Abstract
With increasing availability of drugs for impotence and advanced reproductive technologies for the treatment of subfertility, more men are fathering children at advanced ages. We conducted a study of the chromosomal content of sperm of healthy men aged 24–57 years to (a) determine whether father’s age was associated with increasing frequencies of aneuploid sperm including XY, disomy X, disomy Y, disomy 21, and sperm diploidy, and (b) examine the association between the frequencies of disomy 21 and sex-chromosomal aneuploidies. The study group consisted of 38 fathers of boys with Klinefelter syndrome (47, XXY) recruited nationwide, and sperm aneuploidy was assessed using multicolor X-Y-21 sperm FISH (∼10,000 sperm per donor). Paternal age was significantly correlated with the sex ratio of sperm (Y/X; P=.006) and with the frequency of XY sperm (P=.02), with a clear trend with age by decades (P<.006). Compared with fathers in their 20s (who had an average frequency of 7.5 XY sperm per 10,000), the frequencies of XY sperm were 10% higher among fathers in their 30s, 31% higher among those in their 40s, and 160% higher among those in their 50s (95% CI 69%–300%). However, there was no evidence for age effects on frequencies of sperm carrying nullisomy sex; disomies X, Y, or 21; or meiosis I or II diploidies. The frequencies of disomy 21 sperm were significantly associated with sex-chromosomal aneuploidy (P=.04)—in particular, with disomy X (P=.004), but disomy 21 sperm did not preferentially carry either sex chromosome. These findings suggest that older fathers produce higher frequencies of XY sperm, which may place them at higher risk of fathering boys with Klinefelter syndrome, and that age effects on sperm aneuploidy are chromosome specific.
Introduction
Trisomy is the most common chromosomal abnormality among human live births (Hook 1985; Hecht and Hecht 1987; Hassold et al. 1993; Wyrobek 1994). Although the autosomal trisomies (i.e., trisomy 21, 18, 13, etc.) are primarily of maternal origin, sex-chromosome aneuploidies—such as Klinefelter syndrome (KS; 47,XXY), Turner syndrome (45,XO), and XYY—have more-substantial paternal contributions (Juberg and Mowrey 1983; Magenis 1988; Antonarakis 1993; Hassold et al. 1993). Constitutive aneuploidies are thought to arise in male or female germ cells at meiosis I or II (Hook 1985; Hassold et al. 1993; Wyrobek 1994; Bishop et al. 1996). However, the underlying molecular mechanism of induction and the contributing risk factors remain uncertain. In addition, there is uncertainty as to whether the mechanisms that lead to aneuploidy affect all chromosomes equally.
It is well established that increased maternal age has adverse effects on pregnancy outcome and aneuploidy (Hook 1976; Creasy and Polani 1978; Juberg and Mowrey 1983; Hassold et al. 1993; Huether et al. 1998). However, the evidence for paternal age as a risk factor for aneuploid births remains ambiguous (Bordson and Leonardo 1991; Lorda-Sanchez et al. 1992; de Michelena et al. 1993; MacDonald et al. 1994). Unlike women, men are able to produce gametes over their entire lifetime. Life expectancy has increased dramatically over the past century (Kranczer 1998), and the proportion of births with fathers aged >35 years has markedly increased in the past decades (Ventura et al. 1997). In addition, the availability of new drugs for male impotence (e.g., sildenafil [Viagra]) and advanced technologies for the treatment of male subfertility (e.g., in vitro fertilization and intracytoplasmic sperm injection [ICSI]) have increased the opportunities for older fathers to have children. Several studies have shown increased incidences of sex-chromosome abnormalities in children conceived by ICSI (In’t Veld et al. 1995, 1997; Liebaers et al. 1995), raising concern over the chromosomal quality of sperm among the donors.
We conducted a study of the effects of paternal age on the incidence of sperm aneuploidy in a special group of fathers who have at least one young son with Klinefelter syndrome (KS; 47,XXY). The populationwide incidence of KS is ∼1/600 among live-born male infants, and the phenotypic traits associated with KS include infertility, physical abnormalities, and mental deficiency (Lorda-Sanchez et al. 1992; Hinney et al. 1997; Estop et al. 1998). Previous literature indicated that 40%–50% of KS cases are of paternal origin (Hassold et al. 1993; MacDonald et al. 1994). A companion study has addressed the relationships between parental origin of the extra X chromosome and paternal sperm disomy (Eskenazi B, Wyrobek AJ, Kidd SA, Lowe X, Moore D II, Weisiger K, Aylstock M, unpublished data). The current study used a multicolor sperm FISH procedure designed to detect sperm carrying any of four categories of disomies, three categories of nullisomies, and three categories of diploidies, using probes for chromosomes X, Y, and 21 (table 1). The sperm aneuploidies measured in this study are clinically relevant; for example, disomy X is related to triple X, disomy Y to XYY syndrome, disomy 21 to Down syndrome, XY to Klinefelter syndrome, XO to Turner syndrome, and the sperm diploidies to triploid embryos. This study was designed to investigate (1) whether there are age-dependent increases in aneuploid sperm in these fathers, specifically XY sperm; (2) whether there were associations between the frequencies of sperm with disomy 21 and sex-chromosomal disomies; and (3) whether there was a preferential segregation of disomy 21 sperm with either sex chromosome.
Table 1.
Average Frequencies of Aneuploid Sperm Detected in Each Age Group Using X-Y-21 Sperm FISH Assay
|
Sex Chromosomea |
Chromosome 21a |
Diploidya |
||||||||||||||||||
|
Normal |
HyperhaploidyMeiosis IError |
HyperhaploidyMeiosis IIError |
Nullisomy |
Disomy |
Nullisomy |
Meiosis IError |
Meiosis IIError |
|||||||||||||
| Total Cells Scored | Y-21 | X-21 | SexRatio(Y/X) | All | X-Y-21c | X-X-21 | Y-Y-21 | 21_ | X-21-21 | Y-21-21 | Total | X_ | Y_ | Total | X-Y-21-21 | X-X-21-21 | Y-Y-21-21 | Total | DoubleErrorsd | |
| Age group (n): | ||||||||||||||||||||
| 20–29 (4) | 40,266 | 19,902 | 20,086 | .99 | 66.1 (13.2) | 7.5 (0.6) | 5.0 (1.4) | 4.0 (2.9) | 5.2 (1.2) | 4.7 (1.7) | 4.2 (1.2) | 8.9 (1.3) | .7 (0.5) | 1.2 (2.5) | 2.0 (2.7) | 9.2 (4.9) | 5.2 (1.7) | 7.2 (5.3) | 21.6 (9.5) | 1.5 (1.7) |
| 30–39 (16) | 157,653 | 77,905 | 78,683 | .99 | 66.9 (28.6) | 9.3 (4.8) | 4.7 (2.1) | 4.1 (3.6) | 5.9 (5.1) | 3.9 (2.8) | 2.8 (2.4) | 6.6 (4.7) | 2.0 (1.7) | 1.3 (1.8) | 3.2 (3.1) | 6.9 (4.2) | 3.6 (2.9) | 3.7 (3.0) | 14.3 (8.4) | 1.1 (0.8) |
| 40–49 (14b) | 150,573 | 74,555 | 74,528 | 1.00 | 94.2 (100.0) | 12.7 (8.0) | 4.8 (1.7) | 6.4 (3.7) | 6.9 (4.9) | 3.3 (1.9) | 3.2 (2.6) | 6.5 (4.2) | 1.8 (1.7) | 2.0 (1.7) | 3.9 (3.2) | 19.4 (39.5) | 8.8 (14.2) | 8.8 (14.3) | 36.9 (67.5) | 1.0 (1.8) |
| 50–59 (4b) | 50,189 | 24,858 | 24,913 | 1.00 | 69.2 (11.4) | 17.6 (8.0) | 4.2 (1.7) | 2.6 (2.5) | 6.0 (1.2) | 2.9 (0.6) | 2.9 (1.2) | 5.7 (1.7) | 2.0 (1.4) | 2.7 (2.2) | 4.7 (3.6) | 7.8 (1.0) | 5.1 (1.4) | 6.4 (5.1) | 19.3 (5.6) | .4 (0.5) |
| Total | 398,681 | 197,220 | 198,210 | 3,032 | 454 | 186 | 186 | 248 | 143 | 124 | 267 | 70 | 68 | 138 | 459 | 224 | 241 | 924 | 41 | |
| Frequency/10,000 cells | 1.00 | 76.0 | 11.4 | 4.7 | 4.7 | 6.2 | 3.6 | 3.1 | 6.7 | 1.8 | 1.7 | 3.5 | 11.5 | 5.6 | 6.0 | 23.2 | 1.0 | |||
| P for trende | .068 | .650 | .006 | .620 | .600 | .660 | .190 | .540 | .270 | .190 | .240 | .150 | .770 | .670 | .810 | .710 | .140 | |||
| Change/year | .05% | 1.20% | 3.50% | .09% | .25% | 1.30% | −1.10% | .53% | −.34% | 2.50% | 4.30% | 3.70% | 1.80% | 1.80% | 1.50% | 1.80% | −3.10% | |||
| P for changee | .021 | .290 | .002 | .930 | .870 | .370 | .400 | .720 | .780 | .160 | .097 | .059 | .380 | .370 | .470 | .340 | .210 | |||
| Kendell's τ | .310 | .130 | .260 | .013 | .078 | .130 | −.083 | .044 | −.036 | .170 | .260 | .260 | .041 | .130 | .063 | .092 | −.110 | |||
| P for correlatione | .006 | .240 | .023 | .910 | .490 | .270 | .460 | .700 | .750 | .130 | .018 | .020 | .710 | .260 | .580 | .410 | .340 | |||
Average of rates per 10,000 cells over donors in age group (standard deviation of rates in parenthesis). Hypohaploidy is represented by an underline.
One specimen in this group was rehybridized and rescored for quality control, and all counts from the two hybridizations were combined.
Compared with that in the 20s age group, the average rate of XY sperm is significantly increased with age in the 40s group (P=.08) and the 50s group (P=.01). Full FISH genotypes are shown (e.g., X-Y-21 for XY sperm, X-X-21 for disomy X, etc.).
Double errors include XX_, YY_, XY_, 21 21_.
P values <.05 appear in bold italics.
Material and Methods
Participants
A total of 38 men who had fathered boys with KS were recruited nationwide through the membership list of Klinefelter Syndrome and Associates, an advocacy and education group, and by referral from genetic counselors. Fathers were eligible for enrollment if they were healthy by self report and their affected child was <6 years old. This study was approved by the institutional review boards of the State of California, the University of California at Berkeley, and the Lawrence Livermore National Laboratory (LLNL); written informed consent was obtained. Semen samples were obtained by masturbation, were frozen within 2 h of collection, were shipped to LLNL on dry ice, and then were coded and stored at −80°C, without fixative, until use.
Multicolor Sperm FISH
Using the X-Y-21 assay described by Baumgartner et al. (1999), which utilizes DNA probes for chromosomes X, Y, and 21, we were able to detect sperm in four categories of hyperhaploidy, three categories of hypohaploidy, and three categories of diploidy, as well as complex sperm genotypes involving these three chromosomes (table 1). This assay also allows us to assess whether the disomy and diploidy errors occurred during meiosis I (X-Y-21 and X-Y-21-21) or meiosis II (X-X-21, Y-Y-21, X-X-21-21, and Y-Y-21-21). The overall hybridization efficiency across all the men of our study was ∼99.9% (data not shown).
Semen aliquots were thawed at room temperature and were mixed thoroughly, but gently, with a pipette. Five microliters of seminal fluid were smeared in a thin layer onto ethanol-cleaned glass slides and were air dried overnight at room temperature. Slides were then used for hybridization or were stored at −20°C with a desiccant in a nitrogen atmosphere.
Decondensation of sperm chromatin (Wyrobek et al. 1990; Robbins et al. 1993) was achieved by placing the semen smear in 10 mM dithiothreitol (DTT) on ice for 30 min, followed by incubation in 4 mM lithium 3,5-diiodosalicylic acid (LIS) at room temperature for 90 min. Slides were air dried and then were denatured for 6 min in 70% formamide, 2× salt and sodium citrate [SSC], pH 7.0, at 78°C. A mix of labeled probes was used: 1 μL each of SpectrumGreen- and SpectrumOrange-labeled chromosome enumerated probe X for chromosome X (Vysis), 1 μL fluorescein isothiocynate–labeled probe for the DYZ1 locus of chromosome Y (Oncor), and 1 μL locus-specific probe that hybridizes to chromosome 21, q22.13-q22.2 (LSI) labeled with SpectrumOrange (Vysis). Probes were added to a hybridization solution so that the final concentration was 55% formamide, 10% dextran sulfate, 2× SSC, pH 7.0. After denaturation at 78°C for 6 min, the probe mixture was incubated with denatured smears over two nights at 37°C.
Posthybridization washes were performed for 10 min each in 50% formamide, 2× SSC, pH 7.0, at 42°C, 2× SSC at 37°C, and 2× SSC at room temperature. Five microliters of DAPI (4′-6-diamidino-2-phenylindole; Sigma) in Vectashield (Vector Laboratories) was applied in a concentration of .01 μg/ml for DNA counterstaining.
Data Collection under the Microscope
A Zeiss Axioplan fluorescence microscope with an Osram HBO 100W/2 mercury lamp was used, equipped with various filter sets as described by Robbins et al. (1995). All slides were coded, and a minimum of 10,000 sperm per semen sample were analyzed manually by a single scorer using Cytoscore, a computer program developed at the LLNL, for data entry and data management. Cytoscore is also designed to prevent the scorer from retrieving the data during scoring to avoid bias. The following blinded replicate coding procedure was used: slides were randomized and encoded by a second person (who was not a scorer), and 5,000 sperm were scored in a specified region of the hybridization area; then, every slide was recoded, and an additional 5,000 sperm were scored on a separate area of the same slide by the same scorer. The two data sets for each slide were accepted if they did not differ according to χ2 analyses.
Strict scoring criteria were applied to the multicolor sperm FISH assays. Normal sperm were 21-X (cells containing one red and one yellow signal) and 21-Y (containing one red and one green signal). For a sperm nucleus to be considered to have an abnormal number of fluorescent domains, the characteristics (intensities and sizes) of the affected domains were required to be similar to those of the domains of nearby normal cells, and the domains were positioned within the nuclear perimeter. When two domains of the same color were present within a cell, they were required to be separated by a distance of at least one half of their average diameter (for chromosomes X and Y) or equal to it (for chromosome 21) and to be of similar sizes and intensities. All cells with abnormal fluorescent phenotypes were inspected under single- and dual-band-pass filters to determine whether one signal was located below or above a larger one. All abnormal cells were also evaluated under simultaneous dual-band-pass fluorescence and phase-contrast imaging, to determine how many flagella were attached to the nucleus. All diploid sperm were distinguished from somatic cells by the presence of flagella or a tail-implantation site under phase-contrast microscopy.
The reproducibility of hybridization and scoring criteria of our sperm FISH procedure was evaluated by a complete repeat analysis, performed 1 year later (including rehybridization and rescoring), of two subjects, whose fractions of aneuploid sperm were at the high and low ends, respectively, of the range of aneuploidy frequencies. One year after the first slide was scored, a second slide from each man that had been stored at −20°C for ∼1 year was then hybridized, coded, and scored under the microscope by the same scorer without her knowledge. Approximately 10,000 sperm per subject were scored each time. The first donor showed consistently higher-than-average frequencies of aneuploid sperm in both the first and second analyses for XY sperm (33 per 10,000 for the first analysis vs. 20 for the second analysis), disomy 21 (9 vs. 7), total hyperhaploidy (48 vs. 34), and total diploidy (16 vs. 13). The second donor demonstrated consistently lower-than-average frequencies of aneuploid sperm for both analyses for XY sperm (6 vs. 8), disomy 21 (3 vs. 3), total hyperhaploidy (20 vs. 22), and total diploidy (9 vs. 9). For both men, the data of the first analysis did not significantly differ from those of the second analysis, indicating the reliability and consistency of our hybridization and scoring procedure.
Statistical Analyses
All statistical calculations were performed using S-PLUS. A nonparametric estimate of correlation (Kendell’s tau) was used to calculate correlations among the 38 individuals, between age and frequencies of aneuploid sperm and between frequencies of different types of aneuploid sperm. This nonparametric estimator was used because some aneuploidy frequencies of three participants were substantially higher than those of the rest of the participants, with one in particular having an overall frequency fivefold higher than all the others. All P values are two-sided.
To summarize the relationship between age and any given aneuploidy frequency, age was coded in three separate ways: as a categorical variable by decade; as an ordinal variable, ordered by decade; and as a continuous variable. The categorical encoding was used to calculate simple average aneuploidy frequencies and standard deviations for each decade. The average frequency for a decade was calculated by taking the arithmetic mean of all per-subject aneuploidy frequencies among the subjects for that decade. The standard deviation of the aneuploidy frequencies for a decade was calculated by taking the sample standard deviation of all per-subject aneuploidy frequencies among the subjects for that decade.
The ordinal encoding for age decades was used to derive a test for trend. All of the subjects were used to fit a negative binomial generalized linear model (GLIM) with a log link and the ordinal encoding of age in decades (1, 2, 3, and 4) as the only independent variable. A negative binomial GLIM accommodates the overdispersion seen in these data. The significance of the linear term was used to evaluate the presence of a trend. The software used was the glm.nb function described by Venables and Ripley (1999).
The continuous encoding of age was used to derive a per-year percent increase in frequency. Similar to the ordinal fits, all of the subjects were used to fit a negative binomial GLIM with a log link, but with a continuous encoding of age as the only independent variable. The regression coefficient was converted into a percent increase by exponentiation, subtracting 1, and multiplying by 100.
We evaluated the effect of outlying observations on the negative binomial GLIM fit of XY frequencies vs. age by plotting a quantile-quantile plot of the ordered deviance residuals after the fit versus quantiles of a normal distribution. If the model were correct, the resulting scatter plot would be roughly linear. The plot was quite linear, except for three points, which were dramatically off the line produced by the other points. Removing these points also eliminated the overdispersion that was present in the XY versus age plots. Consequently, we removed the points and refitted the model, this time using a Poisson GLIM to estimate standard errors and confidence intervals. Confidence intervals for a percent increase were calculated by calculating the confidence interval for the regression coefficient and then converting the interval to percent increases using the same formula as described above. Tests of (1) preferential association between disomy 21 and either sex chromosome and (2) symmetry of the two types of meiosis II errors were performed using a binomial permutation test of the hypothesis that the proportion of each of the outcomes was 0.5.
Results
At the time of semen collection, the mean age of the study population was 39 years (SD 7.4 years; range 24–57 years). As shown in table 1, there were 4 men in their 20s, 16 in their 30s, 14 in their 40s, and 4 in their 50s. A total of ∼400,000 sperm were evaluated for sperm aneuploidy. The overall ratio of sperm bearing X or Y chromosomes did not differ from unity; however, there was a significant positive correlation with age (P=.006)—that is, there were slightly more Y-bearing sperm with increasing age (table 1, column 5).
There was an increase of ∼1.2% per year in the aggregate frequency of sperm carrying any numerical anomalies detected by the X-Y-21 sperm FISH method (i.e., sum of 4 disomies, 3 diploidies, 3 nullisomies, plus any complex numerical abnormality), albeit this increase was not statistically significant (P=.29; table 1, column 6). This increase was almost entirely the result of a significant increase of ∼3.5% per year in the frequencies of XY sperm, which ranged from an average of 7.5 per 10,000 sperm for men in their 20s to 17.6 per 10,000 sperm for men in their 50s (table 1, column 7). Neither the frequencies of sex-chromosome-null sperm (21-O genotype) nor meiosis II disomies (X-X-21 and Y-Y-21 genotypes) showed age-related trends. However, the frequencies of chromosome-21-null sperm (sum of Y-O and X-O genotypes) increased significantly, at ∼3.7% per year (P=.02), but this effect was almost entirely the result of an increase in Y-O sperm.
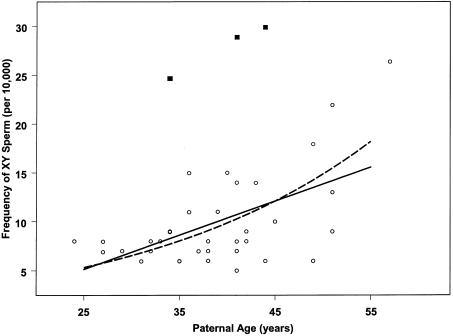
Figure 1 shows a scatter plot of frequency of XY sperm versus age. Three donors are identified whose frequencies of XY sperm were abnormally high, as measured by the graphical evaluation of goodness-of-fit described in the Material and Methods section. To evaluate the extent to which these three donors influenced the XY frequencies in table 1, the donors were removed from the data set, and the relationship between XY sperm aneuploidy and age was re-evaluated using two different models: one in which the frequencies of XY sperm are linearly related to age and another in which the logarithms of the frequencies of XY sperm are linear with age. Although we found very little quantitative difference in the results of the two models, the log-linear model produced a slightly better fit and is described further. After the three outliers were removed, the per-year increase in the frequency of XY sperm increased from 3.5% to 4.2% (95% CI 2.9%–5.6%). Compared with those in the 20s age group, the frequencies of XY sperm were 10% higher in the 30s group (95% CI −27% to 67%), 31% higher in the 40s group (95% CI −14% to 98%), and 160% higher in the 50s group (95% CI 69%–300%), with a clear trend with age by decades (P<.0001). We used a tree-base regression program (the rpart library in S-Plus) to screen the questionnaires, which each contained 360 variables, to consider the effects of potential confounders, including the stress of having a chromosomally abnormal child, job-related hazardous exposures, age-related health problems, etc. However, we were unable to identify any variable that significantly improved the model.
Figure 1.
Comparison of two different fits for the relationship of XY aneuploidy frequency (anomalies per 10,000 sperm scored) to paternal age (in years). Each point corresponds to one individual. For exploratory purposes, two different GLIMs were used to fit aneuploidy frequency to age. One model assumed that the average aneuploidy frequency increased exponentially with age (i.e., a constant percentage increase per year). The other model assumed that the average aneuploidy frequency increased linearly with age (i.e., a constant amount of increase per year). The three labeled points were determined from initial quantile-quantile plots to be outliers and were omitted from the final fits for both models. The dashed line shows the final fitted exponential model, whereas the solid line shows the final fitted linear model. The exponential model produced a slightly better fit than the linear model.
Independent of age, we found an association between the frequencies of sperm with sex-chromosomal aneuploidy (i.e., the sum of disomy X, disomy Y, and XY sperm) versus sperm with disomy 21, (τ=0.23; P=.04). Analyzing the specific categories of sex-chromosomal aneuploidies, we found the strongest association between disomy 21 and disomy X (τ=0.33; P=.004), less association with XY sperm (τ=0.17; P=.12), and no detectable association with disomy Y (τ=0.06; P=.57).
We also investigated whether disomy 21 was more likely to occur in X- or Y-bearing sperm, irrespective of age. Of a total of 267 sperm with disomy 21, 143 carried an X chromosome (X-21-21), and 124 carried a Y chromosome (Y-21-21). Although there was a slight shift toward X-carrying sperm, we found no statistical evidence in this study of a preferential association between disomy 21 and either sex chromosome (P=.22).
Also independent of age, disomy 21 sperm were about twice as frequent as nullisomy 21 sperm (267 vs. 138; P<.01). We also found slightly more sperm with meiosis I (i.e., 454 X-Y-21 sperm) than sperm with meiosis II aneuploidies (i.e., 372 X-X-21 or Y-Y-21 sperm). There was an extraordinary symmetry between the two categories of meiosis II disomies (186 each of disomy Y and disomy X). Meiosis I diploidy (i.e., X-Y-21-21 sperm) occurred at about the same frequency as meiosis II diploidy (i.e., sum of X-X-21-21 and Y-Y-21-21 sperm) (459 vs. 465). The two categories of meiosis II diploidies occurred at similar frequencies (224 vs. 241; P=.4).
Discussion
In a study of fathers of young boys with Klinefelter syndrome, we found that a man’s age was associated with an increase in the frequency of XY sperm but not other clinically relevant sperm aneuploidies (disomy 21, disomy X, disomy Y, or sex-chromosomal nullisomy). There was an average increase of 3.5% in XY sperm per year (i.e., ∼30 years for a doubling). The effect of age appears to be nonlinear; there is an upward change in the slope of the curve, with a concordant increase in variability among men. We also found significant associations, independent of age, between the frequencies of sperm with disomies X and 21, but we did not observe a preferential segregation of either sex chromosome with disomy 21. Our study is unique, in that (a) it consisted of fathers of aneuploid children, (b) it included a relatively large number of donors, (c) it included subjects with a relatively wide range of ages (24–57 years), (d) the health of the donors was checked by a detailed questionnaire, and (e) the combination of probes used allows us to compare and contrast data for a relatively large number of categories of numerical chromosomal abnormalities in sperm (table 1) involving chromosomes which are associated with liveborn aneuploidy syndromes, namely Down, Turner, Klinefelter, triple X, and XYY syndromes.
Aneuploid sperm are thought to arise from meiotic errors during spermatogenesis (Hassold et al. 1993; Wyrobek 1994; Griffin 1996; Spriggs et al. 1996), and paternally transmitted aneuploidy has been associated with abnormal recombination (Weissenbach et al. 1987), size differences in chromosomes, nonhomologous pairing of the sex chromosomes (Hassold et al. 1991), and defects in the nucleolar organizer regions and heterochromatin (Spriggs et al. 1996). More specifically, Shi et al. (2001) demonstrated, by single-sperm PCR, that aneuploid XY sperm have a decreased frequency of recombination in the pseudoautosomal region compared with normal sperm. Although several prior studies have found age-related effects on chromosomal abnormalities in somatic cells (Galloway and Buckton 1978; Guttenbach et al. 1995; Nath et al. 1995; Stone and Sandberg 1995; Bolognesi et al. 1997; Catalan et al. 1998), studies of male germ cells remained inconsistent in their findings (Martin and Rademaker 1987; Bordson and Leonardo 1991; Rosenbusch et al. 1992; Griffin 1996; Guttbach et al. 1997). Using the hamster-egg technique, Rosenbusch et al. (1992) found a positive correlation between the donor age and aneuploidy, whereas Martin and Rademaker (1987) found a significant negative correlation. When sperm FISH was used, small inconclusive age-related increases were suggested for disomy 1 by Martin et al. (1995), by Kinakin et al. (1997), and, for disomy 21, by Rousseaux et al. (1998). Miharu et al. (1993) reported a negative relationship between age and sperm aneuploidy, whereas others have found positive associations, especially for meiosis II disomies (Wyrobek et al. 1994; Griffin et al. 1995; Martin et al. 1995; Robbins et al. 1995; Kinakin et al. 1997). Previous evidence supporting an age effect on the frequency of XY spermatozoa was reported by Griffin et al. (1995) and Martin (1998) but not Robbins et al. (1995). Age effects on the frequencies of sperm with sex-chromosomal aneuploidies were also reported in mice (Lowe et al. 1995).
Prior studies that used molecular methods to identify the parent of origin of the abnormal chromosome (Jacobs et al. 1988; Lorda-Sanchez et al. 1992; MacDonald et al. 1994) provide contradictory evidence of increased paternal age for the group of boys with KS whose mutations were paternal in origin. A companion report to our current study (Eskenazi B, Wyrobek AJ, Kidd SA, Lowe X, Moore D II, Weisiger K, Aylstock M, unpublished data) divided the fathers by parent of origin of the abnormal X chromosome and noted a significant age effect for XY sperm in both the maternal and paternal groups of fathers, suggesting that the age-related findings of our current study may apply to the general population.
The reason for a positive association between age and XY sperm (but not disomy X, Y, or 21) may be associated with special aspects of X and Y pairing during meiosis. Eichenlaub-Ritter (1994) speculated that obligatory crossover of the XY pair was required for normal disjunction. Thus, the absence of XY pairing or the presence of abnormal recombination in pseudoautosomal region during meiosis may be responsible for XY aneuploidy in sperm and this effect may change with age (Hassold et al. 1993). Our study also found associations between the frequencies of sperm carrying disomy 21 and disomy X, which suggests a transchromosomal mechanism affecting nondisjunction at meiosis II. Meiosis II errors leading to two types of disomies and two types of diploidies appear to arise with a high degree of symmetry (table 1), but it is not known whether the mechanisms or predisposing factors for meiosis II disomies and diploidies might be the same.
Use of three-chromosome sperm FISH allowed us to compare the frequencies of various disomic and nullisomic sperm within the same samples. The frequencies of XY and other sperm aneuploidies are well within the range of those reported by others similar sperm FISH methods (Wyrobek et al. 2000). In prior studies, the frequencies of nullisomic sperm were generally higher than those of disomic sperm, which was thought to result from technical errors associated with the difficulties in distinguishing between biological loss of a chromosome and lack of a hybridization signal. Thus, in the past, some laboratories estimated the incidence of nullisomy by doubling the incidence of disomy (Spriggs et al. 1995; Blanco et al. 1996). Notably, in our study (table 1), we found that disomic sperm was two- to fourfold more frequent than nullisomic sperm, and that this was true for chromosome 21 as well as the sex chromosomes. Our finding of excess sperm disomy is consistent with FISH analyses of nuclei in human-hamster systems (Ponsa et al. 1998). Our results are also consistent with earlier findings from our laboratory for a different group of men (Baumgartner et al. 1999). The excess of disomic sperm, relative to nullisomic sperm, might result from chromosome lagging or selection against hypohaploid sperm.
There is a continuing question of whether disomy 21 is preferentially associated with the Y chromosome in sperm. Trisomy 21 is 1.15–1.25 more prevalent at birth among males than among females (Huether et al. 1996; Bishop et al. 1997). We found 267 disomy 21 sperm among 38 donors, with no evidence of an association with the Y chromosomes like that reported by Griffin (1996). The discrepancy between our study and Griffin’s may result from the difference in the numbers of donors and disomic sperm examined (9 donors and ∼100 disomy 21 sperm in Griffin’s study vs. 38 donors and 267 disomy 21 sperm in our study). It may also result from the different types of probes used in the two studies. For the Y chromosome, we utilized a centromeric alpha-satellite probe (DYZ1), which produces a less-diffuse and smaller signal than the satellite III (DYZ3) used by Griffin et al. (1996). Our findings suggest that the excess of males among live-born infants with Down syndrome is not generally attributable to the preferential segregation of Y with disomy 21 sperm. This suggests that postfertilization and developmental mechanisms may be involved that select in favor of male trisomy 21 or against female trisomy 21.
Inspection of figure 1 shows that there were three men (all aged >35 years) who were outliers, for reasons that we could not explain from their questionnaire information. It is possible that these men may be stable variants of sperm aneuploidy—that is, that they consistently produce high levels of sperm aneuploidy. Further studies are needed that analyze multiple samples per donor to examine the characteristics of stable variants of sperm aneuploidy. Because our study population was preselected for having an aneuploid child, which may affect the generality of our findings, it would be very important to determine if these finding can be replicated in future large studies of normal healthy men with normal reproductive histories.
Acknowledgments
We thank Dr. Dan Moore II for help with statistical analysis and discussion. Work was performed by the LLNL under the auspices of the United States Department of Energy, under contract W-7405-ENG-48, with support by National Institute of Environmental Health Sciences Superfund grant P4ZES04705.
References
- Antonarakis SE (1993) Human chromosome 21: genome mapping and exploration, circa 1993. Trends Genet 9:142–148 [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Van Hummelen P, Lowe XR, Adler ID, Wyrobek AJ (1999) Numerical and structural chromosomal abnormalities detected in human sperm with a combination of multicolor FISH assays. Environ Mol Mutagen 33:49–58 [DOI] [PubMed] [Google Scholar]
- Bishop JB, Dellarco VL, Hassold T, Ferguson LR, Wyrobek AJ, Friedman JM (1996) Aneuploidy in germ cells: etiologies and risk factors. Environ Mol Mutagen 28:159–66 [DOI] [PubMed] [Google Scholar]
- Bishop J, Huether CA, Torfs C, Lorey F, Deddens J (1997) Epidemiologic study of Down syndrome in a racially diverse California population, 1989–1991. Am J Epidemiol 145:134–147 [DOI] [PubMed] [Google Scholar]
- Blanco J, Egozcue J, Vidal F (1996) Incidence of chromosome 21 disomy in human spermatozoa as determined by fluorescent in-situ hybridization. Hum Reprod 11:722–726 [DOI] [PubMed] [Google Scholar]
- Bolognesi C, Abbondandolo A, Barale R, Casalone R, Dalpra L, De Ferrari M, Degrassi F, Forni A, Lamberti L, Lando C, Migliore L, Padovani P, Pasquini R, Puntoni R, Sbrana I, Stella M, Bonassi S (1997) Age-related increase of baseline frequencies of sister chromatid exchanges, chromosome aberrations, and micronuclei in human lymphocytes. Cancer Epidemiol Biomarkers Prev 6:249–256 [PubMed] [Google Scholar]
- Bordson BL, Leonardo VS (1991) The appropriate upper age limit for semen donors: a review of the genetic effects of paternal age. Fertil Steril 56:397–401 [DOI] [PubMed] [Google Scholar]
- Catalan J, Autio K, Kuosma E, Norppa H (1998) Age-dependent inclusion of sex chromosomes in lymphocyte micronuclei of man. Am J Hum Genet 63:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy MR, Polani PE (1978) High risk of Down's syndrome at advanced maternal age. Lancet 8066:716–717 [DOI] [PubMed] [Google Scholar]
- de Michelena MI, Burstein E, Lama JR, Vasquez JC (1993) Paternal age as a risk factor for Down syndrome. Am J Med Genet 45:679–682 [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U (1994) Mechanisms of nondisjunction in mammalian meiosis. Curr Top Dev Biol 29:281–324 [DOI] [PubMed] [Google Scholar]
- Estop AM, Munne S, Cieply KM, Vandermark KK, Lamb AN, Fisch H (1998) Meiotic products of a Klinefelter 47,XXY male as determined by sperm fluorescence in-situ hybridization analysis. Hum Reprod 13:124–127 [DOI] [PubMed] [Google Scholar]
- Galloway SM, Buckton KE (1978) Aneuploidy and ageing: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet 20:78–79 [DOI] [PubMed] [Google Scholar]
- Griffin DK (1996) The incidence, origin, and etiology of aneuploidy. Int Rev Cytol 167:263–296 [DOI] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Feingold E, Hassold TJ (1996) Sex ratio in normal and disomic sperm: evidence that the extra chromosome 21 preferentially segregates with the Y chromosome. Am J Hum Genet 59:1108–1113 [PMC free article] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ (1995) Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet 4:2227–2232 [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Engel W, Schmid M (1997) Analysis of structural and numerical chromosome abnormalities in sperm of normal men and carriers of constitutional chromosome aberrations: a review. Hum Genet 100:1–21 [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M (1995) Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet 57:1143–1150 [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt PA, Sherman S (1993) Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev 3:398–403 [DOI] [PubMed] [Google Scholar]
- Hassold TJ, Sherman SL, Pettay D, Page DC, Jacobs PA (1991) XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet 49:253–260 [PMC free article] [PubMed] [Google Scholar]
- Hecht F, Hecht BK (1987) Aneuploidy in humans: dimensions, demography, and dangers of abnormal numbers of chromosomes. In: Vig BK, Sandberg AA (eds) Aneuploidy, part A: incidence and etiology. Alan R. Liss, New York, pp 9–49 [Google Scholar]
- Hinney B, Guttenbach M, Schmid M, Engel W, Michelmann HW (1997) Pregnancy after intracytoplasmic sperm injection with sperm from a man with a 47,XXY Klinefelter's karyotype. Fertil Steril 68:718–720 [DOI] [PubMed] [Google Scholar]
- Hook EB (1976) Risk of Down syndrome in relation to maternal age. Lancet 7983:465 [DOI] [PubMed] [Google Scholar]
- Hook EB (1985) The impact of aneuploidy upon public health: mortality and morbidity associated with human chromosome abnormalities In: Dellarco VL, Voytek PE, Hollaender A (eds) Aneuploidy: etiology and mechanisms. Plenum Press, New York, pp 7–33 [DOI] [PubMed] [Google Scholar]
- Huether CA, Ivanovich J, Goodwin BS, Krivchenia EL, Hertzberg VS, Edmonds LD, May DS, Priest JH (1998) Maternal age specific risk rate estimates for Down syndrome among live births in whites and other races from Ohio and metropolitan Atlanta, 1970–1989. J Med Genet 35:482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether CA, Martin RL, Stoppelman SM, D'Souza S, Bishop JK, Torfs CP, Lorey F, May KM, Hanna JS, Baird PA, Kelly JC (1996) Sex ratios in fetuses and liveborn infants with autosomal aneuploidy. Am J Med Genet 63:492–500 [DOI] [PubMed] [Google Scholar]
- In't Veld P, Brandenburg H, Verhoeff A, Dhont M, Los F (1995) Sex chromosomal abnormalities and intracytoplasmic sperm injection. Lancet 346:773 [DOI] [PubMed] [Google Scholar]
- In't Veld P Kinakin B, Rademaker A, Martin R (1997) Paternal age effect of YY aneuploidy in human sperm, as assessed by fluorescence in situ hybridization. Cytogenet Cell Genet 78:116–119 [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Hassold TJ, Whittington E, Butler G, Collyer S, Keston M, Lee M (1988) Klinefelter's syndrome: an analysis of the origin of the additional sex chromosome using molecular probes. Ann Hum Genet 52:93–109 [DOI] [PubMed] [Google Scholar]
- Juberg RC, Mowrey PN (1983) Origin of nondisjunction in trisomy 21 syndrome: all studies compiled, parental age analysis, and international comparisons. Am J Med Genet 16:111–116 [DOI] [PubMed] [Google Scholar]
- Kinakin B, Rademaker A, Martin R (1997) Paternal age effect of YY aneuploidy in human sperm, as assessed by fluorescence in situ hybridization. Cytogenet Cell Genet 78:116–119 [DOI] [PubMed] [Google Scholar]
- Kranczer S (1998) Banner year for U.S. longevity. Stat Bull Metrop Insur Co 79:8–14 [PubMed] [Google Scholar]
- Liebaers I, Bonduelle M, Van Assche E, Devroey P, Van Steirteghem A (1995) Sex chromosome abnormalities after intracytoplasmic sperm injection. Lancet 346:1095–1097 [PubMed] [Google Scholar]
- Lorda-Sanchez I, Binkert F, Maechler M, Robinson WP, Schinzel AA (1992) Reduced recombination and paternal age effect in Klinefelter syndrome. Hum Genet 89:524–530 [DOI] [PubMed] [Google Scholar]
- Lowe X, Collins B, Allen J, Titenko-Holland N, Breneman J, van Beek M, Bishop J, Wyrobek AJ (1995) Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat Res 338:59–76 [DOI] [PubMed] [Google Scholar]
- MacDonald M, Hassold T, Harvey J, Wang LH, Morton NE, Jacobs P (1994) The origin of 47,XXY and 47,XXX aneuploidy: heterogeneous mechanisms and role of aberrant recombination. Hum Mol Genet 3:1365–1371 [DOI] [PubMed] [Google Scholar]
- Magenis RE (1988) On the origin of chromosome anomaly. Am J Hum Genet 42:529–533 [PMC free article] [PubMed] [Google Scholar]
- Martin RH (1998) Genetics of human sperm. J Assist Reprod Genet 15:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RH, Rademaker AW (1987) The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet 41:484–492 [PMC free article] [PubMed] [Google Scholar]
- Martin RH, Spriggs E, Ko E, Rademaker AW (1995) The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am J Hum Genet 57:1395–1399 [PMC free article] [PubMed] [Google Scholar]
- Miharu N, Best RG, Berete MS, Liu WH, Young SR (1993) Study of aneuploidy frequency of spermatozoa in old and young men, using multi-color fluorescence in situ hybridization (FISH). Am J Hum Genet Suppl 53:A581 [Google Scholar]
- Nath J, Tucker JD, Hando JC (1995) Y chromosome aneuploidy, micronuclei, kinetochores and aging in men. Chromosoma 103:725–731 [DOI] [PubMed] [Google Scholar]
- Ponsa I, Tusell L, Alvarez R, Genesca A, Miro R, Egozcue J (1998) A new assay to assess aneuploidy in human-hamster embryos. Adv Exp Med Biol 444:185–190 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Baulch JE, Moore D 2d, Weier HU, Blakey D, Wyrobek AJ (1995) Three-probe fluorescence in situ hybridization to assess chromosome X, Y, and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod Fertil Dev 7:799–809 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Segraves R, Pinkel D, Wyrobek AJ (1993) Detection of aneuploid human sperm by fluorescence in situ hybridization: evidence for a donor difference in frequency of sperm disomic for chromosomes 1 and Y. Am J Hum Genet 52:799–807 [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch B, Strehler E, Sterzik K (1992) Cytogenetics of human spermatozoa: correlations with sperm morphology and age of fertile men. Fertil Steril 58:1071–1072 [DOI] [PubMed] [Google Scholar]
- Rousseaux S, Hazzouri M, Pelletier R, Monteil M, Usson Y, Sele B (1998) Disomy rates for chromosomes 14 and 21 studied by fluorescent in-situ hybridization in spermatozoa from three men over 60 years of age. Mol Hum Reprod 4:695–699 [DOI] [PubMed] [Google Scholar]
- Shi Q, Spriggs E, Field L, Ko E, Barclay L, Martin R (2001) Single sperm typing demonstrates that reduced recombination is associated with the production of aneuploid 24,XY human sperm. Am J Med Genet 99:34–38 [DOI] [PubMed] [Google Scholar]
- Spriggs EL, Rademaker AW, Martin RH (1995) Aneuploidy in human sperm: results of two-and three-color fluorescence in situ hybridization using centromeric probes for chromosomes 1, 12, 15, 18, X, and Y. Cytogenet Cell Genet 71:47–53 [DOI] [PubMed] [Google Scholar]
- Spriggs EL, Rademaker AW, Martin RH (1996) Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet 58:356–362 [PMC free article] [PubMed] [Google Scholar]
- Stone JF, Sandberg AA (1995) Sex chromosome aneuploidy and aging. Mutat Res 338:107–113 [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD (1999) Modern applied statistics with S-PLUS, third edition. Springer Verlag, New York [Google Scholar]
- Ventura S, Martin J, Curtin S, Mathews T (1997) Report of final natality statistics, 1995. Mon Vital Stat Rep 45:1–81 [PubMed] [Google Scholar]
- Weissenbach J, Levilliers J, Petit C, Rouyer F, Simmler MC (1987) Normal and abnormal interchanges between the human X and Y chromosomes. Development Suppl 101:67–74 [PubMed] [Google Scholar]
- Wyrobek AJ (1994) Methods and concepts in detecting abnormal reproductive outcomes of paternal origin. In: Mattison D, Olsham A (eds) Male mediated developmental toxicology. Plenum Press, New York, pp 1–22 [Google Scholar]
- Wyrobek AJ, Alhborn T, Balhorn R, Stanker L, Pinkel D (1990) Fluorescence in situ hybridization to Y chromosomes in decondensed human sperm nuclei. Mol Reprod Dev 27:200–208 [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Marchetti F, Sloter E, Bishop J (2000) Chromosomally defective sperm and their developmental consequences. In: Anderson D, Karakaya AE, Sram RJ (eds) Human monitoring after environmental and occupational exposure to chemical and physical agents. Amsterdam, IOS Press, pp 134–150 [Google Scholar]
- Wyrobek AJ, Robbins WA, Mehraein Y, Pinkel D, Weier HU (1994) Detection of sex chromosomal aneuploidies X-X, Y-Y, and X-Y in human sperm using two-chromosome fluorescence in situ hybridization. Am J Med Genet 53:1–7 [DOI] [PubMed] [Google Scholar]