). CdMDR1 hybridization signals in the resistant organisms CM2, CD57R, and CD51-II were 50- to 100-fold higher than those observed in matched fluconazole-susceptible isolates. Hybridization of these RNAs with a probe specific for the CdCDR1 gene (homologous to the region from +1 to +421 of the CdCDR1 ORF) revealed that several of these isolates and derivatives with reduced susceptibility to fluconazole also displayed comparatively minor increases in levels of CdCDR1 mRNA expression relative to matched fluconazole-susceptible isolates (Fig. 4B). The oral clinical isolate CM2 (fluconazole MIC, 32 μg/ml) displayed a twofold-higher level of CdCDR1 mRNA compared to the matched fluconazole-susceptible isolate CM1 (fluconazole MIC, 0.5 μg/ml) recovered from the same patient. Isolates CM1 and CM2 both express the full-length CdCdr1p (Fig. 2). Also, the in vitro-generated derivative CD57R (fluconazole MIC, 32 μg/ml), which also expresses the full-length CdCdr1p, displayed a ninefold-higher level of CdCDR1 mRNA than the fluconazole-susceptible parental isolate CD57. Interestingly, the oral clinical isolate CD47-IIb (fluconazole MIC, 16 μg/ml), which was found to express the nonfunctional truncated CdCdr1p, expressed a twofold-higher level of CdCDR1 mRNA than the matched clinical isolate CD47-I (fluconazole MIC, 8 μg/ml). However, in vitro-generated derivatives of the fluconazole-susceptible C. dubliniensis isolates CD51-II and CD36 (fluconazole MIC, 0.25 μg/ml), which displayed reduced susceptibility to fluconazole (fluconazole MICs were 32 to 64 μg/ml for CD51-IIR, CD36R1, and CD36R2), displayed levels of CdCDR1 mRNA that were similar to or reduced in comparison to their respective fluconazole-susceptible parental isolates (Fig. 4B). Both CD36 and CD51-II were shown to express the truncated 85-kDa CdCdr1p (Fig. 2).
FIG. 4.
Northern blot showing expression levels of CdCDR1, CdMDR1, and CdTEF3 mRNAs in matched pairs of C. dubliniensis clinical isolates and in vitro-generated derivatives exhibiting reduced susceptibility to fluconazole. (A) Total RNA was extracted from C. dubliniensis isolates and derivatives grown to the mid-exponential phase in YEPD broth cultures and analyzed by Northern hybridization analysis with [α-32P]dATP-labeled DNA probes homologous to CdCDR1, CdMDR1, and the constitutively expressed internal control CdTEF3 gene (see Materials and Methods). (B) Graphical representation of CdCDR1 and CdMDR1 mRNA expression levels. Hybridization signals were analyzed by scanning densitometry and normalized against levels of CdTEF3 expression.
Total RNA from these isolates and derivatives was also hybridized with sequences specific for CdCDR2 (homologous to the region from +1 to +418 of the CdCDR2 ORF). However, expression of CdCDR2 mRNA was not detected in any of the isolates or derivatives tested.
Targeted disruption of CdCDR1.
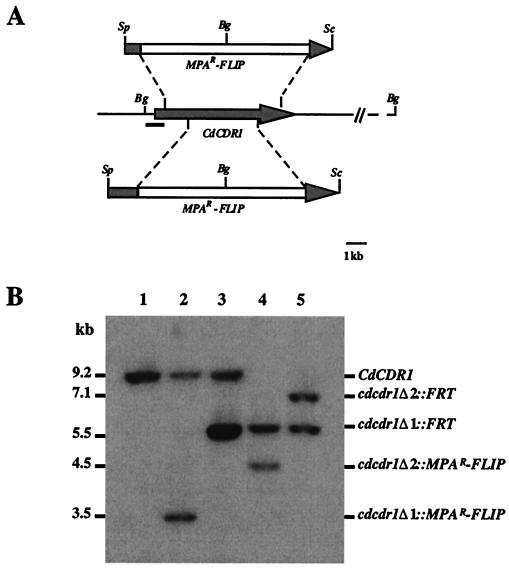
In order to ascertain the role of the CdCDR1 gene in determining susceptibility to azole antifungal drugs in C. dubliniensis, we disrupted both copies of the CdCDR1 gene in a C. dubliniensis strain by using the MPAR-flipper technique described by Wirsching et al. (34, 35). These experiments were carried out in an in vitro-generated derivative of the clinical isolate CD57 (fluconazole MIC, 0.5 μg/ml), termed CD57R, which exhibits reduced susceptibility to fluconazole (fluconazole MIC, 32 μg/ml). CD57R was originally generated by successive subculture of the fluconazole-susceptible isolate CD57 on fluconazole-containing agar medium (13). CD57R expresses the full-length CdCdr1p and showed a ninefold increase in CdCDR1 mRNA expression levels relative to a matched fluconazole-susceptible isolate (CD57) (Fig. 4B) and also exhibits increased CdCdr1p levels in Western immunoblots (data not shown). In addition, like all C. dubliniensis isolates with reduced susceptibility to fluconazole, CD57R exhibits an approximately 88-fold increase in expression of CdMDR1 mRNA (Fig. 4), and this isolate was chosen in order to determine the influence of a CdCDR1 gene disruption on fluconazole susceptibility in this genetic background. For disruption of the CdCDR1 gene, a cassette (MPAR-flipper) containing the MPA resistance gene and the FLP site-specific recombinase fused to the inducible C. albicans SAP2 promoter (27), was inserted into the CdCDR1 ORF to create plasmids pCDRΔ1 and pCDRΔ2, thereby deleting the regions from nucleotide coordinates +538 to +4248 and +1468 to +3532, respectively (Fig. 5A). C. dubliniensis CD57R was first transformed with the insert from pCDRΔ1, and MPA-resistant transformants were analyzed by Southern blotting. In strain CD57R (CdCDR1/CdCDR1), a BglII DNA fragment of 9.2 kb hybridized with the CdCDR1 probe (Fig. 5B). Insertion of the fragment from pCDRΔ1 into one of the CdCDR1 alleles generated a new BglII fragment of 3.5 kb in strain 57RM1 (CdCDR1/cdcdr1Δ::MPAR-FLIP) due to the presence of a BglII site in the MPAR-flipper. Deletion of the cassette by FLP-mediated recombination resulted in the creation of a new 5.5-kb fragment, 3.7 kb smaller than the fragment in the wild-type strain CD57R; this strain was designated 57RM2 (CdCDR1/cdcdr1Δ::FRT). The insert from pCDRΔ2 (Fig. 5A) was then used in a second round of transformation in order to create a deletion in the second CdCDR1 allele in strain 57RM3 (cdcdr1Δ::FRT/cdcdr1Δ::MPAR-FLIP). The MPAR-flipper was excised again from this transformant to produce a fragment of 7.2 kb in strain 57RM4 (cdcdr1Δ::FRT/cdcdr1Δ::FRT).
FIG. 5.
Inactivation of the CdCDR1 gene by the MPAR-flipper procedure. (A) Restriction map of the CdCDR1 locus from strain CD57R and allelic replacements with the inserts from pΔCDR1 (top) and pΔCDR2 (bottom). The shaded arrow in the central portion of the figure represents the CdCDR1 coding region; solid lines represent the CdCDR1 flanking sequences. The open box represents the MPAR-flipper cassette. Restriction endonuclease cleavage sites: Bg, BglII; Sc, SacI; Sp, SphI. The solid bar represents the region which was used as a probe in Southern hybridization experiments. (B) Southern hybridization of BglII-digested genomic DNA from C. dubliniensis CD57R and mutant derivatives with the 5′ CdCDR1 probe indicated in panel A. The molecular sizes of the fragments are shown in kilobases to the left of the blot, and the identities of the fragments are shown to the right. Lane 1, CD57R (CdCDR1/CdCDR1); lane 2, 57RM1 (CdCDR1/cdcdr1Δ::MPAR-FLIP); lane 3, 57RM2 (CdCDR1/cdcdr1Δ::FRT); lane 4, 57RM3 (cdcdr1Δ::FRT/cdcdr1Δ::MPAR-FLIP); lane 5, 57RM4 (cdcdr1Δ::FRT/cdcdr1Δ::FRT).
Disruption of CdCDR1 affects itraconazole and ketoconazole susceptibility.
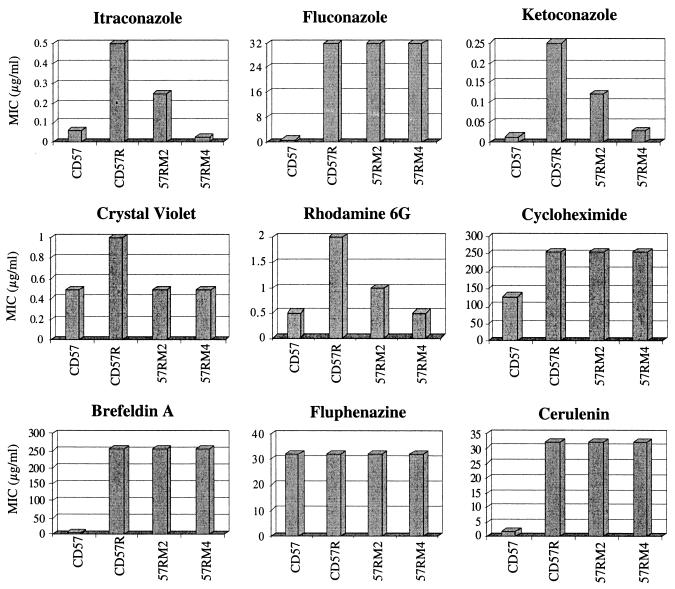
Heterologous expression of CdCDR1 in S. cerevisiae results in resistance to the azole antifungal drugs fluconazole, itraconazole, and ketoconazole. In order to assess the contribution of the CdCDR1 gene towards resistance to these agents in C. dubliniensis, we compared the azole susceptibility of the cdr1-null mutant strain 57RM4 with that of its azole-resistant parental strain CD57R. Broth dilution susceptibility tests were carried out in RPMI 1640 medium against azole drugs for the azole-susceptible clinical isolate CD57, its in vitro-generated fluconazole-resistant derivative CD57R, and the cdr1 disruptants of CD57R, 57RM2 (CdCDR1/cdcdr1Δ::FRT) and 57RM4 (cdcdr1Δ::FRT/cdcdr1Δ::FRT). Disruption of both copies of CdCDR1 in 57RM4 resulted in an eightfold increase in susceptibility to both itraconazole (change in MIC from 0.5 to 0.06 μg/ml) and ketoconazole (change in MIC from 0.25 to 0.03 μg/ml) (Fig. 6). The ketoconazole and itraconazole MICs for the double cdr1 disruptant (57RM4) and CD57, the azole susceptible parental isolate of CD57R, were similar, indicating that expression of the CdCDR1 gene was responsible for the reduction in susceptibility to these drugs in CD57R. However, this disruption did not affect susceptibility to fluconazole (Fig. 6). As fluconazole, unlike ketoconazole and itraconazole, is also a substrate for the CdMDR1 multidrug transporter, we hypothesized that the very high levels of expression of the CdMDR1 transporter in CD57R may mask any effect a deletion of CdCDR1 may have on fluconazole susceptibility. In order to determine if CdMDR1 could compensate for the cdr1 disruption in the presence of other drugs, we analyzed the susceptibility of the cdr1 null mutant to a range of other metabolic inhibitors. We did not observe any change in the MICs of other drugs that are substrates for both CdCdr1p and CdMdr1p in the cdr1 disruptant strain 57RM4, including brefeldin A, cerulenin, and cycloheximide (Fig. 6). However, we observed increased susceptibility to crystal violet and rhodamine 6G in strain 57RM4, two drugs that are substrates for CdCdr1p but are not transported by CdMdr1p (Fig. 6). In addition, we did not observe any differences in fluphenazine susceptibility among the isolates and derivatives tested (Fig. 6), indicating that this drug is not a substrate for CdCdr1p.
FIG. 6.
Susceptibility of C. dubliniensis strain CD57, its fluconazole-resistant derivative CD57R, and CdCDR1 disruptants of CD57R (57RM2 [CdCDR1/cdcdr1Δ::FRT] and 57RM4 [cdcdr1Δ::FRT/cdcdr1Δ::FRT]) to fluconazole, itraconazole, ketoconazole, and metabolic inhibitors. MICs were determined by broth microdilution susceptibility testing in RPMI 1640 supplemented with 2% (wt/vol) glucose.
DISCUSSION
The close relatedness of C. dubliniensis to C. albicans and the ease with which fluconazole-resistant derivatives of susceptible C. dubliniensis clinical isolates can be generated in vitro make C. dubliniensis a useful organism to study the development of fluconazole resistance in Candida species (13, 14). We and others have isolated C. dubliniensis isolates exhibiting fluconazole resistance from the oral cavities of HIV-infected patients, making the investigation of the molecular basis of resistance in this organism worthy of investigation in its own right (10, 14, 20). Recently, molecular genetic techniques, including targeted gene disruption, have been adapted for use in C. dubliniensis and will greatly aid in the dissection of this organism's biology (27). Wirsching et al. recently used targeted gene disruption to demonstrate the importance of the major facilitator CdMdr1p in the development of fluconazole resistance in C. dubliniensis (35). CdMDR1 mRNA is invariably overexpressed in C. dubliniensis strains exhibiting reduced susceptibility to fluconazole, and deletion of this gene in a fluconazole-resistant C. dubliniensis isolate rendered the isolate susceptible to fluconazole. In the present study we investigated the role of the second multidrug transporter, CdCdr1p, in fluconazole resistance in C. dubliniensis. In C. albicans, overexpression of Cdr1p is the most commonly reported mechanism of resistance to azole antifungal drugs (16, 25). Because of this apparent divergence in fluconazole resistance mechanisms in C. albicans and C. dubliniensis, we decided to investigate in detail the role played by CdCDR1 in fluconazole resistance in C. dubliniensis.
Sequence analysis of the C. dubliniensis CdCDR1 and CdCDR2 genes reveals that these genes are highly homologous (>90% identity at the nucleotide sequence level) to their C. albicans counterparts (19, 24). The promoter regions of the C. dubliniensis genes were also found to contain motifs similar to that of the DRE in C. albicans described by de Micheli et al., indicating that expression of these genes may be regulated by similar mechanisms in C. dubliniensis (5). However, 35% (14 of 40) of C. dubliniensis isolates examined in the present study were found to contain a nonsense mutation approximately midway through the CdCDR1 ORF. This mutation introduces a premature translation termination signal in the ORF which leads to the translation of a truncated 85-kDa protein (rather than the full-length protein of 169.6 kDa), which we detected in Western immunoblots with an anti-Cdr1p antiserum. We demonstrated that the full-length CdCdr1p and CdCdr2p proteins are functionally equivalent to their C. albicans counterparts, as when they are heterologously expressed in a S. cerevisiae background they render the host strain less susceptible to a similar range of drugs and metabolic inhibitors. However, expression of CdCDR1 ORFs containing the nonsense mutation in S. cerevisiae did not render the host strain less susceptible to any of the drugs tested, indicating that the truncated CdCdr1p is not capable of mediating a multidrug resistance phenotype. Since the C. dubliniensis isolates harboring the nonsense codon containing CdCDR1 ORFs were obtained from clinical sources, this finding suggests that a fully functional, full-length CdCdr1p is not essential for the normal growth of C. dubliniensis in vivo. Furthermore, all of the C. dubliniensis isolates containing this nonsense mutation were found to belong to a group of closely related strains recently identified as C. dubliniensis genotype 1, as described in the epidemiological study of Gee et al. (8). In the study by Gee et al. (8), four distinct genotypes of C. dubliniensis were identified based on DNA fingerprint analysis with the C. dubliniensis-specific probe Cd25 and by sequence analysis of the internal transcribed spacer regions of the rRNA genes. Gee et al. found that genotype 1 isolates predominated in an analysis of 98 C. dubliniensis isolates. Genotype 1 isolates are mainly recovered from HIV-infected patients, and DNA fingerprint analysis with the C. dubliniensis-specific Cd25 probe indicates that they are a closely related group of organisms (mean similarity coefficient value of 0.80 ± 0.06) despite being recovered from disparate geographic areas, indicating that these represent a more recent, and therefore a more homogenous subgroup, of C. dubliniensis that has become predominant worldwide (8). The most likely explanation for why the CdCDR1 TAG mutation is unique to the C. dubliniensis genotype 1 population, is that it probably appeared in this subgroup after these organisms separated from the other C. dubliniensis genotypes and that it has subsequently spread throughout the human population worldwide. In addition, several of the clinical isolates in which the nonsense mutation was identified in the present study were originally recovered from patients with oral candidiasis who had received fluconazole therapy, indicating that the presence of a truncated CdCdr1p does not appear to adversely affect the ability of these organisms to cause disease. In order to investigate whether the nonsense mutation might have any effect on virulence or on strain selection in patients treated with fluconazole, we propose to conduct growth competition experiments comparing the fitness of strains with and without the CdCDR1 mutation by using in vivo models with and without fluconazole. Of greater interest is the fact that two of these isolates (CD36 and CD51-II) were capable of yielding fluconazole-resistant derivatives (MIC, 64 μg/ml) upon exposure to fluconazole in vitro, indicating that the full-length CdCdr1p is not essential for the development of fluconazole resistance. Whether the truncated CdCdr1p protein could carry out some alternative cellular function other than drug efflux is as yet unknown.
We have shown that some C. dubliniensis isolates and derivatives exhibiting reduced susceptibility to fluconazole that possess the full-length CdCDR1 ORF exhibit increased transcription of this gene (e.g., CM2 and CD57R) (Fig. 4). We have also observed that fluconazole-resistant derivatives of C. dubliniensis isolates possessing the CdCDR1 ORF containing the nonsense mutation often do not exhibit increases in CdCDR1 mRNA expression (e.g., CD36R1 and CD36R2) (Fig. 4), and in some cases even exhibit decreased transcription of this gene (e.g., CD51-IIR) (Fig. 4). CdCDR2 expression has not been detected in vitro in any of the C. dubliniensis isolates tested to date, indicating that this gene is stringently repressed.
In order to further investigate the role of functional alleles of CdCDR1 in the development of fluconazole resistance in C. dubliniensis, we carried out targeted disruption of both alleles of this gene in a derivative of the C. dubliniensis clinical isolate CD57, termed CD57R, which exhibited reduced susceptibility to fluconazole. As CD57R displays a ninefold-higher level of CdCDR1 mRNA expression (in addition to overexpression of CdMDR1), we anticipated that if CdCDR1 is significantly involved in the fluconazole resistance phenotype that its disruption would affect susceptibility to all of the azole antifungal drugs, including fluconazole. We found the double cdr1 disruptant 57RM4 showed increased susceptibility to itraconazole and ketoconazole, and the MICs of these drugs for 57RM4 were similar to those observed for the clinical isolate CD57, the azole-susceptible parent of CD57R, indicating that CdCDR1 overexpression was the main mediator of reduced susceptibility to these drugs in CD57R (Fig. 6). This phenotype was expected, as both ketoconazole and itraconazole are transported by the heterologously expressed gene in S. cerevisiae (Fig. 3). In addition, the double cdr1 disruptant 57RM4 displayed increased susceptibility to rhodamine 6G and crystal violet (Fig. 6), two metabolic inhibitors which are also transported by CdCdr1p in S. cerevisiae (Fig. 3). However, the double cdr1 disruption did not affect fluconazole susceptibility in 57RM4 when compared to its parental strain CD57R (Fig. 6). However, as the cdr1 mutant also displayed increased levels of CdMDR1 mRNA, which encodes a transporter capable of fluconazole efflux, it is perhaps not surprising that fluconazole susceptibility was not affected in this mutant (13). It is likely that such high levels of CdMDR1 expression are capable of mediating the fluconazole resistance phenotype alone and may mask the effect of the cdr1 disruption on fluconazole susceptibility. The cdr1 mutant was also unaffected in susceptibility to cycloheximide, cerulenin, and brefeldin A (Fig. 3), three compounds which, like fluconazole, are transported by both CdCdr1p and CdMdr1p (13). The findings presented here, which implicate CdMDR1 gene expression as the most important resistance mechanism to fluconazole and other metabolic inhibitors in C. dubliniensis is supported by the findings of Wirsching et al. (35). In that study, Wirsching et al. demonstrated that disruption of the CdMDR1 gene in the C. dubliniensis clinical isolate CM2, which exhibits increased expression of CdMDR1 mRNA and also exhibits a twofold increase in CdCDR1 mRNA expression (Fig. 4), was sufficient to render the null mutant susceptible to fluconazole (35). The ketoconazole MICs for the cdmdr1-disrupted strain were still elevated, probably due to the elevated CdCDR1 expression levels seen in this isolate.
In the present study, increased expression of a functional CdCDR1 gene in the C. dubliniensis derivative CD57R mediated an eightfold increase in ketoconazole and itraconazole MICs but did not affect the susceptibility of the isolate to fluconazole (Fig. 6). Previously, many investigators have suggested that multiple efflux mechanisms may contribute to fluconazole resistance in a single isolate (6, 16, 31). It has been suggested that simultaneous activation of multiple fluconazole efflux mechanisms in a single cell would lead to an increased rate of fluconazole efflux and therefore increased resistance to this compound. However, the present study demonstrates that the effects of MDR1 and CDR1 overexpression on fluconazole susceptibility in a single strain are not necessarily additive. It is not clear from these data why the Cdr1p and Mdr1p transporters do not have a cumulative effect on fluconazole susceptibility when expressed in concert. Perhaps there is a threshold level of efflux activity, above which the activation of further efflux proteins has no further effect on fluconazole susceptibility. Alternatively, there may be competition between CdCdr1p and CdMdr1p for available substrate (i.e., fluconazole). In this latter scenario, if one transporter has a higher affinity for fluconazole (perhaps in this case CdMdr1p), it may sequester all of the available substrate, rendering transporters with lower affinity for fluconazole redundant. The precise contribution of CdMDR1 overexpression in fluconazole resistance in specific strains (e.g., CD57RM4 [cdcdr1Δ::FRT/cdcdr1Δ::FRT]) will be determined by inactivating the CdMDR1 gene and determining the effect of this mutation on fluconazole susceptibility.
At present, it is not possible for us to analyze the role of CdCDR1 as a sole mechanism of fluconazole resistance in C. dubliniensis as we have not encountered any CdCDR1-overexpressing fluconazole-resistant clinical isolates or in vitro-generated derivatives which do not also coexpress the CdMDR1 gene to date. In C. albicans however, several investigators have described isolates in which CDR1 is the sole efflux pump activated, indicating that CDR1-type efflux pumps are capable of mediating fluconazole resistance in this species (16, 25). However, coactivation of Mdr1p- and Cdr1p-type pumps has been described in fluconazole-resistant isolates of C. albicans and Candida glabrata and conceivably in these species, as in C. dubliniensis, the effects of both pumps on the resistance phenotype may not be additive (6, 16, 23, 25, 31). The data presented here indicate that the roles of individual transporters in the fluconazole resistance phenotypes observed in C. albicans and C. glabrata species will have to be dissected by targeted disruption of the individual genes in question.
Acknowledgments
This study was supported by the Irish Health Research Board (grant no. 04-99).
We thank Dominique Sanglard for the gift of Cdr1p antiserum and for advice on Western immunoblot analysis.
REFERENCES
- 1.Berrouane, Y. F., R. J. Hollis, and M. A. Pfaller. 1996. Strain variation among and antifungal susceptibilities of isolates of Candida krusei. J. Clin. Microbiol. 34:1856-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle, B. M., D. J. Sullivan, C. Forkin, F. Mulcahy, C. T. Keane, and D. C. Coleman. 2002. Candida dubliniensis candidaemia in an HIV-positive patient in Ireland. Int. J. STD AIDS 13:55-57. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, M. E., L. H. Harrison, M. Pass, A. N. Sofair, S. Huie, R. K. Li, C. J. Morrison, D. W. Warnock, and R. A. Hajjeh. 2000. Candida dubliniensis fungemia: the first four cases in North America. Emerg. Infect. Dis. 6:46-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman, D. C., D. J. Sullivan, D. E. Bennett, G. P. Moran, H. J. Barry, and D. B. Shanley. 1997. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS 11:557-567. [DOI] [PubMed] [Google Scholar]
- 5.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC-transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 6.Franz, R., S. Kelly, D. Lamb, D. Kelly, M. Ruhnke, and J. Morschhäuser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher, P. J., D. E. Bennett, M. C. Henman, R. J. Russell, S. R. Flint, D. B. Shanley, and D. C. Coleman. 1992. Reduced azole susceptibility of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J. Gen. Microbiol. 138:1901-1911. [DOI] [PubMed] [Google Scholar]
- 8.Gee, S. F., S. Joly, D. R. Soll, J. F. G. M. Meis, P. E. Verweij, I. Polacheck, D. J. Sullivan, and D. C. Coleman. 2002. Identification of four distinct genotypes of Candida dubliniensis and detection of microevolution in vitro and in vivo. J. Clin. Microbiol. 40:556-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, E. M., D. W. Warnock, J. Luker, S. R. Porter, and C. Scully. 1995. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J. Antimicrob. Chemother. 35:103-114. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick, W. R., S. G. Revankar, R. K. McAtee, J. L. Lopez-Ribot, A. W. Fothergill, D. I. McCarthy, S. E. Sanche, R. A. Cantu, M. G. Rinaldi, and T. F. Patterson. 1998. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar candida screening and susceptibility testing of isolates. J. Clin. Microbiol. 36:3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maenza, J. R., W. G. Merz, M. J. Romagnoli, J. C. Keruly, R. D. Moore, and J. E. Gallant. 1997. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin. Infect. Dis 24:28-34. [DOI] [PubMed] [Google Scholar]
- 12.Meis, J. F., M. Ruhnke, B. E. De Pauw, F. C. Odds, W. Siegert, and P. E. Verweij. 1999. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg. Infect. Dis. 5:150-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran, G. P., D. Sanglard, S. M. Donnelly, D. B. Shanley, D. J. Sullivan, and D. C. Coleman. 1998. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob. Agents Chemother. 42:1819-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran, G. P., D. J. Sullivan, M. C. Henman, C. E. McCreary, B. J. Harrington, D. B. Shanley, and D. C. Coleman. 1997. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob. Agents Chemother. 41:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulligan, J. T., and S. J. Elledge. 1994. The construction and use of cDNA libraries for genetic selections, p. 65-81. In J. R. Johnston (ed.), Molecular genetics of yeast: a practical approach, vol. 141. Oxford University Press, Oxford, United Kingdom.
- 16.Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martinez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 18.Polacheck, I., J. Strahilevitz, D. Sullivan, S. Donnelly, I. F. Salkin, and D. C. Coleman. 2000. Recovery of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Israel. J. Clin. Microbiol. 38:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad, R., P. De Wergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterisation of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 20.Ruhnke, M., A. Schmidt-Westhausen, and J. Morschhäuser. 2000. Development of simultaneous resistance to fluconazole in Candida albicans and Candida dubliniensis in patients with AIDS. J. Antimicrob. Chemother. 46:291-295. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Sangeorzan, J. A., S. F. Bradley, X. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonisation, infection, treatment, and emergence of fluconazole-resistance. Am. J. Med. 97:339-346. [DOI] [PubMed] [Google Scholar]
- 23.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterisation of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 25.Sanglard, D., K. Kuchler, F. Ischer, J.-L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhäuser. 2000. Differential activation of a Candida albicans virulence gene family during infection. Proc. Natl. Acad. Sci. USA 97:6102-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staib, P., G. P. Moran, D. J. Sullivan, D. C. Coleman, and J. Morschhäuser. 2001. Isogenic strain construction and gene targeting in Candida dubliniensis. J. Bacteriol. 183:2859-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan, D., D. Bennett, M. Henman, P. Harwood, S. Flint, F. Mulcahy, D. Shanley, and D. Coleman. 1993. Oligonucleotide fingerprinting of isolates of Candida species other than C. albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J. Clin. Microbiol. 31:2124-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan, D., and D. Coleman. 1998. Candida dubliniensis: characteristics and identification. J. Clin. Microbiol. 36:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 31.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White, T. C., S. H. Miyasaki, and N. Agabian. 1993. Three distinct secreted aspartyl proteinases in Candida albicans. J. Bacteriol. 175:6126-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirsching, S., S. Michel, and J. Morschhäuser. 2000. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 36:856-865. [DOI] [PubMed] [Google Scholar]
- 35.Wirsching, S., G. P. Moran, D. J. Sullivan, D. C. Coleman, and J. Morschhäuser. 2001. MDR1-mediated drug resistance in Candida dubliniensis. Antimicrob. Agents Chemother. 45:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]