Abstract
The efficacy and safety of intravenous (i.v.) ertapenem (1 g once a day) with the option to switch to an oral agent for treatment of adults with complicated urinary tract infections (UTIs) were compared with that of i.v. ceftriaxone (1 g daily) with the same oral switch option in a multicenter, double-blind, prospective, randomized study. At entry, 592 patients were assigned to one of two strata: acute pyelonephritis or other complicated UTI without acute pyelonephritis. After a minimum of 3 days, patients could be switched to an oral antimicrobial agent. A total of 159 patients in the ertapenem group and 171 patients in the ceftriaxone group were microbiologically evaluable. Approximately 95% of patients in each treatment group were switched to oral therapy. The most common pathogens were Escherichia coli and Klebsiella pneumoniae. At the primary efficacy endpoint 5 to 9 days after treatment, 91.8% of patients who received ertapenem and 93.0% of those who received ceftriaxone had a favorable microbiological response (95% confidence interval for the difference, adjusting for strata, −7.6 to 5.1%), indicating that outcomes in the two treatment groups were equivalent. Microbiological success rates for the two treatment groups were similar when compared by stratum and also by severity of infection. The frequency and severity of drug-related adverse events were generally similar in both treatment groups. In this study, ertapenem was as effective as ceftriaxone for the initial treatment of complicated UTIs in adults, was generally well tolerated, and had a similar overall safety profile.
Urinary tract infections (UTIs) are responsible for over 7 million physician office visits in the United States each year (14). Most of these are uncomplicated infections of the urinary bladder in otherwise healthy young women and are easily managed with short-term antimicrobial therapy. Treatment of complicated UTIs, however, is generally less successful. Ten to 14 days of therapy with an agent active against a more extensive list of gram-negative bacilli is recommended (14, 15). For serious infections, initial empirical therapy with a broad-spectrum parenteral antimicrobial agent followed by an oral agent to which the responsible uropathogen is susceptible has become standard therapy (3, 13-15). Follow-up urine cultures are important because bacterial eradication is more difficult to achieve, and recurrence several weeks posttherapy is not uncommon.
Ertapenem (formerly MK-0826; Merck & Co., Inc.) is a once a day parenteral β-lactam agent with excellent in vitro activity against many gram-positive and gram-negative aerobes and anaerobes generally associated with community-acquired infections, including most members of the family Enterobacteriaceae, pathogens most commonly responsible for UTIs (6, 9). Ertapenem is not indicated for Pseudomonas aeruginosa or enterococci, which are more often associated with nosocomial infections.
The objectives of this study were to compare the efficacy, tolerability, and safety of 1 g of ertapenem once a day with those of 1 g of ceftriaxone once a day, both followed by optional oral therapy, for the treatment of adult patients with complicated UTIs.
(These data were presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 17 December 2001.)
MATERIALS AND METHODS
Patients.
Patients aged ≥18 years with a complicated UTI (i.e., acute pyelonephritis, UTI in men, or UTI associated with obstruction, foreign bodies, or urologic abnormalities) were eligible for the study if they required initial parenteral antimicrobial therapy and if the infection was caused by a pathogen susceptible to the study drugs. Criteria for acute pyelonephritis included fever, flank pain or costovertebral angle tenderness, pyuria (≥10 white blood cells), and positive urine culture (≥105 CFU of a uropathogen/ml) within 48 h of enrollment. Criteria for other complicated UTIs were signs or symptoms of UTI, pyuria, and positive urine culture in a male; female patients were additionally required to have either an indwelling catheter, current bladder catheterization or instrumentation of the urinary tract, or functional or anatomical abnormality of the urinary tract.
Patients with any of the following were excluded from the study: pregnancy or lactation in women, history of serious allergy to study therapy (patients with a history of mild rash to β-lactams could be enrolled), complete obstruction of the urinary tract, perinephric or intrarenal abscess, prostatitis, any rapidly progressive disease, immune-compromising illness or therapy, the need for concomitant antimicrobials, acute hepatic failure, requirement for peritoneal dialysis or hemodialysis, treatment with a systemic antimicrobial agent for ≥24 h within 72 h prior to the baseline urine culture, creatinine clearance of <30 ml/min, aspartate aminotransferase or alanine aminotransferase levels of >6 times the upper limit of normal (ULN), bilirubin or alkaline phosphatase levels of >3 times the ULN, absolute neutrophil count of ≤1,000 per μl, platelet concentration of <75,000 per μl, hematocrit level of <25%, or coagulation tests of >1.5 times the ULN.
Study design and antimicrobial therapy.
This prospective, double-blind (with sponsor blinding), randomized, multicenter study was conducted from April 1998 to February 2000. Written consent was obtained from all patients, and the institutional review board at each participating site approved the protocol. Eligible patients from 31 centers in the United States, Central and South America, and Europe were stratified to pyelonephritis (with or without an abnormality of the urinary tract), or other complicated UTI without acute pyelonephritis. Equal randomization to one of the two treatment arms was accomplished by using a computer-generated random number allocation schedule.
Both ertapenem and ceftriaxone were given once a day as a 1-g intravenous (i.v.) dose infused over 30 min. To avoid unblinding due to slight color differences between the study drugs, patients also received a second color-matched saline placebo infusion. After at least 2 days of hospital or clinic-based infusion therapy, study therapy could be completed in the hospital, a clinic, or at home. Consistent with current pyelonephritis treatment guidelines (14), at the investigator's discretion and after a minimum of 3 days of i.v. therapy, patients could be switched to oral ciprofloxacin (500 mg twice daily) if they were afebrile; nausea and vomiting had resolved; signs, symptoms, and leukocytosis had improved; and a urine culture was obtained. Other oral agents were permitted if the patient could not tolerate ciprofloxacin or if the causal pathogen was resistant. The suggested total duration of i.v. plus optional oral therapy was 10 to 14 days.
Clinical assessments.
Patients were evaluated within 24 h of enrollment and daily thereafter while on parenteral study therapy. The clinical response was measured on day 3 to 5 of parenteral therapy, at the discontinuation of i.v. therapy (DCIV), 5 to 9 days posttherapy (test of cure [TOC] visit), and 4 to 6 weeks posttherapy (late follow-up [LFU]). Each infection was assessed prior to unblinding and considered severe if there was bacteremia, signs of sepsis (diastolic blood pressure of <60 mm Hg, altered mental status, or use of vasopressors), or at least three of the following: moderate to severe flank pain, fever of >101°C, chills, nausea or vomiting, or a white blood cell count of ≥15,000 per μl.
Microbiological assessments.
Urine culture with quantitation and blood culture were performed at the baseline. All isolates were identified at the site laboratory, and pathogens were tested for in vitro susceptibility to ertapenem, ceftriaxone, and ciprofloxacin, following the guidelines of the National Committee for Clinical Laboratory Standards (11).
Microbiologic efficacy was assessed at each time point by quantitative urine culture. After at least 48 h of study i.v. therapy, failure was defined as a urine culture with a ≥104-CFU/ml concentration of any uropathogen present in the admission culture at a concentration of ≥105 CFU/ml. Microbiologic responses during therapy and at the TOC visit were defined as eradication (the urine culture showed that a uropathogen present at a concentration of ≥105 CFU/ml at entry was reduced to a concentration of <104 CFU/ml), persistence (the urine culture grew ≥104 CFU of an original uropathogen/ml), persistence with acquisition of resistance, superinfection (the urine culture grew ≥105 CFU of a uropathogen other than the baseline pathogen during therapy per ml), or new infection (≥105 CFU of a pathogen other than the baseline pathogen cultured after completion of therapy per ml). Additional responses at LFU were recurrence (eradication at TOC but ≥104 CFU of an original uropathogen/ml at LFU) and recurrence with acquisition of resistance.
Populations for analysis.
The treated population included patients who received at least one dose of study therapy. The microbiologic modified intent-to-treat (MITT) population included patients who received at least one dose of i.v. therapy, had a baseline uropathogen in any quantity, and had a follow-up quantitative urine culture after DCIV. Microbiologically evaluable patients had to meet the following criteria: clinical evidence of a UTI, baseline urine pathogen at a concentration of ≥105 CFU/ml (≥104 CFU/ml if the patient was bacteremic), at least one baseline pathogen susceptible or intermediate to both i.v. study antimicrobials, and a urine culture at the TOC visit. The LFU evaluable population included evaluable patients successfully treated at the TOC visit who also had a follow-up assessment within the LFU window.
Efficacy variables.
The primary efficacy variable was the assessment of bacteriologic eradication in the microbiologically evaluable population at the TOC visit. Additional assessments were the microbiologic response rates in the MITT population and the clinical and microbiologic responses in evaluable patients at DCIV. Microbiologic recurrence and clinical relapse were also assessed in evaluable patients at the LFU visit.
Safety and tolerability assessment.
Patients in the treated population were monitored for adverse experiences daily during parenteral therapy and for 14 days after all study therapy (parenteral plus oral) was completed. The investigator categorized the intensity of the adverse event (mild, moderate, or severe) and the likelihood of its relation to the study drug (definitely not, probably not, possibly, probably, or definitely). The tolerability of the parenteral study drug at the local infusion site was evaluated by the investigator daily.
Statistical analyses.
The study was designed to test for equivalence in the efficacy of the ertapenem and ceftriaxone microbiologically evaluable treatment groups. The sample size (minimum of 150 evaluable patients per group) was calculated with Blackwelder's formula (1) and for the following values: alpha, 0.025; beta, 0.20; and the expected response rate in both groups, 90%. In accordance with the Food and Drug Administration's guidance for industry on developing antimicrobial drugs—General Consideration for Clinical Trials (July 1998), equivalence for this study was determined by the 95% (two-sided) confidence interval (CI) for the difference in response rates between the two treatment groups (ertapenem minus ceftriaxone) (7). If the observed response rate in the comparator group was >90%, for equivalence to be demonstrated, the CI of the difference had to contain zero and its lower limit could not be less than −10 percentage points. CIs about the difference were calculated by using the normal approximation to the binomial distribution and were adjusted for strata by using the Cochran-Mantel-Haenzel approach (2). The treatment by stratum interaction was investigated using the Breslow-Day test of homogeneity of odds ratios and the Gail-Simon test, if needed. Kaplan-Meier analyses were also performed to explore the time to DCIV in the microbiologically evaluable population. The SAS, version 6.12, statistical package was used for analysis. No formal tests were performed based on baseline demographics or disease characteristics.
RESULTS
Patients.
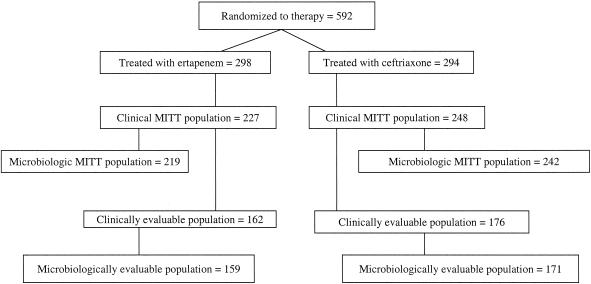
The distribution of the study patients is summarized in Fig. 1. A total of 592 patients were randomized: 298 to the ertapenem group and 294 to the ceftriaxone group. A total of 159 (53.4%) patients in the ertapenem group and 171 (58.2%) patients in the ceftriaxone group were microbiologically evaluable. The most common reason patients were not evaluable was failure to isolate ≥105 CFU of a uropathogen/ml at baseline.
FIG. 1.
Profile of patient enrollment.
Baseline demographics and disease characteristics of the two treatment groups in the randomized and microbiologically evaluable populations were generally similar (Table 1). Approximately 30% of the patients in each treatment group of both populations were male. In the evaluable population, 83 (52.2%) patients in the ertapenem group and 92 (53.8%) patients in the ceftriaxone group had one or more urinary tract abnormalities; indwelling bladder catheters or stents were present in 18 (11.3%) and 20 (11.7%) patients in the ertapenem and ceftriaxone treatment groups, respectively. Approximately half of the evaluable patients in each treatment group had severe infection.
TABLE 1.
Baseline characteristics and therapy of randomized and microbiologically evaluable patients with complicated UTI
| Characteristics | Value for group
|
|||
|---|---|---|---|---|
| Randomized patients
|
Microbiologically evaluable patients
|
|||
| Ertapenem (n = 298) | Ceftriaxone (n = 294) | Ertapenem (n = 159) | Ceftriaxone (n = 171) | |
| Male (n [%]) | 92 (30.9) | 97 (33.0) | 43 (27.0) | 50 (29.2) |
| Caucasian (n [%]) | 194 (65.1) | 206 (70.1) | 114 (71.7) | 116 (67.8) |
| Black (n [%]) | 32 (10.7) | 21 (7.1) | 15 (9.4) | 14 (8.2) |
| Hispanic (n [%]) | 32 (10.7) | 29 (9.9) | 13 (8.2) | 17 (9.9) |
| Other ethnic group (n [%]) | 40 (13.4) | 38 (12.9) | 17 (10.7) | 24 (14.0) |
| Mean age ± SD (yr) | 51.3 ± 20.8 | 53.0 ± 20.5 | 51.8 ± 20.3 | 53.0 ± 20.2 |
| No. (%) ≥ 65 yr | 93 (31.2) | 103 (35.0) | 52 (32.7) | 61 (35.7) |
| No. (%) infected with | ||||
| Acute pyelonephritis | 143 (48.0) | 118 (40.1) | 75 (47.2) | 78 (45.6) |
| Other complicated UTI | 155 (52.0) | 176 (59.9) | 84 (52.8) | 93 (54.4) |
| Severe disease | 134 (45.0) | 116 (39.5) | 75 (47.2) | 79 (46.2) |
| No. of days on therapy (mean ± SD)a | ||||
| i.v. | 4.0 ± 1.9 | 4.1 ± 2.0 | 4.4 ± 1.9 | 4.3 ± 1.8 |
| Oral | 7.6 ± 2.6 | 7.8 ± 2.4 | 7.7 ± 2.2 | 7.8 ± 2.1 |
| Total | 10.0 ± 4.1 | 10.8 ± 3.6 | 11.7 ± 2.2 | 11.8 ± 2.2 |
Among randomized patients, for ertapenem, n = 293 for i.v. treatment and n = 235 (80.2%) for oral treatment; for ceftriaxone, n = 289 for i.v. treatment and n = 248 (85.8%) for oral treatment.
Therapy.
The duration of parenteral, oral, and total (parenteral plus oral) study therapy in the treated and microbiologically evaluable patients was comparable in the ertapenem and ceftriaxone treatment groups (Table 1). Of the evaluable patients, 95.6% (152 of 159) of those treated with ertapenem and 94.7% (162 of 171) of those treated with ceftriaxone were switched to oral therapy, most commonly ciprofloxacin (approximately 94% in each treatment group). The Kaplan-Meier curves for the time to the switch to oral therapy were similar for ertapenem and ceftriaxone (data not shown). Most patients in both treatment groups were switched to oral therapy by study day 4.
Baseline microbiology.
The distribution of the pathogens in each treatment and their susceptibility profiles were comparable. The most frequently isolated pathogen was Escherichia coli, recovered from 111 (69.8%) patients in the ertapenem group and 117 (68.4%) patients in the ceftriaxone group, followed by Klebsiella pneumoniae, which was cultured from 22 (13.8%) patients in the ertapenem group and 21 (12.3%) patients in the ceftriaxone group. For all other species, there were fewer than 10 isolates per group. All isolates were susceptible to ertapenem, and all were susceptible or intermediate to ceftriaxone, with the exception of two (33.3%) of six enterococcus isolates in the ertapenem group and three Pseudomonas isolates (two of six isolates in the ertapenem group and one of two isolates in the ceftriaxone group). Twenty microbiologically evaluable patients who received ertapenem and 15 patients treated with ceftriaxone were bacteremic at the baseline; the etiologic agent in 27 (75.0%) of these patients was E. coli.
Efficacy.
At the primary efficacy endpoint, 91.8% of the microbiologically evaluable patients in the ertapenem group and 93.0% of those in the ceftriaxone group had a favorable microbiologic response assessment (95% CI for the difference, adjusting for strata, −7.6 to 5.1%), indicating equivalence of the two treatments. In the supportive microbiologic MITT analysis, which included 80% of all patients randomized, 92.5% treated with ertapenem and 91.1% treated with ceftriaxone had a favorable microbiologic response (95% CI for the difference, −4.0 to 6.9%), supporting the results of the primary analysis.
Overall bacterial eradication rates in the evaluable population are summarized by stratum, subgroup, and time of assessment (DCIV and the TOC visit) in Table 2. There were no patients in either group who failed treatment prior to receiving three doses of i.v. therapy. Eradication rates by pathogen are shown in Table 3. One persistent pathogen (E. coli) in the ertapenem group developed resistance to the oral study drug (ciprofloxacin), none had developed resistance to ertapenem. In the ceftriaxone group, one persistent pathogen (Citrobacter freundii) developed resistance to ceftriaxone and two pathogens (Proteus mirabilis and E. coli) had developed resistance to the oral agent (ciprofloxacin). Seventeen of the 20 (85.0%) bacteremic patients in the ertapenem group and 13 of the 15 (86.7%) in the ceftriaxone group had a favorable overall microbiologic response. Failures were a result of persistent bacteriuria; persistent bacteremia was not documented in any of these patients.
TABLE 2.
Favorable microbiologic response assessments in microbiologically evaluable patients with complicated UTI
| Time of evaluation and stratum or subgroup | Ertapenem
|
Ceftriaxone
|
||
|---|---|---|---|---|
| No. cured/ no. assessed | % Response (95% CI) | No. cured/ no. assessed | % Response (95% CI) | |
| DCIV | ||||
| Acute pyelonephritis | 69/71 | 97.2 (93.3-100) | 73/74 | 98.6 (96.0-100) |
| Other complicated UTI | 78/78 | 100 (95.4-100) | 84/90 | 93.3 (88.2-98.5) |
| Overall | 147/149 | 98.7 (96.8-100) | 157/164 | 95.9 (92.7-98.9) |
| TOC | ||||
| Acute pyelonephritis | 71/75 | 94.7 (89.5-99.8) | 74/78 | 94.9 (89.9-99.8) |
| Other complicated UTI | 75/84 | 89.3 (82.6-95.9) | 85/93 | 91.4 (85.7-97.1) |
| Mild to moderate infection | 77/84 | 91.7 (85.7-97.6) | 85/92 | 92.4 (86.9-97.8) |
| Severe infection | 69/75 | 92.0 (85.8-98.2) | 74/79 | 93.7 (88.3-99.1) |
| Age < 75 yr | 122/135 | 90.4 (85.4-95.4) | 129/137 | 94.2 (90.2-98.1) |
| Age ≥ 75 yr | 24/24 | 100 (85.8-100) | 30/34 | 88.2 (77.2-99.2) |
| Overall | 146/159 | 91.8 (87.5-96.0) | 159/171 | 93.0 (89.2-96.9) |
TABLE 3.
Eradication rates at TOC in microbiologically evaluable patients with complicated UTI
| Isolate | Ertapenem (n = 159)
|
Ceftriaxone (n = 171)
|
||
|---|---|---|---|---|
| No. with favorable assessment/ no. assessed at TOC | % Response (95% CIa) | No. with favorable assessment/ no. assessed at TOC | % Response (95% CIa) | |
| Gram-positive cocci | ||||
| Enterococci | 3/6 | 50.0 | 0/2 | 0.0 |
| Other | 3/3 | 100 | 4/5 | 80.0 |
| Total | 6/9 | 66.7 | 4/7 | 57.1 |
| Gram-negative bacilli | ||||
| E. coli | 104/111 | 93.7 (89.2-98.2) | 112/117 | 95.7 (92.0-99.4) |
| K. pneumoniae | 21/22 | 95.5 (77.2-99.9) | 20/21 | 95.2 (76.2-99.9) |
| P. mirabilis | 8/9 | 88.9 | 5/6 | 83.3 |
| Other Enterobacteriaceae | 9/10 | 90.0 | 22/24 | 91.7 |
| P. aeruginosa | 3/5 | 60.0 | 1/1 | 100 |
| Pseudomonas spp. | 1/1 | 100 | 1/1 | 100 |
| Total | 146/158 | 92.4 (88.3-96.5) | 161/170 | 94.7 (91.3-98.1) |
CIs were only calculated for species with at least 10 isolates.
Bacterial recurrence rates in patients evaluable for the LFU assessment (n = 105 for ertapenem; n = 121 for ceftriaxone) were 7.6% of patients treated with ertapenem and 8.3% of those treated with ceftriaxone (95% CI for the difference, −8.7 to 7.4%), indicating that recurrence rates in the two treatment groups were similar. In the ertapenem group none of these recurrent pathogens had developed resistance to either the parenteral or oral study drug, whereas one recurrent pathogen (E. coli) in the ceftriaxone group developed resistance to the oral agent (ciprofloxacin). Clinical relapse rates at the LFU visit (5.4% of patients treated with ertapenem [n = 114] and 7.9% of those treated with ceftriaxone [n = 135]) were also similar.
Nonbaseline, emergent infections included new infections, which occurred in 27 (17.0%) microbiologically evaluable patients in the ertapenem group and 21 (12.3%) patients in the ceftriaxone group, and superinfections, which developed in one patient in each treatment group. The most common pathogens causing new infections in both groups were enterococci, E. coli, and K. pneumoniae, in descending order of frequency. One isolate each of Pantoea agglomerans and P. aeruginosa was responsible for superinfection in the ertapenem and ceftriaxone groups, respectively.
Safety and local tolerability.
A total of 293 patients in the ertapenem group and 289 patients in the ceftriaxone group received at least one dose of study i.v. therapy and were evaluated for adverse experiences. During i.v. therapy, one or more drug-related adverse experiences were reported for 86 (29.4%) patients in the ertapenem group and 76 (26.3%) patients in the ceftriaxone group, none of which was considered to be serious. The most common drug-related adverse events in the treated population were diarrhea (ertapenem, 21 [7.2%] patients; ceftriaxone, 19 [6.6%] patients), headache (ertapenem, 19 [6.5%] patients; ceftriaxone, 13 [4.5%] patients), and nausea (ertapenem, 16 [5.5%] patients; ceftriaxone, 10 [3.5%] patients). Parenteral study therapy was discontinued due to a drug-related adverse experience in 8 (2.7%) patients in the ertapenem group and none in the ceftriaxone group. Events resulting in discontinuation of ertapenem were rash in three patients, one of whom reported a prior penicillin allergy, and pruritus, vomiting, paresthesia, diarrhea, and syncope in one patient each.
Drug-related laboratory adverse experiences were reported in 27 (9.7%) patients in the ertapenem group and 20 (7.2%) patients in the ceftriaxone group. None of these events was considered serious, and none resulted in discontinuation of study therapy. The most common drug-related adverse laboratory events were elevations of alanine aminotransferase levels (3.8% [9 of 240 patients] in the ertapenem group and 4.6% [11 of 238 patients] in the ceftriaxone group) and aspartate aminotransferase levels (3.7% [10 of 268 patients] and 3.4% [9 of 261 patients] in the ertapenem and ceftriaxone groups, respectively).
Twenty-seven (9.2%) patients in the ertapenem group and 20 (6.9%) patients in the ceftriaxone group experienced reactions of moderate to severe intensity at the local infusion site. The most common symptom in both treatment groups was pain followed by tenderness.
DISCUSSION
In this double-blind, randomized study, the efficacy and safety of ertapenem were compared with those of ceftriaxone for the empirical treatment of serious complicated UTI judged by the investigator to require initial therapy with a parenteral antimicrobial agent. The study design was consistent with standard clinical practice (13-15). After a minimum of 3 full days of parenteral study therapy, investigators had the option to switch to oral ciprofloxacin (or other agent) if the patient had clinically improved. Approximately 95% of the evaluable patients in both treatment groups were switched to oral therapy (ciprofloxacin in about 94% of patients in each treatment group), usually by study day 4. The Kaplan-Meier curves for the time to switch to oral therapy in the microbiologically evaluable population appeared similar for both study drugs, indicating that the time to defervescence and improvement of clinical signs and symptoms was comparable for both ertapenem and ceftriaxone.
Results of this study show that ertapenem therapy, 1 g once a day, was highly effective in all patient populations, including those with pyelonephritis or severe infection and the elderly, and was equivalent to treatment with ceftriaxone. Over 90% of the evaluable patients in both treatment groups had a favorable microbiologic response assessment at the TOC visit 5 to 9 days posttherapy. These rates are similar to or better than those reported in previous studies of patients with complicated UTIs (3-5, 8, 10, 12), and they exceed the cure rates expected when evaluating a new anti-infective drug for the treatment of UTI (>80% for acute pyelonephritis and >65% for complicated UTI) (13). As expected, success rates were higher at the completion of i.v. therapy than at the TOC visit for both study drugs. This difference was more apparent for patients with other complicated UTIs than for those with acute pyelonephritis, which suggests that the few additional failures posttherapy were due to the underlying urinary tract abnormality or inadequate oral therapy rather than failure of the parenteral antimicrobial agent.
Ertapenem has excellent activity against many gram-positive and gram-negative aerobic, facultative, and anaerobic bacteria generally associated with infections acquired in the community. Several studies have shown that most members of the family Enterobacteriaceae are highly susceptible to ertapenem, including isolates resistant to several other antimicrobial agents (6, 9). In this study, members of the family Enterobacteriaceae, all of which were susceptible to ertapenem, accounted for 93.0% of all isolates from microbiologically evaluable patients in both treatment groups. Similar to this study, other studies have shown that E. coli and K. pneumoniae account for the majority of responsible pathogens in complicated UTIs (5, 8, 10, 12). Ertapenem is thus a reasonable choice for treatment of complicated UTIs when infection is not caused by P. aeruginosa or enterococci, both of which were recovered from very few patients in this study.
The overall safety and tolerability profile of ertapenem in this study was similar to that of ceftriaxone. Drug-related clinical adverse events most frequently reported for both agents were related to the gastrointestinal tract. The most common drug-related laboratory adverse event for both drugs was mild to moderate elevation of transaminase levels, which tended to be transient and without clinical consequence.
In summary, in adult patients with moderate to severe complicated UTI requiring initial parenteral therapy, ertapenem (1 g once a day), with the option to switch to an appropriate oral antimicrobial agent after clinical improvement, was generally well tolerated and highly effective both clinically and microbiologically.
Acknowledgments
Financial support for this study was provided by Merck & Co., Inc.
REFERENCES
- 1.Blackwelder, W. C. 1982. “Proving the null hypothesis” in clinical trials. Control. Clin. Trials 3:345-353. [DOI] [PubMed] [Google Scholar]
- 2.Cochran, W. G. 1954. Some methods for strengthening the common chi-square tests. Biometrics 10:417-451. [Google Scholar]
- 3.Cox, C. E. 1989. Sequential intravenous and oral ciprofloxacin versus intravenous ceftazidime in the treatment of complicated urinary tract infections. Am. J. Med. 87(Suppl. 5A):157S-159S. [DOI] [PubMed] [Google Scholar]
- 4.Fang, G. D., C. Brennen, M. Wagener, D. Swanson, M. Hilf, L. Zadecky, J. De Vine, and V. L. Yu. 1991. Use of ciprofloxacin versus use of aminoglycosides for therapy of complicated urinary tract infection: prospective, randomized clinical and pharmacokinetic study. Antimicrob. Agents Chemother. 35:1849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friman, G., O. Cars, E. Back, H. Bechman, M. Carlsson, J. Forsell, H. Fredlund, R. Neringer, I. Odenholt-Tornqvist, C. Ryden, and R. Svensson. 1989. Randomized comparison of aztreonam and cefuroxime in gram-negative upper urinary tract infections. Infect. 5:284-289. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2001. In vitro activities of ertapenem (MK-0826) against clinical bacterial isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1915-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauck, W. W., and S. Anderson. 1999. Some issues in the design and analysis of equivalence trials. Drug Information J. 33:109-118. [Google Scholar]
- 8.Klimberg, I. W., C. E. Cox, Jr., C. L. Fowler, W. King, S. S. Kim, and S. Callery-D'Amico. 1998. A controlled trial of levofloxacin and lomefloxacin in the treatment of complicated urinary tract infection. Urology 51:610-615. [DOI] [PubMed] [Google Scholar]
- 9.Livermore, D. M., M. W. Carter, S. Bagel, B. Wiedemann, F. Baquero, E. Loza, H. P. Endtz, N. van Den Braak, C. J. Fernandes, L. Fernandes, N. Frimodt-Moller, L. S. Rasmussen, H. Giamarellou, E. Giamarellos-Bourboulis, V. Jarlier, J. Nguyen, C. E. Nord, M. J. Struelens, C. Nonhoff, J. Turnidge, J. Bell, R. Zbinden, S. Pfister, L. Mixson, and D. L. Shungu. 2001. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob. Agents Chemother. 45:1860-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naber, K. G., F. D. Silverio, A. Geddes, and J. Guibert. 1996. Comparative efficacy of sparfloxacin versus ciprofloxacin in the treatment of complicated urinary tract infections. J. Antimicrob. Chemother. 37(Suppl. A):135-144. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Performance standard for antimicrobial disk susceptibility tests. Approved standard M2-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Nicolle, L. E., T. J. Louie, J. Dubois, A. Martel, G. K. Harding, and C. P. Sinave. 1994. Treatment of complicated urinary tract infections with lomefloxacin compared with that with trimethoprim-sulfamethoxazole. Antimicrob. Agents Chemother. 38:1368-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin, R. H., E. D. Shapiro, V. T. Andriole, R. J. Davis, and W. E. Stamm. 1992. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Clin. Infect. Dis. 15(Suppl. 1):S216-S227. [DOI] [PubMed] [Google Scholar]
- 14.Stamm, W. E, and T. M. Hooton. 1993. Management of urinary tract infections in adults. N. Engl. J. Med. 329:1328-1334. [DOI] [PubMed] [Google Scholar]
- 15.Warren, J. W., E. Abrutyn, J. R. Hebel, J. R. Johnson, A. J. Schaeffer, and W. E. Stamm. 1999. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin. Infect. Dis. 29:745-758. [DOI] [PubMed] [Google Scholar]