Abstract
The objective of this study was to characterize the relationship between gentamicin concentrations during surgery and the development of wound infection following colorectal operations. Despite decades of research in surgical prophylaxis, the relationship between intraoperative antibiotic concentrations and postoperative infection and the concentrations required for effective prophylaxis have not been established. A pharmacodynamic analysis was conducted using data from a previous prospective, randomized, double-blind clinical study which compared two dosage regimens of gentamicin plus metronidazole for prophylaxis in connection with elective colorectal surgery. Univariate and multivariate analyses of risk factors for postoperative wound infection were conducted, and the relationship between intraoperative gentamicin concentrations and surgical outcome was characterized. The gentamicin concentration at the time of surgical closure was one of the strongest independent risk factors for infection (P = 0.02), along with the presence of diabetes mellitus (P = 0.02), stoma (P = 0.04), and advanced age (P = 0.05). Gentamicin concentrations at closure of less than 0.5 mg/liter were associated with an infection rate of 80% (representing 8 of 10 patients with concentrations below that level) (P = 0.003). Receiver operating characteristic curve analysis identified a critical closure concentration of 1.6 mg/liter for effective surgical prophylaxis (P = 0.002; sensitivity, 70.8%; specificity, 65.9%). This study provides new and important information on antibiotic pharmacodynamics in surgical prophylaxis. It demonstrates the critical effect of the antibiotic concentration at closure on wound infection and suggests a significant association between the concentration and other well-established risk factors, like the timing of preoperative antibiotic administration and surgery duration.
Surgical site infections, a significant postoperative complication, can lead to considerable patient morbidity and mortality (5, 14). Wound infections account for 38% of surgical infections and 17% of all nosocomial infections (14, 22). The benefits of preoperative antibiotics, which reduce bacterial contamination during clean-contaminated and contaminated operations, are well known (8, 14). However, the relationship between intraoperative antibiotic concentrations and postoperative infection and the concentrations required for effective prophylaxis have not been established. Over the past decade, pharmacodynamic research has advanced the treatment of infectious diseases by characterizing relationships between antibiotic concentrations and clinical response (7, 12, 20). Although the application of similar principles to surgical prophylaxis has been suggested, there is a notable lack of supportive study (1, 16, 18). It is probable that low antibiotic concentrations during surgery due to inappropriate timing of the preoperative antibiotic (3), prolonged surgery (9, 11, 21), and patient obesity (13, 17) contribute to the high infection rates associated with these factors. However, the direct effect of intraoperative antibiotic concentrations on surgical outcome has been largely overlooked by clinical studies, which have not included this variable in risk factor analyses. Pharmacodynamic data which characterize effective antibiotic concentrations during surgery could change the approach to surgical prophylaxis.
In a previous prospective, randomized, double-blind clinical study, regimens of single high doses of gentamicin (4.5 mg/kg of body weight preoperatively) and of multiple standard doses of gentamicin (1.5 mg/kg preoperatively and at 8, 16, and 24 h postoperatively), both in combination with metronidazole, were compared for prophylaxis in connection with colorectal surgery (24). Several observations suggested an association between low serum gentamicin concentrations during surgery and clinical failure. First, a trend towards fewer wound infections in the high-dose group suggested improved efficacy when higher antibiotic concentrations were achieved during surgery. Second, a strong association between prolonged surgery, which is a well-documented risk factor, and infection in the standard-dose but not in the high-dose group also supported an association between intraoperative antibiotic concentrations and clinical outcome. Our goal was to conduct a pharmacodynamic analysis of data from the original clinical study to characterize the relationship between intraoperative gentamicin concentrations and the development of wound infection following colorectal surgery. To our knowledge, this is the first such study in the area of surgical prophylaxis.
MATERIALS AND METHODS
Previous clinical study.
Data were obtained from a previous prospective, randomized, double-blind clinical study (number of patients, 146) of antibiotic prophylaxis for elective colorectal surgery (24). Study treatments consisted of either single high doses of gentamicin (4.5 mg/kg) plus metronidazole (500 mg) preoperatively or multiple standard doses of gentamicin (1.5 mg/kg) plus metronidazole (500 mg) preoperatively and at 8, 16, and 24 h postoperatively. Only those patients with serum creatinine levels of less than 150 μmol/liter were enrolled in the clinical study. Gentamicin doses were based on actual body weight or on dosing weight for subjects weighing more than 120% of their ideal body weight. Dosing weights were calculated according to the formula [0.40 × (actual body weight − ideal body weight)] + ideal body weight. The ideal body weight was defined as 50 kg plus 2.3 kg for each inch of height over 5 ft for males and 45.5 kg plus 2.3 kg for each inch of height over 5 ft for females. Metronidazole and gentamicin were infused over 30 min and administered sequentially. All patients received polyethelene glycol electrolyte lavage (Golytely; Baxter Corp., Mississauga, Ontario, Canada) or phosphate soda solution (Phosphasoda; Merck Frosst Canada Inc., Pointe Claire, Quebec, Canada) the day prior to surgery.
The primary clinical outcome was the development of surgical site infection within 30 days of surgery. Incisional infections were classified according to the Centers for Disease Control's standard definitions of superficial infections involving skin or subcutaneous tissues and deep infections involving deep tissue, including fascia or muscle (10). Subjects were monitored for systemic (e.g., fever, chills, or leukocytosis) and local (e.g., erythema, swelling, or purulent drainage) signs of infection.
Pharmacokinetics.
Two blood samples were collected from the first 35 participants after the administration of the preoperative gentamicin dose. The first sample was drawn at least 30 min after the infusion, and the second sample was collected in the recovery room. Concentrations of gentamicin in serum were measured by fluorescence immunoassay (TDx; Abbott, Chicago, Ill.). The limit of detection was 0.2 μg/liter, and the coefficients of variation were 10.8% at 2.2 μg/liter and 6.5% at 13.4 μg/liter. The gentamicin assay results were concealed until the end of the study.
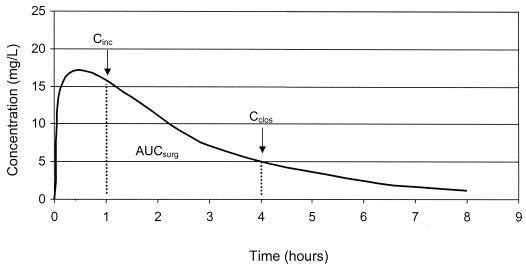
A reference group (n = 16) of subjects was constructed; for this group, two measurements of serum gentamicin concentrations from appropriately collected blood samples were available. Parameters, including the elimination rate constant (kel) and volume of distribution (V), were calculated by using a one-compartment, linear pharmacokinetic model. Correlations between estimated creatinine clearance (CLCR) (4) and kel and between weight and V were determined by linear regression analysis. A validation group (n = 19) was composed of subjects other than those in the reference group, with at least one measurement of gentamicin concentration. These individuals were excluded from the reference group because samples were not collected in 1 case, drawn too close to the administration of the dose in 12 cases, drawn too closely together in 4 cases, and not documented in 2 cases. The pharmacokinetic model was derived from data from the reference group and was tested with the validation group. The model was then used to construct profiles of serum gentamicin concentration versus time and to predict the concentration at the time of incision, the concentration at the time of closure, and the area under the concentration-versus-time curve during surgery (AUCsurg) for all other subjects (Fig. 1).
FIG. 1.
Profile of serum gentamicin concentration versus time (Cinc, incision concentration; Cclos, closure concentrations).
Pharmacodynamics.
Separate risk factor analyses were conducted with the high-dose and standard-dose groups. Univariate analyses were used to test standard variables (i.e., age, gender, weight, inflammatory bowel disease, malignancy, diabetes mellitus, chronic corticosteroid use, surgeon, operation, stoma, intraoperative core temperature, timing of the preoperative antibiotic, and surgery duration) in addition to pharmacodynamics parameters (i.e., gentamicin concentration at the time of incision, concentration at closure, and AUCsurg). Two-tailed t tests were used for continuous data, Wilcoxon rank tests were used for ordinal data, and Fisher's exact or chi-square tests were used for nominal variables (α = 0.05). Multivariate logistic regression analysis with backward elimination was used to identify independent risk factors. The polynomial equation from the model was then used to predict the probability of infection as follows:
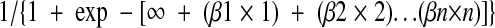 |
(1) |
Receiver operating characteristic (ROC) curves were used to further analyze significant gentamicin concentration parameters. All statistical analyses were performed with SPSS for Windows, release 10 (SPSS Inc., Chicago, Ill.).
RESULTS
Twelve of the clinical study participants were excluded because the timing of the preoperative antibiotic was not documented. Therefore, 134 subjects, of which 68 received the high-dose regimen and 66 received the standard-dose regimen, were included in the pharmacodynamic study. As shown in Table 1, there were no significant differences in characteristics between the high-dose and standard-dose regimens.
TABLE 1.
Patient characteristics
| Characteristic | Valuea for group |
P value | |
|---|---|---|---|
| High dose (n = 68) | Standard dose (n = 66) | ||
| Age (yr) | 56.6 ± 18.9b | 56.8 ± 17.7 | 1.0 |
| Gender (male) | 37 (54.4) | 39 (59.1) | 0.6 |
| Normalized wt (actual/ideal wt) | 1.16 ± 0.18 | 1.17 ± 0.24 | 0.8 |
| CLCR (ml/min/1.72 m2) | 102 ± 25 | 104 ± 22 | 1.0 |
| Presence of: | |||
| Inflammatory bowel disease | 25 (36.8) | 15 (22.7) | 0.09 |
| Malignancy | 35 (51.5) | 42 (63.6) | 0.2 |
| Diabetes mellitus | 5 (7.4) | 8 (12.3) | 0.5 |
| Chronic corticosteroid use | 11 (16.2) | 10 (15.2) | 0.9 |
| Right colon operation | 18 (26.5) | 19 (28.8) | 0.9 |
| Stoma | 33 (48.5) | 22 (33.3) | 0.09 |
| Intraoperative core temp (°C) | 35.6 ± 0.8 | 35.3 ± 0.7 | 0.10 |
| Timing of preoperative antibiotic (min) | 71 ± 33 | 67 ± 35 | 0.5 |
| Surgery duration (min) | 196 ± 81 | 186 ± 65 | 0.5 |
| Surgical site infections (total) | 19 (27.9) | 24 (36.4) | 0.27 |
| Deep | 6 (8.8) | 4 (6.1) | |
| Superficial | 13 (19.1) | 20 (30.3) | |
Except where otherwise indicated, values are given in number (percent) of patients.
Values expressed as means ± standard deviations.
Pharmacokinetics.
In the reference group (n = 16), the mean kel was 0.34 h−1 (95% confidence interval [CI95], 0.29 to 0.39 h−1), which corresponds to a mean harmonic half-life of 2 h, and the mean V was 0.23 liter/kg (CI95, 0.19 to 0.26 liter/kg). The pharmacokinetic model was described by the following equations:
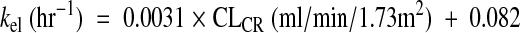 |
(2) |
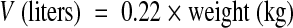 |
(3) |
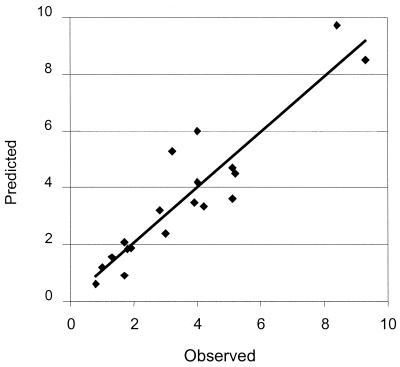
In the validation analysis (Fig. 2), the pharmacokinetic model showed excellent correlations between predicted and measured serum gentamicin concentrations (r2 = 0.85), with good measures of precision (0.92 mg/liter) and bias (0.031 mg/liter). The gentamicin concentration parameters measured for the reference group and predicted for all other subjects are provided in Table 2.
FIG. 2.
Predicted versus observed serum gentamicin concentrations (mg/liter) in the validation analysis (n = 19).
TABLE 2.
Gentamicin concentration parameters
| Dosage group | Concn (mg/liter)a at time of: |
AUCsurg (mg · h/liter)a | |
|---|---|---|---|
| Incision | Closure | ||
| High dose (n = 68) | 14.5 ± 3.8 | 4.7 ± 2.6 | 26.1 ± 8.9 |
| Standard dose (n = 66) | 5.2 ± 1.3 | 1.8 ± 1.0 | 8.8 ± 2.5 |
Values are given as means ± standard deviations. P values for all concentrations were <0.0001.
Pharmacodynamics.
In univariate analyses, several risk factors for infection were identified in the standard-dose group but none were observed in the high-dose group. In the standard-dose group, the gentamicin concentration at the time of closure (P = 0.001), the concentration at incision (P = 0.001), the surgery duration (P = 0.001), the presence of diabetes mellitus (P = 0.003), the presence of stoma (P = 0.03), and the timing of the preoperative antibiotic (P = 0.03) were associated with infection. As detailed in Table 3, however, only the gentamicin concentration at closure (P = 0.02) and the presence of diabetes mellitus (P = 0.02; odds ratio, 18.2; CI95, 1.7 to 193.8), stoma (P = 0.04; odds ratio, 4.3; CI95, 1.1 to 17.3), and advanced age (P = 0.05) were independent risk factors for infection. A concentration at closure of less than 0.5 mg/liter in 10 participants was associated with an 80% infection rate (P = 0.003; odds ratio, 2.8). The overall probability of infection is described by the following equation:
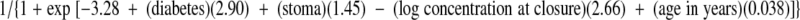 |
(4) |
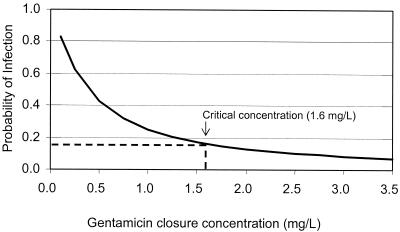
where the values for diabetes and stoma were equal to 1 if present and 0 if absent. Figure 3 simulates the effect of the gentamicin concentration at closure on the probability of surgical site infection in a representative 57-year-old patient population. ROC curve analysis identified a critical value for the gentamicin concentration at closure of 1.6 mg/liter for effective surgical prophylaxis (P = 0.002, sensitivity = 70.8%, specificity = 65.9%).
TABLE 3.
Independent risk factors for infection in the standard-dose group
| Group (n) | Gentamicin concn at time of closure (mg/liter)a | No. (%) with: |
Age (yr)a | |
|---|---|---|---|---|
| Diabetes mellitus | Stoma | |||
| With infection (24) | 1.3 ± 1.0 | 7 (29.2) | 12 (50) | 59 ± 14 |
| Without infection (42) | 2.1 ± 0.9b | 1 (2.4)c | 10 (23.8)d | 55 ± 19e |
Values are given as means ± standard deviations.
Significantly different from results for the group with infection at a P of 0.02.
Significantly different from results for the group with infection at a P of 0.02 (odds ratio, 18.2; CI95, 1.7 to 193.8).
Significantly different from results for the group with infection at a P of 0.04 (odds ratio, 4.3; CI95, 1.1 to 17.3).
Significantly different from results for the group with infection at a P of 0.05.
FIG. 3.
Probability of postoperative wound infection and gentamicin concentration at closure.
DISCUSSION
Although the goal of surgical prophylaxis is to maintain adequate antibiotic exposure during surgery, research establishing effective intraoperative concentrations has not been conducted (1, 16, 18). This study provides new and important information on antibiotic pharmacodynamics in surgical prophylaxis. In the standard-dose group, the risk of wound infection was dependent on the gentamicin concentration at closure and on the presence of diabetes mellitus, stoma, and, to a lesser degree, advanced age. Other well-established risk factors, including the timing of preoperative antibiotic and surgery duration, were identified in univariate tests but were not independently associated with infection. The identification of the gentamicin concentration at closure as an independent variable suggests that a low antibiotic concentration was the predominant risk associated with inappropriate timing of the preoperative antibiotic and with prolonged surgery.
Notably, no risk factors for infection were identified in the otherwise-matched, high-dose group. This result could represent a gentamicin dose (i.e., 4.5 mg/kg) that produced adequate concentrations at closure for surgical prophylaxis and which therefore did not characterize the relationship between low antibiotic concentrations and clinical failure. Furthermore, neither timing of preoperative antibiotic nor surgery duration was significantly associated with infection in the high-dose group. This could also indicate that the gentamicin dose achieved sufficient intraoperative concentrations even in cases where the timing of the preoperative antibiotic administration was too early or the surgery duration was prolonged.
In the treatment of infectious diseases, antibiotic concentrations are usually related to the pathogen and its susceptibility, as indicated by the MIC. Pharmacodynamic indices such as the peak concentration-to-MIC ratio, time above MIC, and AUC divided by MIC are analyzed to identify those which best correlate with the eradication of microbes or with clinical cure. The application of such principles to the prevention of infection is uncertain (1, 16, 18). However, this study shows the importance of gentamicin concentrations at surgical closure and identifies a critical value of 1.6 mg/liter for effective prophylaxis. This suggests that standard gentamicin doses, as used in our clinical study, may be suboptimal for colorectal operations. For example, a 1.5-mg/kg preoperative gentamicin dose would require a second dose in 3.8 h to maintain concentrations above 1.6 mg/liter (assuming a patient weighing 70 kg, a kel of 0.35 h−1, and a V of 0.25 liter/kg). A 4.5-mg/kg dose would extend the coverage to 6.9 h, which may be more appropriate for operations with a mean duration exceeding 3 h. The critical gentamicin concentration of 1.6 mg/liter must be interpreted with some caution. First, the value was determined for a specific antibiotic and patient population and may not apply to other operations, for example. Second, it was derived from a retrospective analysis of clinical study data and therefore may have been influenced by the range of intraoperative concentrations available. Although a prospective investigation of preselected and targeted antibiotic concentrations would provide the most complete pharmacodynamic characterization, it would also require the deliberate administration of low, presumably ineffective, doses resulting in clinical failure. This method of study has obvious ethical barriers.
One limitation of this study was the application of pharmacokinetic data from a reference group to all other subjects. However, the pharmacokinetic model produced pharmacokinetic parameters that were within expected ranges and that demonstrated excellent predictive performance in the validation analysis (6, 23). The model described a relatively homogenous population of participants with normal renal function who were admitted for elective surgery.
This study was based on the assumption that bacterial wound infections are located in interstitial spaces and that concentrations in serum reflect those in interstitial fluids. First, clean incisional sites and other uninfected tissues are represented by high ratios of surface area to volume and by rapid equilibration of antibiotic levels between serum and wound fluid (2, 15, 19). The use of gentamicin, with low protein binding and rapid distribution into extracellular fluid, further justifies the use of concentrations in serum to approximate intraoperative levels in tissue.
This study demonstrates the critical effect of antibiotic concentration at closure on wound infection following colorectal surgery. The results also suggest a significant association between low concentrations and high infection rates found with other well-established risk factors. Finally, this study shows the value of pharmacodynamic research in surgical prophylaxis and the need for investigations of other antibiotics for colorectal and other operations.
Acknowledgments
We acknowledge the contributions of Mary Cheang, Department of Community Health Sciences, Department of Medicine, University of Manitoba, for biostatistical consultation.
REFERENCES
- 1.Bergamini, T. M., and H. C. Polk, Jr. 1989. Pharmacodynamics of antibiotic penetration of tissue and surgical prophylaxis. Surg. Gynecol. Obstet. 168:283-289. [PubMed] [Google Scholar]
- 2.Cars, O. 1990. Pharmacokinetics of antibiotics in tissues and tissue fluids: a review. Scand. J. Infect. Dis. Suppl. 74:23-33. [PubMed] [Google Scholar]
- 3.Classen, D. C., R. S. Evans, S. L. Pestotnik, S. D. Horn, R. L. Menlove, and J. P. Burke. 1992. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N. Engl. J. Med. 326:281-286. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger, E. P., P. A. Gross, T. L. Barrett, P. J. Krause, W. J. Martone, J. E. McGowan, Jr., R. L. Sweet, R. P. Wenzel, and the Infectious Disease Society of America. 1994. Quality standard for antimicrobial prophylaxis in surgical procedures. Clin. Infect. Dis. 18:422-427. [DOI] [PubMed] [Google Scholar]
- 6.Dettli, L. C. 1974. Drug dosage in patients with renal disease. Clin. Pharmacol. Ther. 16:274-280. [DOI] [PubMed] [Google Scholar]
- 7.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyssens, I. C. 1999. Preventing postoperative infections: current treatment recommendations. Drugs 57:175-185. [DOI] [PubMed] [Google Scholar]
- 9.Haley, R. W., D. H. Culver, W. M. Morgan, J. W. White, T. G. Emori, and T. M. Hooton. 1985. Identifying patients at high risk of surgical wound infection. A simple multivariate index of patient susceptibility and wound contamination. Am. J. Epidemiol. 121:206-215. [DOI] [PubMed] [Google Scholar]
- 10.Horan, T. C., R. P. Gaynes, W. J. Martone, W. R. Jarvis, and T. G. Emori. 1992. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 13:606-608. [PubMed] [Google Scholar]
- 11.Kaiser, A. B., J. L. Herrington, Jr., J. K. Jacobs, J. L. Mulherin, Jr., A. C. Roach, and J. L. Sawyers. 1983. Cefoxitin versus erythromycin, neomycin, and cefazolin in colorectal operations. Importance of the duration of the surgical procedure. Ann. Surg. 198:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashuba, A. D., A. N. Nafziger, G. L. Drusano, and J. S. Bertino, Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob. Agents Chemother. 43:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilienfeld, D. E., D. Vlahov, J. H. Tenney, and J. S. McLaughlin. 1988. Obesity and diabetes as risk factors for postoperative wound infections after cardiac surgery. Am. J. Infect. Control 16:3-6. [DOI] [PubMed] [Google Scholar]
- 14.Mangram, A. J., T. C. Horan, M. L. Pearson, L. C. Silver, W. R. Jarvis, and Centers for Disease Control and Prevention Hospital Infection Control Practices Advisory Committee. 1999. Guideline for prevention of surgical site infection, 1999. Am. J. Infect. Control 27:96-134. [PubMed] [Google Scholar]
- 15.Mazzei, T., F. Tonelli, A. Novelli, F. Ficari, C. Mazzoni, A. Anastasi, and P. Periti. 1994. Penetration of cefotetan into suction skin blister fluid and tissue homogenates in patients undergoing abdominal surgery. Antimicrob. Agents Chemother. 38:2221-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novelli, A. 1999. Antimicrobial prophylaxis in surgery: the role of pharmacokinetics. J. Chemother. 11:565-572. [DOI] [PubMed] [Google Scholar]
- 17.Nystrom, P. O., A. Jonstam, H. Hojer, and L. Ling. 1987. Incisional infection after colorectal surgery in obese patients. Acta Chir. Scand. 153:225-227. [PubMed] [Google Scholar]
- 18.Polk, H. C., Jr., and A. B. Christmas. 2000. Prophylactic antibiotics in surgery and surgical wound infections. Am. Surg. 66:105-111. [PubMed] [Google Scholar]
- 19.Ryan, D. M., O. Cars, and B. Hoffstedt. 1986. The use of antibiotic serum levels to predict concentrations in tissues. Scand. J. Infect. Dis. 18:381-388. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Recio, M. M., C. I. Colino, and A. Sanchez-Navarro. 2000. A retrospective analysis of pharmacokinetic/pharmacodynamic indices as indicators of the clinical efficacy of ciprofloxacin. J. Antimicrob. Chemother. 45:321-328. [DOI] [PubMed] [Google Scholar]
- 21.Velasco, E., L. C. Thuler, C. A. Martins, L. M. Dias, and V. M. Conalves. 1996. Risk factors for infectious complications after abdominal surgery for malignant disease. Am. J. Infect. Control 24:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein, R. A. 1998. Nosocomial infection update. Emerg. Infect. Dis. 4:416-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaske, D. 1992. Aminoglycosides. In J. S. W. Evans and W. Jusko (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics, Inc., Vancouver, Wash.
- 24.Zelenitsky, S. A., R. E. Silverman, H. Duckworth, and G. K. Harding. 2000. A prospective, randomized, double-blind study of single high dose versus multiple standard dose gentamicin both in combination with metronidazole for colorectal surgical prophylaxis. J. Hosp. Infect. 46:135-140. [DOI] [PubMed] [Google Scholar]