Abstract
The genetic relationship among fecal vancomycin-resistant Enterococcus faecium (VREF) and vancomycin-susceptible E. faecium (VSEF) isolates (n = 178) from the same populations of pigs, human healthy volunteers, and hospitalized patients (from The Netherlands) and chickens (from The Netherlands and Greece) was studied by amplified-fragment length polymorphism (AFLP). The majority of VREF isolates from pigs, healthy volunteers, and hospitalized patients grouped together (genetic similarity, ≥65%). In a previous AFLP study by our group the VREF isolates from hospitalized patients grouped separately, most likely because these were clinical and not fecal isolates as in the present study. Furthermore, VSEF isolates from humans and pigs were found much more genetically diverse than VREF isolates, whereas VREF and VSEF isolates from chickens clustered together in a separate genogroup (genetic similarity, ≥65%), a pattern clearly distinct from the patterns for human and pig isolates. The present study suggests that pigs are a more important source of VREF for humans than chickens and that human- and pig-derived VSEF isolates seem much more heterogeneous than VREF isolates.
In countries of the European Union (EU) vancomycin-resistant enterococci (VRE) are relatively frequently found in healthy humans in the community and in farm animals and vancomycin resistance is mostly vanA mediated. This considerable pool of possibly transmissible isolates with vanA-mediated glycopeptide resistance in the EU is very likely caused by the use of avoparcin (an analogue of vancomcyin) as a growth promoter in animal husbandry until April 1997 (14, 16). Occasionally, genetically related VRE isolates have been found in food animals, meat products (5), outpatients, and hospitalized patients, suggesting that transmission between animals and humans can occur and may contribute to colonization and subsequent infection in humans (9, 11, 15).
Thus far, molecular comparisons by pulsed-field gel electrophoresis (PFGE) (1, 11, 15, 18) and amplified-fragment length polymorphism (AFLP) analysis (12, 21) of human- and animal-derived enterococci have been done only on VRE strains. These comparisons revealed the existence of a common human and pig genogroup, while poultry-derived vancomycin-resistant Enterococcus faecium (VREF) isolates clustered in a separate genogroup. However, the typing of the Tn1546 transposon (1, 4, 10, 13, 20, 22) showed that identical Tn1546 derivatives were found in humans and poultry, suggesting horizontal spread of the vanA transposon from poultry to humans.
Until now, it was not known whether VREF strains constitute a separate population within the E. faecium population and whether host-specific AFLP genogroups are also found among VSEF strains. Previous studies mainly focused on the genetic relationship among isolates from animals and humans within one country (11, 18). In this study, we used AFLP analysis to study the genetic relationship among 178 VREF and vancomycin-sensitive E. faecium (VSEF) strains isolated from fecal samples from humans and pigs in The Netherlands and from chickens in Greece and The Netherlands. AFLP has the ability to establish genetic relatedness between strains that, by PFGE, would show no similarity at all. PFGE is the reference standard for tracing the transmission of strains in hospital outbreaks but is too discriminatory to determine genetic relatedness among epidemiologically unrelated strains (7, 21).
The AFLP genogroups defined in the present study were compared to our previous results for host-specific genogroups among 255 VREF isolates from hospitalized patients, nonhospitalized persons, and various animal sources (pigs, poultry, calves, dogs, and cats) from nine different countries (21). Including vancomcyin-susceptible enterococci in molecular epidemiology studies might provide more insight into the composition of the enterococcal intestinal flora in humans and animals and help to further elucidate the transmission routes and persistence of E. faecium strains from animals in the human gut (21).
(Part of the study was presented as a poster at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, 15 to 19 December 2001 [abstr. 1875].)
MATERIALS AND METHODS
Bacterial isolates.
All VREF isolates and a comparable number of VSEF isolates were derived from four different populations of (i) healthy individuals (n = 537), (ii) hospitalized patients (n = 100), (iii) pigs (n = 126), and (iv) poultry (n = 139). The fecal samples from healthy individuals were collected from volunteers living in the north (n = 129), south (n = 171), and west (n = 133) of The Netherlands and were selected by using random addresses from the telephone directory in 1999. From the southern region fecal samples from healthy volunteers were also collected in 1996 (n = 104). The fecal samples from hospitalized patients came from patients admitted to the surgical ward of the University Hospital Maastricht in 1999. The poultry samples came from Greek (n = 50) (kindly provided by K. Sarris, Salonika, Greece) and Dutch slaughterhouses (n = 89). The last group of fecal samples were derived from a Dutch pig slaughterhouse. Multiple sampling from both pigs and poultry from the same farm was prevented by collecting feces from at most every 300th pig and by collecting only one chicken sample in the morning and one in the afternoon after evisceration at the slaughtering line.
All fecal samples were diluted 1:10 in 0.9% (wt/vol) NaCl supplemented with 20% (vol/vol) glycerol on the day of arrival at the bacteriological laboratory and were stored at −20°C until assayed. After the samples were thawed, 1:10 and 1:1,000 dilutions (40 μl each) were inoculated on KF-Streptococcus agar plates (CM701; Oxoid, Basingstoke, United Kingdom), with and without vancomycin (concentration: 10 mg/liter), by using a spiral plater (Salm en Kip BV, Utrecht, The Netherlands) as previously described (6, 17). After 48 h of incubation at 42°C, one typical enterococcal colony from each sample dilution was randomly chosen from the plates with and without vancomycin, and colonies were identified by generally accepted methods (2, 17).
In total 178 fecal E. faecium isolates were included into the study: 93 VREF isolates and 85 VSEF isolates. Sixty-four isolates were selected from healthy volunteers, of which 37 were VREF and 27 were VSEF. Seven VREF isolates and 12 VSEF isolates were derived from the hospitalized patients. A total of 35 (VREF, n = 16; VSEF, n = 19) and 34 isolates (VREF, n = 19; VSEF, n = 15) came from Greek and Dutch poultry farms, respectively, and 26 isolates were derived from a Dutch pig slaughterhouse (VREF, n = 15; VSEF, n = 11). None of the isolates in the present study were included in our previous study (21).
AFLP analysis.
All 178 isolates were analyzed by AFLP. DNA was isolated as described elsewhere (20), with the addition of a final ethanol precipitation step to further purify the DNA. The AFLP analysis and the degree of genetic similarity of ≥65% for the AFLP patterns to distinguish genogroups were as previously prescribed (21). This level of 65% was arbitrarily set and is not an absolute limit but distinguished four main genogroups and showed a clear association with the source of the strains. To form a specific genogroup, the minimal number of strains needed was five. The genogroups distinguished were compared to the genogroups of our previous study (21).
Genotypic diversity based on AFLP typing was calculated by the following equation: genotypic diversity (GD) = [n/(n − 1)](1 − Σxi2), where xi is the frequency of the ith identical AFLP type and n is the number of strains (8, 19). Two isolates were considered to have an identical AFLP type when the similarity of banding patterns was >95%. This cutoff of 95% was based on the previous finding that the degree of similarity between quadruplicate isolates of the same organism was 95 to 99% (21). When isolates are highly diverse, no two isolates have an identical AFLP type, and the calculated GD is 1. When all isolates are identical the GD is 0. Thus an increase in GD suggests an increased level of genetic heterogeneity.
Statistical analysis.
The chi square test (two-sided, P < 0.05) was used to analyze the differences in the distributions of the different experimental groups among the AFLP genogroups.
RESULTS
AFLP analysis.
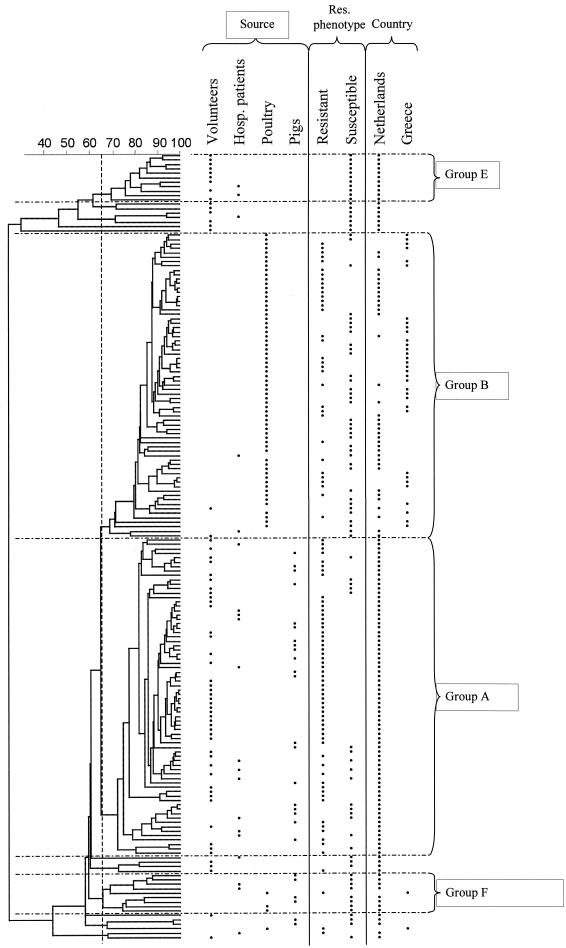
In total 178 strains, 93 VREF strains and 85 VSEF strains, from different animal and human sources, healthy volunteers, hospitalized patients, pigs, and poultry, were subjected to AFLP typing. Four main groups of strains that shared ≥65% of their restriction fragments were formed as a result of grouping by AFLP. Most VREF isolates from healthy volunteers (35 of 36) and hospitalized patients (6 of 7) and all VREF isolates from pigs (15 of 15) clustered in genogroup A (Fig. 1; Table 1), and all of these isolates clustered in our previously reported genogroup A (21).
FIG. 1.
Dendrogram of the genetic similarity of all isolates by AFLP analysis. Groups A, B, E, and F are based on a 65% genetic similarity. The dots indicate the sources, the vancomycin resistance phenotypes, and the countries of origin of the isolates.
TABLE 1.
Distribution of isolates of the different experimental groups among the AFLP genogroups
| AFLP genogroup | No. (%) of isolates of indicated type in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Healthy volunteersa
|
Hospital patients
|
Poultry
|
Pigsa
|
|||||
| VREF | VSEF | VREF | VSEF | VREF | VSEF | VREF | VSEF | |
| A | 35 (97%) | 6 (22%) | 6 (86%) | 5 (38%) | 15 (100%) | 5 (46%) | ||
| B | 2 (7%) | 2 (15%) | 33 (94%) | 32 (94%) | ||||
| E | 9 (33%) | 2 (15%) | ||||||
| F | 2 (15%) | 1 (3%) | 2 (6%) | 4 (36%) | ||||
| Unrelated | 1 (3%) | 10 (37%) | 1 (8%) | 2 (15%) | 1 (3%) | 2 (18%) | ||
| Total | 36 | 27 | 7 | 13 | 35 | 34 | 15 | 11 |
Significant differences (P < 0.05) in the distribution of the genogroups for the VREF and VSEF isolates from human volunteers and pigs were found.
Compared to the VREF isolates, the VSEF isolates from humans and pigs were genetically more heterogeneous and were dispersed over the entire dendrogram (Fig. 1). A significant difference in the distributions of genogroups between the VREF and VSEF isolates from human volunteers and pigs was found (P < 0.05; Table 1). New genogroups E and F, mainly consisting of VSEF isolates (11 of 11 and 8 of 9, respectively), were identified in addition to the previous published genogroups (21). The isolates that clustered in genogroup E were derived from healthy volunteers (9 of 11) and hospitalized patients (2 of 11), and the isolates in genogroup F were from hospitalized patients (2 of 9), pigs (4 of 9), and poultry (3 of 9) (Fig. 1; Table 1).
Fifteen strains were not assigned to any of the genogroups since they did not cluster in a group comprising at least 5 strains. The majority of these strains (12 of 15) were vancomycin sensitive and were isolated from healthy volunteers (10 of 12) and hospitalized patients (2 of 12) (Fig. 1; Table 1). The genetic heterogeneity of VSEF was also confirmed by the greater genotypic diversity of the vancomycin-sensitive enterococci isolated from human volunteers, hospitalized patients, and pigs (GD, 1.00, 0.97, and 0.95, respectively) than of the vancomycin-resistant isolates (GD, 0.77, 0.87, and 0.84, respectively) (Table 2).
TABLE 2.
Genotypic diversity found in the different experimental groups
| Source | Countrya | GDb of:
|
|
|---|---|---|---|
| VREF | VSEF | ||
| Healthy volunteers | NL | 0.77 | 1.00 |
| Hospitalized patients | NL | 0.87 | 0.97 |
| Pigs | NL | 0.84 | 0.95 |
| Poultry | NL, GR | 0.88 | 0.93 |
NL, The Netherlands; GR, Greece.
The formula for GD is given in Materials and Methods.
The vast majority of the poultry isolates (65 of 69) grouped in genogroup B (Fig. 1), and all clustered within the previously reported genogroup B (21). The poultry isolates grouped clearly separately from isolates recovered from humans and pigs, irrespective of whether they were VREF or VSEF and of Dutch or Greek origin (Fig. 1; Table 1). This clustering of poultry-related VREF and VSEF isolates also means that the difference in genotypic diversity between VSEF and VREF isolates from poultry (GD, 0.93 and 0.88, respectively) was smaller than that between VSEF and VREF isolates from humans and pigs (Table 2).
DISCUSSION
In our previous study, 255 VREF strains from different human and animal sources were subjected to AFLP typing. Four genogroups (A to D), with clustering of isolates from nonhospitalized volunteers and pigs in genogroup A, chickens in genogroup B, hospitalized patients in genogroup C, and vealers in genogroup D, were discriminated (21). In the present study, the VREF strains from (nonhospitalized) healthy volunteers and pigs also clustered in the same genogroup A. However, the strains from hospitalized patients also clustered in genogroup A, whereas in the previous study they formed genetically distinct genogroup C. This observed difference between the two studies may be explained by the fact that in this study the VREF isolates derived from hospitalized patients were not clinical isolates from infections or associated with hospital outbreaks but rather were isolates from the fecal flora. Therefore, it is very likely that the VREF isolates derived from hospitalized patients were already acquired in the community, as also found by Endtz et al. (3).
The VREF strains derived from healthy volunteers in 1996 and 1999 and from different regions in The Netherlands showed no specific clustering of AFLP patterns by year or place and mainly clustered in genogroup A (specific data not shown).
The genetic similarity between human and pig VREF isolates did not extend to VSEF isolates. Especially, the VSEF isolates derived from healthy volunteers were genetically more diverse then their resistant counterparts and were spread among genogroups A, B, and E. In addition, 38% did not cluster in one of the four main genogroups at all. Even though the numbers of VSEF strains derived from hospitalized patients (n = 12) and pigs (n = 11) were smaller, these strains also showed more genetic diversity than the resistant strains.
In contrast to the clear distinction between the relatively homogeneous VREF strains and relatively heterogeneous VSEF strains derived from both humans and pigs, the vast majority of VREF and VSEF strains derived from chickens clustered in genogroup B and displayed a more or less identical genotypic diversity. Perhaps the antibiotics especially used in poultry farming have already selected for a specific genetic poultry E. faecium population.
The finding that strains isolated from Dutch and Greek chickens clustered in genogroup B suggests that the clustering of strains may be more related to the type of animal they were isolated from than their geographic origin. However most broilers in the world not only are genetically related but also descend from only a few breeding centers. Hence a common source of contamination cannot be excluded.
The finding of a separate (VREF) poultry group (by AFLP analysis) distinct from human isolates was also reported in our previous study (21). Also, van den Braak et al. found two major PFGE types of VRE among poultry-derived strains that were not found in the fecal flora of patients (18). These results suggest that clonal transmission of VREF via the food chain from chickens to humans is less important than transmission from pigs to humans. This might be due to the fact that chicken-specific VREF strains may have difficulty in persisting in the human intestinal tract. Nevertheless, similarity of PFGE and AFLP types between turkeys and turkey farmers (11, 21) and between poultry and poultry farmers (21) has been found, indicating that clonal dissemination of poultry strains to corresponding farmers does occur.
For comparison among different typing methods a subset of strains from this study were subjected to a multilocus sequence typing (MLST) scheme developed by our group, and an article describing this MLST scheme for E. faecium has been published (3a).
In conclusion, human- and pig-derived VSEF strains are much more heterogeneous than their resistant counterparts and do not form a single genogroup. The VREF strains derived from humans and pigs are genetically different from the majority of the VSEF strains from pigs and humans and VREF and VSEF strains from poultry. These results suggest that pigs are a more important VREF source for humans than chickens by way of clonal dissemination. Why strains derived from chickens do not seem to persist or survive as well as pig strains in the human gut remains to be elucidated. More research on the strain-specific traits of strains derived from different human and animal sources, such as virulence factors and colonization and persistence abilities, is warranted, as they may play a crucial role in the transmission and survival of resistant bacteria in the human gut.
Acknowledgments
This work was financially supported by ZorgOnderzoek Nederland (ZON), The Netherlands (97-1-104).
REFERENCES
- 1.Descheemaeker, P. R. M., S. Chapelle, L. A. Devriese, P. Butaye, P. Vandamme, and H. Goossens. 1999. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob. Agents Chemother. 43:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devriese, L. A., A. V. D. Kerckhove, R. Klipper-Balz, and K. H. Schleifer. 1987. Characterization and identification of Enterococcus species isolated from the intestines of animals. Int. J. Syst. Bacteriol. 37:257-259. [Google Scholar]
- 3.Endtz, H. P., N. van den Braak, A. van Belkum, J. A. J. W. Kluytmans, J. G. M. Koeleman, L. Spanjaard, A. Voss, A. J. L. Weersink, C. M. J. E. Vandenbroucke-Grauls, A. G. M. Buiting, A. van Duin, and H. A. Verbrugh. 1997. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J. Clin. Microbiol. 35:3026-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. A. van Embden, and R. J. L. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen, L. B. 1998. Differences in the occurrence of two base pair variants of Tn1546 from vancomycin-resistant enterococci from humans, pigs, and poultry. Antimicrob. Agents Chemother. 42:2463-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klare, I., H. Heier, H. Claus, G. Böhme, S. Marin, G. Seltmann, R. Hakenbeck, V. Antanassova, and W. Witte. 1995. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb. Drug Resist. 1:265-272. [DOI] [PubMed] [Google Scholar]
- 6.London, N., R. Nijsten, A. van den Bogaard, and E. Stobberingh. 1993. Antibiotic resistance of fecal enterobacteriaceae isolated from healthy volunteers, a 15-week follow-up study. J. Antimicrob. Chemother. 32:83-91. [DOI] [PubMed] [Google Scholar]
- 7.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, L. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence tping: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nei, M., and F. Tajima. 1981. DNA polymorphism detectable by restriction endonucleases. Genetics 97:145-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robredo, B., K. V. Singh, F. Baquero, B. E. Murray, and C. Torres. 2000. Vancomycin-resistant enterococci isolated from animals and food. Int. J. Food Microbiol. 54:197-204. [DOI] [PubMed] [Google Scholar]
- 10.Schouten, M. A., R. J. L. Willems, W. A. G. Kraak, J. Top, J. A. A. Hoogkamp-Korstanje, and A. Voss. 2001. Molecular analysis of Tn1546-like elements in vancomycin-resistant enterococci isolated from patients in Europe shows geographic transposon type clustering. Antimicrob. Agents Chemother. 45:986-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stobberingh, E., A. van den Bogaard, N. London, C. Driessen, J. Top, and R. Willems. 1999. Enterococci with glycopeptide resistance in turkeys, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of The Netherlands: evidence for transmission of vancomycin resistance from animals to humans. Antimicrob. Agents Chemother. 43:2215-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vancanneyt, M., A. Lombardi, C. Andrighetto, E. Knijff, S. Torriani, K. J. Blorkroth, C. M. A. P. Franz, M. R. Foulquié Moreno, H. Revets, L. De Vuyst, J. Swings, K. Kersters, F. Dellaglio, and W. H. Holzapfel. 2002. Intraspecies genomic groups in Enterococcus faecium and their correlation with origin and pathogenicity. Appl. Environ. Microbiol. 68:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bogaard, A. E., L. B. Jensen, and E. E. Stobberingh. 1997. Vancomycin-resistant enterococci in turkeys and farmers. N. Engl. J. Med. 337:1558-1559. [DOI] [PubMed] [Google Scholar]
- 14.van den Bogaard, A. E., and E. E. Stobberingh. 1999. Antibiotic use in animals: impact on bacterial resistance and public health. Drugs 58:589-607. [DOI] [PubMed] [Google Scholar]
- 15.van den Bogaard, A. E., N. London, and E. E. Stobberingh. 2000. Antimicrobial resistance in pig fecal samples from The Netherlands (five abattoirs) and Sweden. J. Antimicrob. Chemother. 45:663-671. [DOI] [PubMed] [Google Scholar]
- 16.van den Bogaard, A. E., N. Bruinsma, and E. E. Stobberingh. 2000. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:145-153. [DOI] [PubMed] [Google Scholar]
- 17.van den Bogaard, A. E., M. Hazen, M. Hoyer, P. Oostenbach, and E. E. Stobberingh. 2002. Effects of flavophospholipol on resistance in fecal Escherichia coli and enterococci of fattening pigs. Antimicrob. Agents Chemother. 46:110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Braak, N., A. van Belkum, M. van Keulen, J. Vliegenhart, H. A. Verbrugh, and H. P. Endtz. 1998. Molecular characterization of vancomycin-resistant enterococci from hospitalized patients and poultry products in The Netherlands. J. Clin. Microbiol. 36:1927-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Loo, I. H. M., H. G. J. van der Heide, N. J. D. Nagelkerke, J. Verhoef, and F. R. Mooi. 1999. Temporal trends in the population structure of Bordetella pertussis during 1949-1996 in a highly vaccinated population. J. Infect. Dis. 179:915-923. [DOI] [PubMed] [Google Scholar]
- 20.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, D. J. Mevius, G. Hendriks, M. van Santen-Verheuvel, and J. D. A. van Embden. 1999. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob. Agents Chemother. 43:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van den Bogaard, and J. D. A. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]
- 22.Woodford, N., A.-M. A. Adebiyl, M.-F. I. Palepou, and B. D. Cookson. 1998. Diversity of VanA glycopeptide resistance elements in enterococci from humans and nonhuman sources. Antimicrob. Agents Chemother. 42:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]