Abstract
The objective of this study was to determine the susceptibility breakpoint of a new carbapenem, ertapenem (MK-0826), against Streptococcus pneumoniae strains based on bacterial density and survival studies in a murine thigh infection model. Sixteen S. pneumoniae isolates for which MICs ranged from 0.015 to 4.0 mg/liter were tested with neutropenic ICR mice. Animals were infected with bacteria at 105 to 106 CFU per thigh and were treated with ertapenem starting at 2 h postinfection for 4 days. Ertapenem was given subcutaneously at 50 mg/kg of body weight every 6 h, which simulates the human pharmacodynamic profile (in particular, the duration of time that the concentration of free drug remains above the MIC of 2 mg/liter). At 0 and 24 h postinfection, thighs were harvested for bacterial density determination. Survival was assessed during 4 days of therapy and 3 days after the therapy. A protein binding study was conducted with mice by use of the ultrafiltration method. Protein binding in mice was approximately 95%, which is comparable to that in humans. The average change in bacterial density ranged from −0.22 to −4.4 log CFU per thigh over 24 h compared to 0-h controls. The extent of microbial eradication was dependent on the MIC for the S. pneumoniae isolate. Substantial bactericidal activities (i.e., killing of approximately 2 log CFU per thigh) were consistently observed against isolates for which MICs were ≤2 mg/liter, which also resulted in nearly 100% survival during the 4 days of drug dosing and 3 days after the therapy. Less-pronounced and highly variable bactericidal activities were detected against isolates for which the MIC was 4 mg/liter. Substantial enhancement in bactericidal activity was observed for CBA/J mice and is attributed to the contribution of the host defenses in the immunocompetent species. Assessment of the effectiveness of ertapenem by bacterial-density reduction over 24 h and by survival over 4 days of therapy in the murine thigh infection model reveals that the drug maintains maximal efficacy against S. pneumoniae isolates for which the MIC of this agent is ≤2 mg/liter.
Ertapenem (MK-0826) is a new, long-acting 1-β-methyl carbapenem with potent antimicrobial activity against a variety of pathogenic species including pneumococci (4). While this agent apparently has good in vitro activity against Streptococcus pneumoniae, the pharmacodynamic assessment of the susceptibility breakpoint of ertapenem against this important pathogen has not been fully described. The availability of these data will assist not only in optimizing the effectiveness of the prescribed antimicrobial regimen in clinical practice but also in the assessment of appropriate National Committee for Clinical Laboratory Standards (NCCLS) breakpoints for this antimicrobial agent.
The purpose of the present study was to determine the susceptibility breakpoint of ertapenem against S. pneumoniae strains based on bacterial density and survival studies using a murine model of pneumococcal thigh infection.
MATERIALS AND METHODS
Antimicrobial agents.
Ertapenem (analytical grade standard; lot L-749345-002C089, 98.4% purity) for in vitro and in vivo testing was obtained from Merck Research Laboratory. Ertapenem was reconstituted with a 0.1 M morpholineethanesulfonic acid (MES)-ethylene glycol (1:1, vol/vol) solution for all in vitro testing. For animal dosing, an ertapenem solution was freshly prepared with sterile water for injection prior to drug administration.
Bacterial isolates and susceptibilities.
Sixteen clinical isolates of S. pneumoniae were included in this study. The MIC for ertapenem was determined by the microdilution method according to NCCLS guidelines by using cation-adjusted Mueller-Hinton broth (20 to 25 mg of calcium/liter and 10 to 12.5 mg of magnesium/liter) with 5% lysed horse blood in ambient air (8). Trypticase soy agar with 5% sheep blood was used as the growth medium for S. pneumoniae isolates.
Thigh infection model.
Specific-pathogen-free female ICR mice weighing approximately 25 g (Harlan Sprague Dawley Inc., Indianapolis, Ind.) were utilized throughout the experiment. All care and the experiments described in this report were approved by and performed in accordance with guidelines of the Hartford Hospital Institutional Animal Care and Use Committee (IACUC).
Mice were rendered transiently neutropenic by two intraperitoneal (i.p.) injections of cyclophosphamide, the first at a dose of 150 mg/kg of body weight 4 days before infection and the second at a dose of 100 mg/kg 1 day before infection (1, 5). Renal impairment was induced by a single i.p. injection of uranyl nitrate 3 days before infection (1). Broth cultures of the test organism were grown overnight and subsequently diluted to an inoculum of approximately 106 to 107 CFU/ml. Final inoculum concentrations were confirmed by serial-dilution and plating techniques. Thigh infection with each of the test isolates was produced by injecting 0.1 ml of the inoculum into each mouse thigh 2 h prior to initiation of antimicrobial therapy.
Pharmacokinetic studies and dosing-regimen determination.
Pharmacokinetic studies were undertaken using neutropenic ICR mice in order to find an ertapenem regimen for the murine pneumococcal thigh infection model that simulates the pharmacokinetic profile observed in humans receiving 1-g dosing every 24 h (q24h). In an attempt to optimize the pharmacokinetic profile of ertapenem, renal impairment was induced by a single i.p. injection of uranyl nitrate (5 mg/kg) 3 days before infection. Single doses of ertapenem at 20, 50, and 100 mg/kg were administered by subcutaneous injection in a 0.2-ml volume 2 h after pneumococcal thigh inoculation. Blood samples from four mice per time point were collected by intracardiac puncture at 0.25, 0.5, 1, 2, 4, 6, and 8 h after dosing. Serum was separated by centrifugation at 3,000 × g for 10 min and was then transferred into polypropylene tubes and stored at −80°C until analysis.
Concentrations of ertapenem in murine serum were determined by using a validated reverse-phase high-pressure liquid chromatography (HPLC) assay. The sample extraction method involved liquid-phase extraction. After addition of 50 μl of an internal standard (meropenem, 50 mg/liter) to the mouse serum samples (200 μl), 800 μl of acetonitrile was added. Protein precipitation was achieved by a brief vortexing followed by centrifugation at 2,600 × g for 10 min. Supernatants were transferred into clean labeled tubes, and then 2.5 ml of dichloromethane was added. Again samples were briefly vortexed and subjected to centrifugation. The resultant aqueous solution was separated and transferred into vials for injection into the HPLC system.
Chromatography was performed at ambient temperature on a reversed-phase C18 column (5 μm; 100 by 4.6 mm; Keystone Scientific Inc., Belletonte, Pa.) with an injection volume of 30 μl. The mobile phase, consisting of 25 mM phosphate buffer (pH 6.5) solution-methanol (100:9.5, vol/vol), was delivered via a Waters HPLC pump at a flow rate of 1.0 ml/min. Ertapenem and the internal standard were eluted at approximately 10.5 and 14.9 min, respectively. UV detection was performed at a wavelength of 300 nm.
The assay was linear over a range of 0.15 to 50 mg/liter. Intra-assay coefficients of variation for the low (1.0-mg/liter) and high (40-mg/liter) check samples were 2.2 and 4.0%, respectively. Interassay coefficients of variation for the low and high check samples were 3.6 and 4.3%, respectively.
Individual concentration data from all the dosing regimens were analyzed by using a population approach with the NONMEM computer program, version V, level 1.1; NM-TRAN, version III, level 1.0; and PREDPP, version IV, level 1.0 (NONMEM Project Group, University of California at San Francisco, San Francisco, Calif.). One- and two-compartment structural models with first-order absorption and elimination were compared to fit the data by using the first-order estimation method. Residual error was modeled by using a combined proportional and additive error model. Model selection was based on a likelihood ratio test, Akaike's information criterion, and evaluation of goodness-of-fit plots from the model. The statistical significance level was set a priori at 0.005.
Based on the population parameter estimates from NONMEM analysis, the dosing regimen was calculated in such a way as to simulate the drug exposure found in humans following 1-g q24h dosing (area under the concentration-time curve from 0 to 24 h [AUC0-24], 500 to 600 mg·h/liter; maximum concentration of drug in serum [Cmax], 140 mg/liter; duration of time that the free drug concentration remains above the MIC of 2 mg/liter [T>MICfree], 20%).
Protein binding.
The protein binding of ertapenem was determined by an ultrafiltration (Centrifree) method with ICR mouse specimens. Three concentrations of ertapenem (1, 10, and 100 mg/liter) were tested with freshly collected mouse serum. All spiked serum samples were placed in a 37°C shaking water bath for 10 min before being loaded onto Centrifree filters (30,000-molecular-weight cutoff; Millipore). Filtrates were separated by centrifugation at 1,000 × g for 15 min, and samples were measured by a validated HPLC method.
Therapeutic efficacy as assessed by bacterial density.
Starting at 2 h after infection, ICR mice were treated with ertapenem at 50 mg/kg q6h by subcutaneous injection in a 0.2-ml volume for 24 h. This dosing regimen was calculated on the basis of the preliminary pharmacokinetic analysis to simulate the drug exposure observed in humans. Control animals received water in the same volume (0.2 ml) and on the same schedule as ertapenem. Untreated control mice (four per group) were sacrificed just prior to antibiotic initiation and after 24 h. Ertapenem-treated animals were euthanized by CO2 exposure followed by cervical dislocation after 24 h.
After sacrifice, both thighs were removed and individually homogenized in normal saline. Serial dilutions were plated on Trypticase soy agar with 5% sheep blood for CFU determinations. For the purposes of these studies, efficacy (change in bacterial density) was calculated by subtracting the mean log CFU per thigh of the control mice obtained just prior to antibiotic administration from the log CFU per thigh of each ertapenem-treated mouse at the end of therapy (24 h).
In addition, we compared the bactericidal activities of ertapenem against S. pneumoniae in the murine neutropenic and nonneutropenic thigh infection models. An S. pneumoniae strain (SP-129; MIC, 2 mg/liter) was tested with neutropenic ICR mice and immunocompetent CBA/J mice (Charles River Laboratories, Wilmington, Mass.). The animal infection procedure for CBA/J mice was the same as the procedure described above for ICR mice except that the CBA/J mice received no cyclophosphamide. Animals were inoculated at 105 to 106 CFU per thigh and were treated starting at 2 h postinfection for 24 h. At 0 and 24 h postinfection, thighs were harvested for bacterial density determination. The regimens for ertapenem were 50 mg/kg q6h and q24h. Four animals were used in each study arm.
Therapeutic efficacy as assessed by survival.
Groups of 20 mice were similarly infected with each test strain for evaluation of survival after 96 h of therapy. Ertapenem therapy (50 mg/kg q6h) was initiated 2 h after inoculation in 15 animals. The remaining five animals received the same volume of water q6h and served as the controls. Cumulative mortality was calculated during 96 h of therapy. Although death has historically been used as an end point for studies of this type, this end point is no longer suitable in the present era of animal research. Therefore, our study methodology has been modified to contemporary standards. We utilized the following approach in order to lessen the duration of pain and suffering during the mortality experiment. (i) Animals were monitored four times daily by members of the study team who have been trained and are experienced in recognizing the signs of illness and abnormal behavior. (ii) Animals which appeared to have substantial alterations in posture (e.g., abnormal posture or head tucked into abdomen), coat, exudate around eyes and/or nose, and breathing or movement were removed from the group housing and were euthanized.
The term “mortality” has been used as an end point for this study; however, it should be clearly understood that, when possible, every attempt was made to minimize pain and suffering and that the animals were euthanized prior to naturally succumbing to infection if pain and suffering were observed. For the purposes of this study, death due to the natural infection process and euthanasia were considered the same end point for experimental and statistical purposes.
Data analysis.
Spearman's rank correlation coefficient was used to evaluate the correlation between change in CFU and T>MICfree for ertapenem after 24 h of therapy as well as the correlation between mortality and T>MICfree for ertapenem after 96 h of therapy. In addition, the sigmoid Emax model was applied to further evaluate the relationship between these variables.
RESULTS
The MICs of ertapenem for the S. pneumoniae isolates incorporated into this study are displayed in Table 1. The test organisms selected represent a wide range of sensitivities to ertapenem, with MICs ranging from 0.015 to 4 mg/liter.
TABLE 1.
MICs of ertapenem for the S. pneumoniae test isolates
| Isolate no. | MICa (mg/liter) of:
|
|
|---|---|---|
| Penicillin | Ertapenem | |
| 21 | 0.03 | 0.015 |
| 81 | 0.25 | 0.03 |
| 80 | 0.125 | 0.125 |
| 71 | 8.0 | 0.5 |
| 51 | 2.0 | 1.0 |
| 70 | 3.0b | 1.0 |
| 85 | 1.0 | 1.0 |
| 100 | ND | 1.0 |
| 84 | 2.0 | 2.0 |
| 129 | 4.0 | 2.0 |
| 127 | 8.0 | 2.0 |
| 125 | 8.0 | 3.0b |
| 120 | ND | 4.0 |
| 122 | 4.0 | 4.0 |
| 126 | 8.0 | 4.0 |
| 128 | 8.0 | 4.0 |
ND, not determined.
Multiple duplicate analysis yielded MICs of 2 and 4 mg/liter, respectively. For the purposes of the pharmacodynamic analysis, these data were presented as averaged MICs.
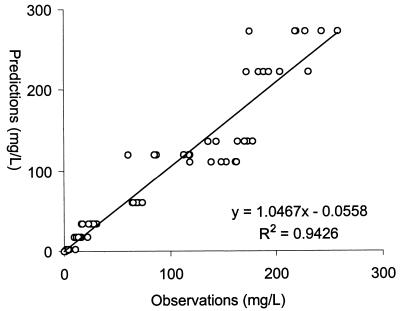
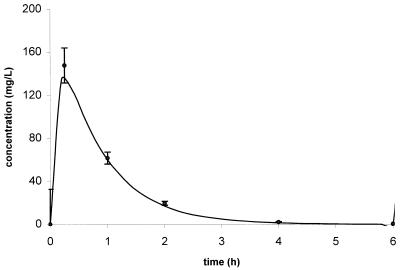
Concentration-independent protein binding levels for ertapenem were observed over the concentrations studied. The average protein binding level in murine serum was 95.5% ± 1.0%, which is comparable to the 94% protein binding at a concentration of 100 mg/liter observed in humans (10; product package insert, 2001, Merck & Co., Inc., Whitehouse Station, N.J.). A total of 130 concentrations were used to compute population pharmacokinetic parameters. The pharmacokinetic disposition of ertapenem in mice was best described with a one-compartment model with first-order absorption and elimination; parameter estimates are presented in Table 2. The population analysis was quite robust, as evidenced by the plot of the observed versus the population predicted concentrations of ertapenem in infected animals (Fig. 1). Based on the population analysis, a 50-mg/kg q6h dosing regimen produced an AUC0-24 of 586 mg · h/liter, a Cmax of 140 mg/liter, and a T> MICfree of 22%, which are comparable to those found in humans after 1-g q24h dosing. This simulated regimen was then administered to another group of mice to confirm the predicted values of the modeling scheme. As displayed in Fig. 2, the regimen selected produced a concentration-versus-time profile in this murine model that was comparable to the NONMEM-predicted profile.
TABLE 2.
Population pharmacokinetic parameter estimates for ertapenem in mice infected in the thigh with S. pneumoniae
| Parameter | Parameter estimate | Relative standard error (%) | 95% Confidence interval |
|---|---|---|---|
| Clearance (liters/h) | 0.0078 | 6.6 | 0.0068-0.0088 |
| Vol of distribution (liters) | 0.0063 | 6.7 | 0.0055-0.0071 |
| Ka (liters/h) | 9.95 | 115 | —a |
This parameter is poorly estimated. The 95% confidence interval estimated from the standard error includes zero. Ka, absorption rate constant.
FIG. 1.
Population predicted concentrations versus concentrations observed by NONMEM analysis.
FIG. 2.
Goodness of fit between the NONMEM simulated ertapenem concentration-time profile after 50-mg/kg dosing and the mean observed serum ertapenem concentration-versus-time profile in the murine thigh infection model.
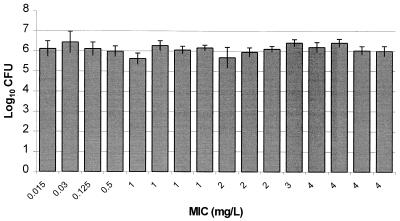
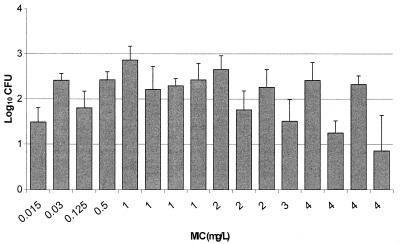
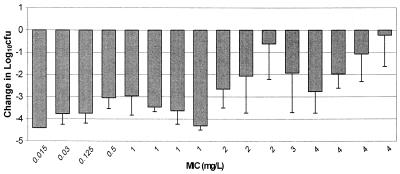
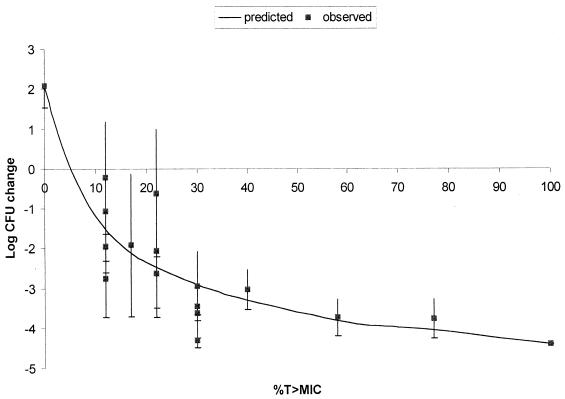
As presented in Fig. 3, excellent recovery of bacteria from infected thighs of control animals prior to the start of therapy was obtained for all isolates, with an average bacterial density of 6.09 ± 0.23 log10 CFU per thigh. These data support the accuracy of inoculum preparation and the consistency of the inoculation procedure. Figure 4 displays the growth of each organism in control animals over the 24-h postinfection period. Average bacterial growth in untreated control animals over 24 h was 2.1 ± 0.55 log10 CFU per thigh, with a range from 0.9 to 2.9 log10 CFU per thigh. The mean changes in bacterial density at the conclusion of 24 h of therapy with 50 mg of ertapenem/kg q6h are presented in Fig. 5. The magnitude of killing ranged from 0.22 to 4.4 log10 CFU per thigh over 24 h, and the extent of microbial eradication appeared to be related to the MIC for the pneumococcal isolate. Substantial bactericidal activities were observed against isolates for which MICs were ≤2 mg/liter, with a mean bacterial kill of 3.1 log10 CFU per thigh. Less-pronounced and highly variable bactericidal activities were detected against isolates for which the MIC was 4 mg/liter. Spearman's rank correlation coefficient calculation revealed a significant correlation between the change in CFU and the T>MICfree for ertapenem after 24 h of therapy (P < 0.05). The relationship between T>MICfree and log10 CFU was further described with a sigmoid Emax model (Fig. 6). As shown in Fig. 6, maximal bacterial killing activity appears to be reached once the T>MICfree exceeds 30%.
FIG. 3.
Densities of S. pneumoniae in the thighs of infected animals at the start of therapy. Each value represents 1 of 16 isolates and is the mean ± standard deviation for 8 thighs.
FIG. 4.
Growth in density of S. pneumoniae in the thighs of infected control animals over 24 h. Each value represents 1 of 16 isolates and is the mean ± standard deviation for 8 thighs.
FIG. 5.
Changes in density of S. pneumoniae in the thighs of infected animals after 24 h of ertapenem therapy. Each value represents 1 of 16 isolates and is the mean ± standard deviation for 8 thighs.
FIG. 6.
Sigmoid Emax evaluation of the correlation between T>MICfree and the bactericidal activity of ertapenem. r2 = 0.88.
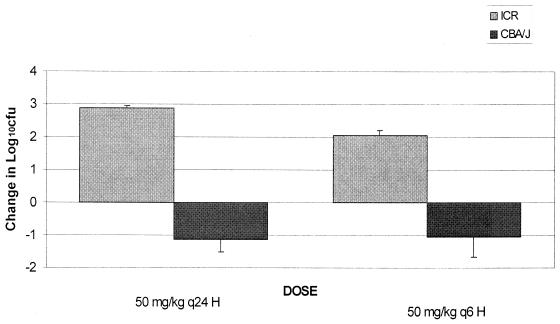
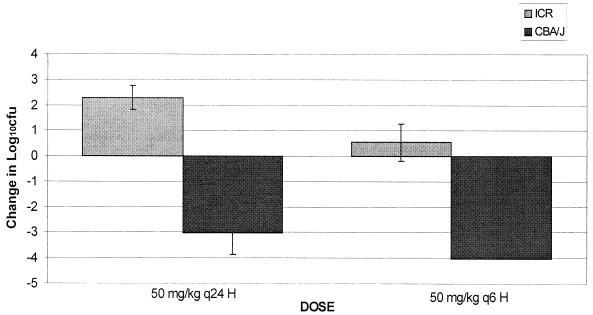
A comparison between the bactericidal activities of ertapenem in neutropenic ICR mice and nonneutropenic CBA/J mice was undertaken. Excellent recovery of bacteria was observed for both ICR and CBA/J mice at 2 h after bacterial inoculation, which is consistent with previous data (6). However, levels of bacterial growth in untreated control ICR mice and untreated control CBA/J mice were significantly different (P < 0.05) after 24 h of inoculation. Organisms grew approximately 2.5 log10 CFU per thigh over 24 h in untreated ICR mice, while an average reduction in bacterial density of 1.1 log10 CFU per thigh was observed for untreated CBA/J mice (Fig. 7). Furthermore, significantly different bactericidal effects were observed for ertapenem-treated ICR versus CBA/J mice. As shown in Fig. 8, the bactericidal activity observed in CBA/J mice was considerably enhanced over that in ICR mice (P < 0.05).
FIG. 7.
Growth in density of S. pneumoniae (SP-129) in the thighs of infected immunocompromised (ICR) and immunocompetent (CBA/J) control animals over 24 h. Values are means ± standard deviations for 8 thighs.
FIG. 8.
Changes in density of S. pneumoniae (SP-129) in the thighs of infected immunocompromised (ICR) and immunocompetent (CBA/J) animals after 24 h of ertapenem therapy. Values are means ± standard deviations for 8 thighs.
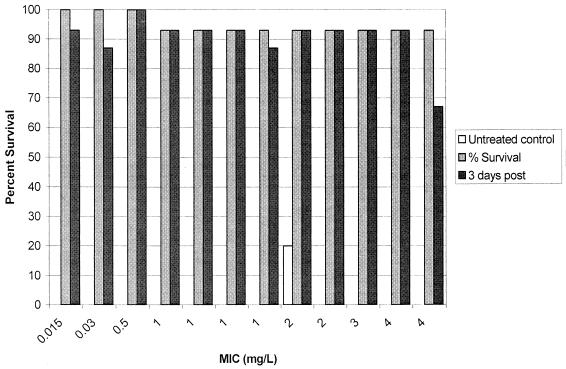
Cumulative mortality over 96 h of ertapenem treatment and 3 days posttherapy is reported in Fig. 9. Observed mortality in untreated control animals was 100% over this observation period except with one isolate. Animals treated with ertapenem survived the infection during the 4 days of treatment with a 93% survival rate. No significant correlation was detected between mortality and T>MICfree.
FIG. 9.
Percentage of survival of pneumococcus-infected animals after 4 days of ertapenem treatment and 3 days posttreatment (n = 15). Each set of bars represents one S. pneumoniae isolate.
DISCUSSION
S. pneumoniae remains one of the leading causes of community-acquired bacterial infections, and severe S. pneumoniae infections such as pneumonia and meningitis have significant morbidity and mortality rates (7). In the present study, we evaluated the pharmacodynamic profile of ertapenem against 16 clinical isolates of S. pneumoniae with a wide range of sensitivities by using the neutropenic murine model of thigh infection. It has been shown that the most important pharmacokinetic/pharmacodynamic parameter for prediction of the antimicrobial effect of ertapenem is T>MIC (M. L. Van Ogtrop, D. Andes, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 999, 1999). In addition, ertapenem has been reported as a highly protein-bound drug, which is in accordance with our observation (95% bound; Merck & Co., Inc., product package insert, 2001). Therefore, the dosing regimen employed in this study was selected based on the drug exposure found in humans following 1-g q24h dosing, in which the T>MICfree is approximately 20%. In the present study, the serum pharmacokinetic profile produced by the 50-mg/kg q6h dosing regimen in our model was quite similar to the target exposure in humans.
Over the course of the study, excellent recovery of bacteria from infected thighs was noted for all the isolates prior to the initiation of therapy. However, the growth of organisms in untreated control animals over the 24-h treatment period was quite variable, revealing the inherent variability of in vivo growth of S. pneumoniae, which is consistent with previous findings (6). Use of the simulated exposure with a number of S. pneumoniae strains for which MICs were varied yielded a wide range of levels of bacterial killing over the 24 h of therapy. The extent of bacterial killing appears to be related to the MIC of ertapenem for the pneumococcal isolate. Substantial bactericidal activities were consistently observed against isolates for which MICs were ≤2 mg/liter. In contrast, less-pronounced and highly variable bactericidal activities were detected against isolates for which the MIC was 4 mg/liter. Despite variable bactericidal activity, the overall survival rate was very high (93%) over the 7-day observation period. While inconsistent, this degree of killing of these organisms (MIC, 4 mg/liter) during the initial 24-h bacterial-density studies was sufficient to prevent mortality, an observation which has been made in similar studies with other compounds (6, 9). Pharmacodynamic evaluation revealed that T>MICfree was closely correlated with efficacy, and the relationship between these two parameters can be adequately described with the sigmoid Emax model. In addition, as shown in Fig. 6, the T>MICfree required for a static effect is approximately 6%, which is in agreement with previous observations (M. L. Van Ogtrop, D. Andes, and W. A. Craig, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-48, 1998). The magnitude of the pharmacokinetic/pharmacodynamic parameter required for a static effect is much less than that observed with penicillin and cephalosporins (2, 3). Moreover, we compared the bacterial-density determinations for the immunocompromised (ICR mice) and the immunocompetent (CBA/J mice) host with the same pneumococcal strain. A substantial enhancement in bactericidal activity was observed for the CBA/J mice; this is attributed to the contribution of the host defenses in the immunocompetent species. This is in accordance with a previous observation, which also demonstrates the host immunocompetency effect on efficacy (6).
In summary, ertapenem demonstrated substantial bactericidal activity against S. pneumoniae in the murine thigh infection model. The relationship between T>MIC and bactericidal activity can be characterized with the sigmoid Emax model. In the context of the isolates and dosing regimen studied, ertapenem therapy protected animals from death. Enhanced bactericidal activities were observed in the immunocompetent animals. Based on these data and the consideration that the presence of neutrophils would be expected to further enhance the activity of ertapenem, the proposed susceptibility breakpoint for ertapenem would be 2 mg/liter.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill, C. H., J. J. Jackson, L. S. Gerckens, B. A. Pelak, R. K. Thompson, J. G. Sundelof, H. Kropp, and H. Rosen. 1998. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749,345). Antimicrob. Agents Chemother. 42:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattoes, H. M., M. Banevicius, D. Li, C. Turley, D. Xuan, C. H. Nightingale, and D. P. Nicolau. 2001. Pharmacodynamic assessment of gatifloxacin against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2092-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mufson, M. A. 1990. Streptococcus pneumoniae, p. 1539-1551. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone Inc., New York, N.Y.
- 8.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Nicolau, D. P., C. O. Onyeji, M. K. Zhong, P. R. Tessier, M. A. Banevicius, and C. H. Nightingale. 2000. Pharmacodynamic assessment of cefprozil against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob. Agents Chemother. 44:1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odenholt, I. 2001. Ertapenem: a new carbapenem. Expert Opin. Investig. Drugs 10:1157-1166. [DOI] [PubMed] [Google Scholar]