Abstract
Heterozygous, de novo mutations in the glial fibrillary acidic protein (GFAP) gene have recently been reported in 12 patients affected by neuropathologically proved Alexander disease. We searched for GFAP mutations in a series of patients who had heterogeneous clinical symptoms but were candidates for Alexander disease on the basis of suggestive neuroimaging abnormalities. Missense, heterozygous, de novo GFAP mutations were found in exons 1 or 4 for 14 of the 15 patients analyzed, including patients without macrocephaly. Nine patients carried arginine mutations (four had R79H; four had R239C; and one had R239H) that have been described elsewhere, whereas the other five had one of four novel mutations, of which two affect arginine (2R88C and 1R88S) and two affect nonarginine residues (1L76F and 1N77Y). All mutations were located in the rod domain of GFAP, and there is a correlation between clinical severity and the affected amino acid. These results confirm that GFAP mutations are a reliable molecular marker for the diagnosis of infantile Alexander disease, and they also form a basis for the recommendation of GFAP analysis for prenatal diagnosis to detect potential cases of germinal mosaicism.
In 1949, W. Stewart Alexander described a “progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant” (Alexander 1949) and suggested that the primary pathogenesis was a specific dysfunction of astrocytes. Since this first description, the dysfunction of astrocytes has been observed in various age groups, with diverse symptoms (Russo et al. 1976; Borrett and Becker 1985; Pridmore et al. 1993; Springer et al. 2000; Messing et al. 2001). Histologically, Alexander disease (MIM 203450) is characterized by the presence in astrocytes of cytoplasmic inclusions, termed “Rosenthal fibers.” These inclusions, which contain the intermediate filament protein GFAP (MIM 137780) in association with small heat-shock proteins, are found predominately in astrocytes located in subependymal, subpial, and periventricular areas. The disease is also usually associated with myelin loss in a rostrocaudal gradient. The most notable features of the infantile form of Alexander disease, which begins during the first two years of life, are macrocephaly (and sometimes hydrocephaly), psychomotor regression, seizures, and spasticity. The patient dies within the first decade. MRI is useful for diagnosis and shows signaling changes in white matter, with frontal predominance, and in some patients abnormalities of the basal ganglia and the thalamus, contrast enhancement, and variable enlargement of the ventricles (Pridmore et al. 1993; Johnson et al. 1996; Springer et al. 2000; van der Knaap et al. 2001). On the basis of the presence of Rosenthal fibers, juvenile and adult forms have been identified. Juvenile patients have a slower clinical course (with bulbar signs, ataxia, and spasticity), and their intellectual abilities are usually preserved (Russo et al. 1976; Borrett and Becker 1985; Reichard et al. 1996). Adult patients have heterogeneous symptoms; some patients have relapsing-remitting neurological symptoms that mimic multiple sclerosis and are only diagnosed as Alexander disease during neuropathological examination (Seil et al. 1968; Herndon et al. 1970; Howard et al. 1993; Schwankhaus et al. 1995). Most of the neuropathologically proved cases of Alexander disease are sporadic, but rare familial cases have been reported (reviewed by Messing et al. 2001). In adults, an autosomal dominant mode of inheritance has been suggested (Howard et al. 1993; Schwankhaus et al. 1995), whereas recessive transmission has been postulated for the few described infants who have affected siblings (Wohlwill et al. 1959; Springer 2000).
The genetic origin of this disease was still controversial when Rosenthal fibers, indistinguishable from those described in Alexander disease, were found in the brain tissue of transgenic mice overexpressing human GFAP (Messing et al. 1998; Eng et al. 1998). Thus, the GFAP gene became a good candidate for Alexander disease, and missense mutations were found in 10 of 11 sporadic, mostly infantile, neuropathologically proved cases in which the GFAP coding region was sequenced (Brenner et al. 2001). All these mutations were heterozygous, appeared de novo, and affected only arginine residues.
We evaluated the GFAP gene for mutations in 15 patients with sporadic infantile disease. Although they had heterogeneous clinical presentations, all 15 patients had MRI abnormalities suggestive of Alexander disease, and 4 had neuropathologically proved Alexander disease (table 1).
Table 1.
Clinical Features and GFAP Mutations of Patients with Progressive Fibrinoid Degeneration
|
PsychomotorDevelopment |
|||||||||||||
| Patient | Age atOnset(mo) | Delay(mo) | Degradation | HeadCircumferencea | Seizuresb | Status and Ageat Follow-Upor Death(years) | Neuro-pathlogy | Exon | Domain | NucleotideChangec | AminoAcidChanged | RestrictionSite | Parentse |
| 1 | 4 | … | 4 mo | 3 | ++ | Dead (5.4) | + | 1 | 1A | 240C→T | L76F* | SacI | NAf |
| 2 | 4 | 8 | … | 1 | … | Alive (1.5) | … | 1 | 1A | 243A→T | N77Y* | … | 2 nl |
| 3 | Birth | Birth | 7 years | 1 | + | Alive (7.5) | … | 1 | 1A | 250G→A | R79H | AciI | 2 nl |
| 4 | 8 | 8 | … | 1.5 | + | Alive (2.6) | … | 1 | 1A | 250G→A | R79H | AciI | … |
| 5 | 6 | 6 | … | 0.5 | + | Alive (4.7) | … | 1 | 1A | 250G→A | R79H | AciI | 2 nl |
| 6 | 6 | 6 | 8 years | 1.5 | + | Alive (20) | … | 1 | 1A | 250G→A | R79H | AciI | … |
| 7 | 3 | 6 | … | 3 | ++ | Alive (5.5) | … | 1 | 1A | 276C→T | R88C* | … | 2 nl |
| 8 | Birth | Birth | … | 3 | ++ | Alive (2.5) | … | 1 | 1A | 276C→T | R88C* | … | 2 nl |
| 9 | 1 | 1 | … | 1 | … | Alive (3.5) | … | 1 | 1A | 276C→A | R88S* | … | … |
| 10 | 6 | 6 | … | 3 | + | Alive (2.2) | … | 4 | 2A | 729C→T | R239C | AciI | 2 nl |
| 11 | 6 | 6 | … | 3.5 | … | Alive (2) | … | 4 | 2A | 729C→T | R239C | AciI | 2 nl |
| 12 | 9 | 12 | 13 mo | 1 | ++ | Dead (4.5) | + | 4 | 2A | 729C→T | R239C | AciI | 2 nl |
| 13 | 18 | 18 | 6 years | 1.5 | + | Alive (8) | … | 4 | 2A | 729C→T | R239C | AciI | 1 nl |
| 14 | 3 | … | 3 mo | 3 | + | Dead (1.2) | + | 4 | 2A | 730G→A | R239H | AciI | 2 nl |
| 15 | 4 | 4 | 8 mo | 4 | +++ | Dead (2.5) | + | … | … | None | … | … | … |
Expressed as standard deviations above the mean.
+ = Occasional seizures; ++ = recurrent seizures; and +++ = severe seizures.
Nucleotide numbers refer to the cDNA sequence reported in Brenner et al. (1990).
* = Novel mutations.
nl = Normal for the mutation in question.
NA = Not available. Parental DNA was not available; however, the mutation was absent in 50 control samples.
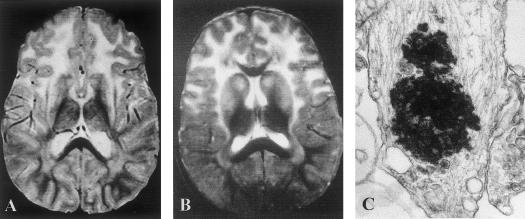
In six patients (patients 1, 7, 8, 10, 14, and 15), the diagnosis of infantile Alexander disease was suspected on the basis of typical clinical presentation characterized by onset at age <6 mo, delay or deterioration of psychomotor development, and the presence of seizures and progressive megalencephaly. In all six patients, typical abnormalities, with a rostrocaudal gradient of the abnormal white matter signal, were observed with the use of cerebral CT scanning and MRI (fig. 1A). Diagnosis was confirmed in three patients by the presence of Rosenthal fibers in tissue samples taken during postmortem examination (patients 14 and 15) or brain biopsy (patient 1) (fig. 1C). For these three patients, the disease course was particularly severe, with onset by age 4 mo, feeding and respiratory difficulties at age 12–24 mo, recurrent seizures, and death at age 15–65 mo. Patient 15 was especially severely affected by hydrocephalus and refractory seizures.
Figure 1.
A and B, Typical T2-weighted MRI images of brains of patients with infantile Alexander disease. A, Classical form (patient 7). B, Mild form (patient 3). Note that both patients show the high signal intensity of white matter, predominately in the frontal area, and of the basal ganglia. C, Rosenthal fiber in Alexander disease viewed by electron microscopy (patient 15); original magnification ×28,500.
In the other nine patients, clinical features were less significant. In patient 12, head circumference was normal, but the course of the disease was severe. In a pattern similar to that of the first group of patients, patient 12 had early psychomotor degradation and recurrent seizures (which appeared at age 13 mo), and death occurred at age 53 mo, with postmortem confirmation of the diagnosis. The eight remaining patients were referred to a neuropediatric unit, either because of seizures (patients 4 and 6) or because of delayed psychomotor development (patients 2, 3, 5, 9, 11, and 13). None had spasticity or ataxia until age 18 mo, and only one had a progressive macrocephaly, observed after the age of 1 year (patient 11). A diagnosis of Alexander disease was suspected when (1) MRI or CT demonstrated an abnormal white matter signal, clearly predominant in the frontal regions, similar to those found in the first group of patients (fig. 1B); and (2) negative results of widespread metabolic screening ruled out known causes of leukodystrophies (especially Canavan, mitochondrial, lysosomal, and peroxysomal diseases). During follow-up, five patients (patients 3–6 and 13) have had only occasional seizures, and three had no epilepsy (patients 2, 9, and 11). All made initial psychomotor progress, three achieving independent walking and the use of a few words (patients 3, 5, and 6). However, three patients (patients 3, 6, and 13) subsequently developed slowly progressive impairment of motor and intellectual abilities, with onset at age 6–8 years. All eight of these patients are still alive at age 1.5–20 years.
Blood samples from the affected patients—and from parents, when available—were collected after obtaining informed consent. For the first nine patients (patients 1–5, 7, 10, 14, and 15), each of the nine GFAP exons was amplified and sequenced (conditions and primers available on request). GFAP mutations were found in eight of these patients (table 1). Each of these mutations was either in exon 1 or 4, and most were the same as ones described elsewhere, which resulted in the loss of an AciI restriction site (Brenner et al. 2001). Because a mutation that removed an AciI site had also previously been found in exon 8, exons 1, 4, and 8 were first amplified and tested for a change in the AciI digestion pattern for the remaining six patients. When a modified restriction pattern was observed, the corresponding exon was sequenced. When no modification was observed, the three exons were sequenced. Using this approach, we found mutations for each of the remaining six patients.
Each of the 14 mutations detected was present in the heterozygous state, and 9 involved two of the previously reported arginine residues (four patients with R79 and five patients with R239); however, in five patients we discovered four novel nonconservative mutations, all located in the first exon: L76F, N77Y, R88C (two patients), and R88S (fig. 2A). None of these mutations was found in the DNA samples of the 19 parents analyzed by restriction digestion or sequencing, including the parents of the patients with the N77Y and R88C mutations. Because parents were not available for the patient with the L76F mutation, we tested for its presence in 50 control DNA samples, by SacI restriction digestion (fig. 2B). All were negative.
Figure 2.
A, Identification of novel mutations in the GFAP gene in four patients with Alexander disease. Segments of the sequencing chromatograms in the region of each mutated nucleotide are shown, with the site of the heterozygous point mutation indicated by an arrow. B, Confirmation of the heterozygous 240C→T point mutation by RFLP analysis. This point mutation results in the loss of a restriction site for SacI. Digestion of a PCR-amplified fragment of genomic DNA from control subjects produced three fragments of 368, 280, and 39 bp (C1 and C2). RFLP analysis of the affected individual generated four fragments of 648, 368, 280, and 39 bp; the 648-bp fragment (368 bp + 280 bp) arose from loss of the SacI site on one of the chromosomes (case 1). The 39-bp restriction fragment is not shown in this gel. ND = nondigested PCR-amplified fragment. C, Schematic representation of the GFAP gene and the corresponding protein, showing the localization of mutations in Alexander disease. The nine exons of the GFAP gene are represented by boxes, and introns are represented by lines. Multiple occurrences of a mutation are indicated by the number shown in parentheses, and mutations described elsewhere (Brenner et al. 2001) are shown in blue. Mutations are clustered within exons encoding the rod domain of GFAP.
Together with the results we reported elsewhere (Brenner et al. 2001), analysis of the GFAP coding region of patients diagnosed with Alexander disease has revealed mutations in 26 of 28 patients (tables 1 and 2). All of these mutations involve exons 1 (50%), 4 (42%), or 8 (8%); and 81% of the mutations involve loss of an AciI restriction site. Therefore, detection of GFAP mutations represents a valuable diagnostic tool for infantile Alexander disease, and GFAP mutations are detected in 93% of patients by amplification of only exons 1, 4, and 8.
Table 2.
Severity of the Disease According to the Type of GFAP Mutation: Results Obtained among Patients in the Present Study and among Patients Reported by Brenner et al.
| Amino Acid Change | Patienta | Macrocephalyb | Disease Duration (years) | Status | Severityc |
| L76F | 1 | + | 5 | Dead | +++ |
| N77Y | 2 | − | 1.2 | Alive | NR |
| R79H | 3 | − | 7.5 | Alive | 0 |
| R79H | 4 | − | 2 | Alive | + |
| R79H | 5 | − | 4 | Alive | + |
| R79H | 6 | − | 20 | Alive | + |
| R79H | 2* | NA | 38 | Dead | + |
| R79H | 13* | − | 12 | Alive | + |
| R79C | 1* | NA | 14 | Dead | + |
| R79C | 12* | NA | 6 | Alive | + |
| R88C | 7 | + | 5 | Alive | ++ |
| R88C | 8 | + | 2.5 | Alive | ++ |
| R88S | 9 | − | 3.5 | Alive | + |
| R239C | 10 | + | 1.7 | Alive | ++ |
| R239C | 11 | + | 1.5 | Alive | ++ |
| R239C | 12 | 0 | 3.5 | Dead | +++ |
| R239C | 13 | 0 | 6.5 | Alive | + |
| R239C | 3* | NA | 5 | Dead | +++ |
| R239C | 4* | NA | 10 | Dead | ++ |
| R239C | 5* | NA | 10 | Dead | ++ |
| R239C | 6* | + | 4 | Dead | +++ |
| R239H | 14 | + | 1 | Dead | ++++ |
| R239H | 7* | NA | .6 | Dead | ++++ |
| R258P | 8* | NA | 6 | Dead | ++ |
| R416W | 9* | NA | 6 | Dead | ++ |
| R416W | 10* | NA | 8 | Dead | ++ |
| None | 15 | + | 2 | Dead | +++ |
| None | 11* | NA | .3 | Dead | ++++ |
* = Patients reported by Brenner et al. (2001).
+ = Maximum head circumference >2 SD above the mean; NA = not available.
+ = Death after >10 years of disease or patient still alive without macrocephaly after 5 years of disease; ++ = death after 5–10 years of disease or patient still alive with macrocephaly after 1 year of disease; +++ = death after 1–5 years of disease ; ++++ = death after <1 year of disease; NR = not rated because of short duration of disease.
All the mutations we have identified involve amino acids that are identical among human, rat, and mouse GFAP, as well as many other intermediate filaments. Each occurs within the helical-rod domain of GFAP (fig. 2C), which is highly conserved among intermediate filaments and is essential for dimerization and organization into a filament network (Fuchs and Cleveland 1998). It has been noted that there are disease-associated mutations in other intermediate filaments homologous to each of those previously reported for GFAP (Quinlan 2001). This is also the case for the new L76 and N77 mutations reported in the present study (e.g., Bonifas et al. 1994; Endo et al. 1997). We have not found any report of a mutation in an arginine homologous to R88; but keratin 9 (MIM 144200), which has a Q at this position, does have a disease-associated mutation at this site (Hennies et al. 1994).
Clinical heterogeneity (including patients without macrocephaly and those with less-severe courses) has been described elsewhere in patients with Alexander disease diagnosed according to neuropathological criteria or, more recently, according to MRI criteria (Russo et al. 1976; Borrett and Becker 1985; Pridmore et al. 1993; Springer et al. 2000; van der Knaap et al. 2001). Our results validate these diagnoses by finding GFAP mutations in a large percentage of patients who had heterogeneous clinical symptoms but were candidates for Alexander disease on the basis of suggestive neuroimaging abnormalities. Many of these mutations were identical to those previously found for pathologically diagnosed Alexander disease, and the others fell into the same pattern observed in the neuropathologically proved cases: missense mutations that are heterozygous and nonconservative and that arise de novo.
A genotype-phenotype correlation can be discerned for the two most frequently mutated arginine residues (R79 [8 patients] and R239 [10 patients]), with the phenotype of the R79 mutations appearing much less severe than that of the R239 mutations (table 2). The number of patients with other mutations is too small to determine a phenotypic pattern (table 1 and 2); however, the four patients we found with R79 mutations appear to be the least-severely affected: none developed macrocephaly, three achieved independent walking, and, at the time of writing, all are alive at age 2.5–20 years. Similarly, among the four patients with R79 mutations who were reported by Brenner et al. (2001), two lived until the ages of 14 and 48 years, and the other two were still alive, at ages 7 and 8 years, when the article was published.
In sharp contrast, our five patients with R239 mutations had a marked impairment of psychomotor development, and three had progressive macrocephaly. Similarly severe phenotypes were displayed by the patients with R239 who were reported by Brenner et al. (2001). Interestingly, the single patient in the present study and the single patient reported by Brenner et al. (2001) who carried an R239H mutation had especially severe clinical courses. Our patient (patient 14) acquired only reactive smiling and head control, with early regression and feeding and respiratory difficulties that led to death at age 15 mo. Similarly, the single patient of Brenner et al. (2001) who had R239H died at age 11 mo. In the two studies, the eight patients who had the R239C mutation had a less-severe clinical course than that of the two patients with the R239H mutation, and none of the eight patients has died before the age of 4 years (tables 1 and 2).
Both patients who had Alexander disease without identified GFAP mutations (patient 11 of Brenner et al. [2001] and patient 10 of the present work) had a histologically proved diagnosis and a severe clinical form of the disease. However, we have not yet rigorously excluded (1) mutations in the promoter or intronic sequences of the GFAP gene or (2) rearrangements of the genomic region containing GFAP. On the other hand, although GFAP gene mutations appear to be the predominant cause of infantile Alexander disease, it is possible that other genes may contribute, particularly in patients with the most-severe (early infantile) or mildest (juvenile and adult) forms of the disease (reviewed in Messing et al. 2001). In other human genetic diseases involving intermediate filaments, mutations have been found in both the intermediate filament protein and associated proteins.
In all patients analyzed, the GFAP mutations are dominant and arise de novo. Affected siblings whose parents were unaffected, including one family with neuropathologically proved Alexander disease (Wohlwill et al. 1959), could result from autosomal recessive transmission or from germinal mosaicism for a dominant mutation. Therefore, in patients with Alexander disease who have de novo GFAP mutations, prenatal diagnosis should be proposed for all further pregnancies. Further GFAP analysis is needed to investigate whether the inheritable dominant forms of Alexander disease that have been described in two families, both of which had late onsets after age 25 years (Howard et al. 1993; Schwankhaus et al. 1995), are also associated with GFAP mutations.
In conclusion, GFAP mutations are a reliable marker for infantile Alexander disease diagnosed according to neuropathological or MRI defined criteria. MRI abnormalities with the characteristic rostrocaudal gradient in white matter signal provide a strong rationale for the analysis of the GFAP gene, even in the absence of macrocephaly or neurological deterioration, when other causes of leukodystrophies have been ruled out.
Acknowledgments
The patients and their families are warmly acknowledged for their participation. We greatly thank L. Dauche and E. Eymard-Pierre (both of INSERM U384) and D. Recan (banque de cellules, Hôpital Cochin) for their help in processing blood samples. This work was supported by grants from the European Leukodystrophy Association, INSERM projet PROGRES, the Centre National de la Recherche Scientifique (to D.R., D.P.D., and A.D.), the National Institutes of Health (to M.B. and A.M.), the Jean Pierre and Nancy Boespflug Myopathic Research Foundation, and a fellowship from the Association Française de Recherche en Génétique (to D.R.). We are also indebted for a grant, to study genetic leukodystrophies, from Ricerca Finalizzata Strategica of the Italian Ministry of Health.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for Alexander disease [MIM 203450], GFAP [MIM 137780], and keratin 9 [MIM 144200] )
References
- Alexander WS (1949) Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain 72:373–381 [DOI] [PubMed] [Google Scholar]
- Bonifas JM, Matsumura K, Chen MA, Berth-Jones J, Hutchison PE, Zloczower M, Fritsch PO, Epstein EH Jr (1994) Mutations of keratin 9 in two families with palmoplantar epidermolytic hyperkeratosis. J Invest Dermatol 103:474–477 [DOI] [PubMed] [Google Scholar]
- Borrett D, Becker LE (1985) Alexander’s disease, a disease of astrocytes. Brain 108:367–385 [DOI] [PubMed] [Google Scholar]
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nature Genet 27:117–120 [DOI] [PubMed] [Google Scholar]
- Brenner M, Lampel K, Nakatani Y, Mill J, Banner C, Mearow K, Dohadwala M, Lipsky R, Freese E (1990) Characterization of human cDNA and genomic clones for glial fibrillary acidic protein. Mol Brain Res 7:277–286 [DOI] [PubMed] [Google Scholar]
- Endo H, Hatamochi A, Shinkai H (1997) A novel mutation of a leucine residue in coil 1A of keratin 9 in epidermolytic palmoplantar keratoderma. J Invest Dermatol 109:113–115 [DOI] [PubMed] [Google Scholar]
- Eng LF, Lee YL, Kwan H, Brenner M, Messing A (1998) Astrocytes cultured from transgenic mice carrying the added human glial fibrillary acidic protein gene contain Rosenthal fibers. J Neurosci Res 53:353–360 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Cleveland DW (1998) A structural scaffolding of intermediate filaments in health and disease. Science 279:514–519 [DOI] [PubMed] [Google Scholar]
- Hennies HC, Zehender D, Kunze J, Kuster W, Reis A (1994) Keratin 9 gene mutational heterogeneity in patients with epidermolytic palmoplantar keratoderma. Hum Genet 93:649–654 [DOI] [PubMed] [Google Scholar]
- Herndon RM, Rubinstein LJ, Freeman JM, Mathieson G (1970) Light and electron microscopic observations on Rosenthal fibers in Alexander's disease and in multiple sclerosis. J Neuropathol Exp Neurol 29:524–551 [DOI] [PubMed] [Google Scholar]
- Howard RS, Greenwood R, Gawler J, Scaravilli F, Marsden CD, Harding AE (1993) A familial disorder associated with palatal myoclonus, other brainstem signs, tetraparesis, ataxia and Rosenthal fibre formation. J Neurol Neurosurg Psychiatry 56:977–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AB (1996) Alexander disease. In: Moser HG (ed) Handbook of clinical neurology. Vol 22. Neurodystrophies and neurolipidoses. Elsevier, Amsterdam, pp 701–710 [Google Scholar]
- Messing A, Goldman JE, Johnson AB, Brenner M (2001) Alexander disease: new insights from genetics. J Neuropathol Exp Neurol 60:563–573 [DOI] [PubMed] [Google Scholar]
- Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, Brenner M (1998) Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol 152:391–398 [PMC free article] [PubMed] [Google Scholar]
- Pridmore CL, Baraitser M, Harding B, Boyd SG, Kendall B, Brett EM (1993) Alexander’s disease: clues to diagnosis. J Child Neurol 8:134–144 [DOI] [PubMed] [Google Scholar]
- Quinlan R (2001) Cytoskeletal catastrophe causes brain degeneration. Nature Genet 27:10–11 [DOI] [PubMed] [Google Scholar]
- Reichard EA, Ball WS Jr, Bove KE (1996) Alexander disease: a case report and review of the literature. Pediatr Pathol Lab Med 16:327–343 [PubMed] [Google Scholar]
- Russo LS, Aron A, Anderson PJ (1976) Alexander’s disease: a report and reappraisal. Neurology 26:607–614 [DOI] [PubMed] [Google Scholar]
- Schwankhaus JD, Parisi JE, Gulledge WR, Chin L, Currier RD (1995) Hereditary adult-onset Alexander's disease with palatal myoclonus, spastic paraparesis, and cerebellar ataxia. Neurology 45:2266–2271 [DOI] [PubMed] [Google Scholar]
- Seil FJ, Schochet SS Jr, Earle KM (1968) Alexander's disease in an adult: report of a case. Arch Neurol 19:494–502 [DOI] [PubMed] [Google Scholar]
- Springer S, Erlewein R, Naegele T, Becker I, Auer D, Grodd W, Krageloh-Mann I (2000) Alexander disease: classification revisited and isolation of a neonatal form. Neuropediatrics 31:86–92 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Naidu S, Breiter SN, Blaser S, Stroink H, Springer S, Begeer JC, van Coster R, Barth PG, Thomas NH, Valk J, Powers JM (2001) Alexander disease: diagnosis with MR imaging. Am J Neuroradiol 22:541–552 [PMC free article] [PubMed] [Google Scholar]
- Wohlwill FJ, Bernstein J, Yakovlev PI (1959) Dysmyelinogenic leukodystrophy: report of a case of a new, presumably familial, type of leukodystrophy with megalencephaly. J Neuropathol Exp Neurol 18:359–383 [DOI] [PubMed] [Google Scholar]