Abstract
The effects of lamivudine (3TC) on in vitro infection of peripheral blood mononuclear cells (PBMC) from healthy donors with human T-cell lymphotropic virus type 1 (HTLV-1) were investigated. Direct measures of viral replication (viral DNA, RNA, and protein) all gave similar, very high 50% inhibitory concentrations in comparison with those previously reported for zidovudine. Nevertheless, 3TC inhibited HTLV-1-driven long-term growth of infected PBMC in vitro at concentrations (6.25 μM) which had poor or no direct antiviral effects, suggesting that another mechanism may be playing a role.
Lamivudine (3TC) is a cytosine nucleoside reverse transcriptase inhibitor (NRTI) that is used, in combination with other nucleoside or nonnucleoside reverse transcriptase inhibitors (NNRTIs) or protease inhibitors (PIs), in the treatment of infections caused by human immunodeficiency virus (HIV) (3, 6, 15). As monotherapy, 3TC is used in hepatitis B virus (HBV) infection (9). Human T-cell lymphotropic virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATL) (14, 17) and of demyelinating diseases characterized by a remarkable inflammatory response, known as HTLV-1-associated human myelopathy/tropic spastic paraparesis (HAM/TSP) (2, 13). ATL has a poor prognosis and shows high resistance to conventional chemotherapy. Recent studies in vivo have shown the potential therapeutic utility of 3TC in the treatment of pathologies associated with HTLV-1 infection (11, 16). In particular, 3TC was found to be a relatively safe drug that caused a rapid fall in proviral load in HAM/TSP patients, although phases of decrease in viral load alternated with phases of active replication of the virus (16). In this study, we have assayed the capacity of 3TC to inhibit infection and long-term growth caused by HTLV-1 in vitro.
HTLV-1 transmission was performed by coculturing peripheral blood mononuclear cells (PBMC) isolated from normal adult donors, seronegative for HIV, HBV, and HCV, with lethally irradiated (120 Cy; cesium gamma cell irradiator 1000; Canada Atomic Energy Ltd., Chalk River, Canada) MT-2 cells at a ratio of 5:1 (12). MT-2 is a cell line chronically infected by HTLV-1 (12). To assay its antiviral activity, 3TC (Wellcome Research Laboratories, Beckenham, United Kingdom) was added at final concentrations ranging from 6.25 to 100 μM at the onset of the culture and then at half the initial concentrations 3, 7, and 10 days postinfection (p.i.) to ensure a constant level of the drug in the culture medium in the early phase of infection. In preliminary experiments, lower concentrations of 3TC did not exert any antiviral effect against HTLV-1 in vitro. Concentrations of 3TC used were close to the peak levels in serum (0.2 to 20 μM) which are detected in patients treated with pharmacological doses of the drug (7). The antiviral effects of 3TC at different concentrations were evaluated 4 weeks p.i. by assaying the presence of proviral DNA, viral RNA, and viral protein expression in untreated and treated cell cultures. To detect proviral DNA, 1 μg of DNA was used as a template and was amplified in a standard PCR mix by using a primer pair specific for the pol region (SK54 and SK56) as previously described (10). To detect viral RNA, total RNA from infected cells was reverse transcribed into cDNA and amplified with primers specific for the HTLV-1 Tax/Rex splicing region (8, 18). Amplified DNA was analyzed by liquid hybridization and probed with specific 32P-end-labeled oligonucleotides (18). Three experiments using PBMC from different donors were performed with similar results.
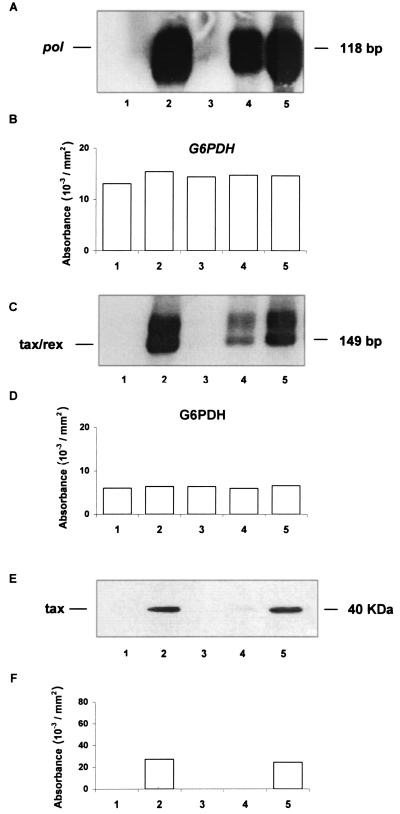
Results of one representative experiment are reported in Fig. 1. The uninfected culture used for this experiment was designated PB-1, while the infected culture was designated PB-1/MT. Addition of 100 μM 3TC caused complete inhibition of proviral DNA (Fig. 1A), as detected by pol primers and viral RNA (Fig. 1C), compared to the untreated control. Conversely, 25 and 6.25 μM 3TC partly inhibited the presence of proviral DNA, and 6.25 μM 3TC did not inhibit viral RNA expression. The housekeeping gene glucose-6-phosphate dehydrogenase (G6PDH) was present at equal levels in all the samples (Fig. 1B and D) for both DNA and RNA. Expression of the virus protein Tax, assayed by Western blot analysis as previously described (18), was completely inhibited at 100 and 25 μM 3TC, while 6.25 μM 3TC was not inhibitory (Fig. 1E and F). Staining with Ponceau dye indicated that equal amounts of proteins were analyzed (data not shown).
FIG. 1.
(A) Presence of HTLV-1 proviral DNA in PB-1/MT cell cultures 4 weeks p.i. (B) Densitometric analysis of genomic DNA from PB-1/MT cultures. Absorbance values (optical density) refer to amplification of genomic DNA with primers specific for G6PDH. (C) Presence of HTLV-1 viral mRNA in PB-1/MT cultures 4 weeks p.i. (D) Densitometric analysis of total RNA from PB-1/MT cultures 4 weeks p.i. Absorbance values (optical density) refer to amplification with primers specific for G6PDH. (E) Expression of the HTLV-1 protein Tax in PB-1/MT cultures 4 weeks p.i. Western blot analysis of cell lysates was performed. Blots were incubated overnight at 4°C with a 1:1,000 dilution of an anti-Tax monoclonal antibody (a generous gift from Elliot Cowan of the U.S. Food and Drug Administration Division of Transfusion-Transmitted Diseases, Bethesda, Md.). (F) Absorbance values (optical density) obtained by densitometric analysis of Tax in PB-1/MT cultures. Lanes for all panels: 1, uninfected control; 2, untreated PB/MT-1 cultures; 3 through 5, PB/MT-1 cultures treated with 100, 25, and 6.25 μM 3TC, respectively.
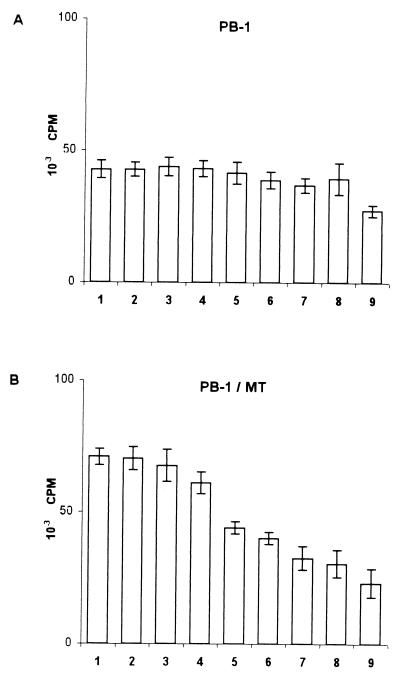
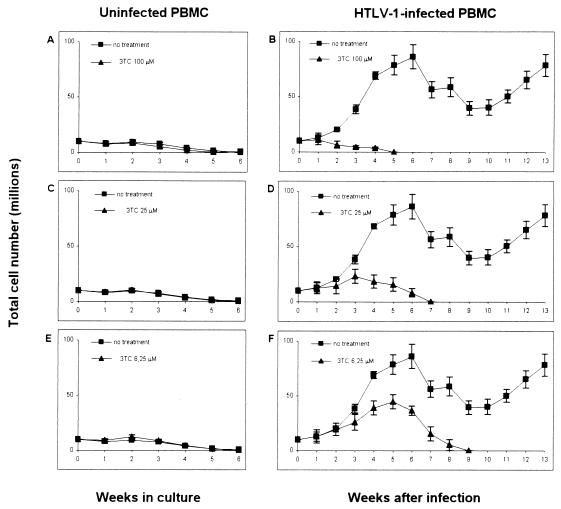
Exposure to HTLV-1 in vitro usually induces proliferation of PBMC, leading in most cases to immortalization of infected cells. Since these are key hallmarks of HTLV-1 pathogenesis, the effects of 3TC on cell proliferation were examined both by a short-term assay and by a long-term growth assessment. To assay proliferation at early times p.i., PBMC were cultivated, alone or with irradiated MT-2 cells, for 7 days in the presence of interleukin 2 (IL-2) without any other stimulation. 3TC was added at the onset of the cultures at concentrations ranging from 2 to 800 μM. Proliferation was measured by [3H]thymidine (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom) uptake during the last 18 h in culture. Figure 2 shows the results of one representative experiment in quadruplicate, out of four performed with cells from different donors. In uninfected cultures, 800 μM 3TC significantly inhibited cell proliferation (P = 0.001 by the two-sided Dunnett's pairwise multiple-comparison t test). There were no significantly different effects at the other 3TC concentrations (Fig. 2A). Cultures were promptly induced to proliferate following HTLV-1 infection (twofold increase), as shown by comparing thymidine uptake in untreated PB-1/MT versus untreated PB-1 cultures (Fig. 2). In infected cultures, high concentrations of 3TC (100 to 800 μM) caused marked inhibition of thymidine uptake (Fig. 2B) (P < 0.001 relative to untreated infected cultures by the two-sided Dunnett's pairwise multiple-comparison t test). In order to examine the consequences of 3TC treatment on the long-term growth of HTLV-1-infected PBMC, cells that were either left untreated or treated with 3TC were maintained in culture for 13 weeks. Cell growth was monitored weekly by evaluating the living-cell count by use of the trypan blue dye exclusion test, calculated as the mean of two independent evaluations. After counting, cell concentrations were adjusted to 1 × 106/ml. Results were expressed as total cell number (TCN), calculated from weekly cell counts as follows. For the first week, TCN was the number of cells (expressed as millions of viable cells per milliliter) detected before the first adjustment. On successive weeks, TCN was calculated as the TCN at the previous weekly passage multiplied by the number of cells (expressed as millions of viable cells per milliliter) detected before weekly adjustment. Figure 3 shows the mean TCNs ± standard deviations (SD) from three different experiments using PBMC from three donors. Uninfected cultures grew poorly when maintained in IL-2 without additional stimuli throughout the time of observation (Fig. 3A, C, and E). Infected cultures treated with 100 or 25 μM 3TC ceased to grow after 5 and 7 weeks, respectively (Fig. 3B and D). Infected cultures treated with 6.25 μM 3TC survived to 9 weeks p.i. (Fig. 3F). Conversely, all infected untreated cultures were still growing at week 13, when the experiments were interrupted.
FIG. 2.
Effects of different concentrations of 3TC on the proliferative responses of PB-1 cell cultures left unexposed (A) or exposed to HTLV-1 (PB-1/MT) (B). Results are expressed as mean counts per minute of radioactivity ± SD from quadruplicate samples. Bars: 1, untreated PB-1 or PB-1/MT; 2 through 9, cultures treated with 3TC at 2 μM (bar 2), 6.25 μM (bar 3), 25 μM (bar 4), 100 μM (bar 5), 200 μM (bar 6), 400 μM (bar 7), 600 μM (bar 8), or 800 μM (bar 9). Results were obtained from a single donor, designated PB-1, representative of four donors tested.
FIG. 3.
Growth curves of uninfected PBMC (A, C, and E) and HTLV-1 infected PBMC (B, D, and F) in the presence of different concentrations of 3TC. Each experimental point represents the mean TCN ± SD determined weekly in cultures from three different experiments using PBMC from three donors. The growth curve of untreated cultures is reported in all graphs, for visual comparison. By one-way analysis of variance, significant differences in HTLV-1-infected PBMC among groups were determined starting by week 3, while no differences in uninfected PBMC among groups were found at any times tested.
Different measures of the antiviral effects of 3TC in HTLV-1 infection were compared by calculating the 50% inhibitory concentrations (IC50s) for proviral DNA, viral RNA, and Tax detection, as previously shown for other NRTIs (10). Means ± SD of three IC50 evaluations, calculated from all data obtained in three experiments performed using PBMC from different donors, were as follows: 29 ± 2 μM for proviral DNA (pol), 24 ± 2 μM for viral RNA (tax/rex), and 14 ± 1 μM for Tax protein (the Pearson coefficient [r2] for the y value was 0.9). IC50s were calculated according to the best-fit curve (y value versus log x, where y is the value of the function examined and x is the drug concentration). These results show that IC50s calculated from proviral DNA and viral RNA assays were about twice as high as the IC50 for Tax expression, suggesting a possible effect of 3TC at the translational level.
3TC completely inhibited proviral DNA, viral mRNA, and protein expression only at a high concentration (100 μM). This concentration is out of the range which can be considered pharmacological on the basis of clinical experience with HIV infection. Moreover, the antiviral potency of 3TC against HTLV-1 transmission is more than 100 times lower than that of zidovudine (AZT), as determined by comparing IC50s presented in this paper with those previously reported (10). This is different from what has been observed for HIV infection, where inhibitory concentrations of 3TC and AZT are similar. The discrepancy between the behaviors of HIV and HTLV-1 when subjected to 3TC treatment might be due to a different capacity of 3TC to interact with the reverse transcriptase (RT) of HIV and HTLV-1, respectively. This hypothesis is strongly supported by biochemical results showing high-level resistance to 3TC in HTLV-1 RT by use of an enzymatic assay (5). The YMDD site, the most frequent source of mutations conferring resistance to 3TC in HIV (1, 4), seems well conserved in the RT of HTLV-1. Therefore, a site other than YMDD could be responsible for a possible low conformational affinity of 3TC to HTLV-1 RT. While 6.25 μM 3TC showed no signs of antiviral or short-term antiproliferative activity, this dose was nevertheless able to arrest growth in the long-term assay of infected cells. This fact suggests that 3TC may counteract long-term growth by mechanisms other than antiviral effect, possibly affecting HTLV-1-infected-cell activity. Inhibition of long-term cell growth in infected cells might be due to relatively high-level incorporation and, possibly, phosphorylation of 3TC in cells chronically infected by the virus. On the other hand, protection against immortalization by 6.25 μM 3TC could be related to the partial, if not complete, inhibitory action on Tax transactivating protein expression caused by the drug at this concentration. Inhibition of long-term cell growth in HTLV-1-infected cells in vitro could explain, at least in part, the effects exerted by 3TC in HTLV-1-infected patients in vivo. Another noteworthy point is that 3TC, in spite of its ability to inhibit long-term growth of HTLV-1-infected cultures, was shown to be less potent than AZT in inhibiting the proliferation of either uninfected or freshly HTLV-1-infected cultures, based on a historical comparison with previous results (10, 18). This observation indicates a low level of immunotoxicity for this drug, which could be potentially beneficial for patients undergoing therapy.
Taken together, these data demonstrate that 3TC has little in vitro antiviral activity against HTLV-1 infection at clinically relevant concentrations and therefore is unlikely to have direct antiviral efficacy in HTLV-1-infected patients. Rather, indirect effects could explain its limited clinical benefit in HTLV-1 infection in vivo.
Acknowledgments
We thank Alison Inglis for linguistic assistance.
This work was supported by grants from the Ministry of Health, AIDS Project, by grants from the University of Rome “Tor Vergata” to B. Macchi, and by grants from the Italian Ministry of Education, University and Research, Research Projects of National Interest, and from the University of Messina, to A. Mastino.
REFERENCES
- 1.Boyer, P. L., H. Q. Gao, P. K. Clark, S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2001. YADD mutants of human immunodeficiency virus type 1 and Moloney murine leukemia virus reverse transcriptase are resistant to lamivudine triphosphate (3TCTP) in vitro. J. Virol. 75:6321-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceroni, M., P. Piccardo, P. Rodgers-Johnson, C. Mora, D. Asher, C. Gajdusek, and C. J. Gibbs. 1988. Intrathecal synthesis of IgG antibodies to HTLV-1 supports an etiological role for HTLV-1 in tropical spastic paraparesis. Ann. Neurol. 23:188-191. [DOI] [PubMed] [Google Scholar]
- 3.Coates, J. A. V., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng, J. Y., and K. S. Anderson. 1999. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry 38:9440-9448. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lerma, J. G., S. Nidtha, and W. Heneine. 2001. Susceptibility of human T cell leukemia virus type 1 to reverse transcriptase inhibitors: evidence for resistance to lamivudine. J. Infect. Dis. 184:507-510. [DOI] [PubMed] [Google Scholar]
- 6.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, M. A. Fischl, et al. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, M. A., K. H. P. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita, T., S. Masanori T. Tobinai, M. Ito, S. Ito, S. Ikeda, K. Tajima, K. Shimotohno, and T. Sugimura. 1989. Detection of the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai, C. L., and M. F. Yuen. 2000. Profound immunosuppression of hepatitis B virus replication with lamivudine. J. Med. Virol. 61:367-373. [DOI] [PubMed] [Google Scholar]
- 10.Macchi, B., I. Faraoni, J. Zhang, S. Grelli, C. Favalli, A. Mastino, and E. Bonmassar. 1997. AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 78:1007-1016. [DOI] [PubMed] [Google Scholar]
- 11.Machuca, A., and V. Soriano. 2000. In vivo fluctuation of HTLV-I and HTLV-II proviral load in patients receiving antiretroviral drugs. J. Acquir. Immune Defic. Syndr. 24:189-193. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shirashi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by cocultivating normal human cord leucocytes and human leukemic cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 13.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, and A. Igata. 1986. HTLV-1 associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed]
- 14.Poiesez, B. J., F. W. Ruscetti, A. F. Gadzar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, D., M. M. Berrey, M. Robertson, D. Mehrotra, M. Markowitz, L. Perrin, N. Clumeck, A. Lazzarin, B. Burckhardt, R. Weber, L. Corey, and D. A. Cooper. 2000. Virological and immunological effects of combination antiretroviral therapy with zidovudine, lamivudine and indinavir during primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:950-954. [DOI] [PubMed] [Google Scholar]
- 16.Taylor, G. P., S. E. Hall, S. Navarrete, C. A. Michie, R. Davis, A. D. Witkover, M. Rossor, M. A. Nowak, P. Rudge, E. Matutes, C. R. M. Bangham, and J. N. Weber. 1999. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-Tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J. Virol. 73:10289-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, J., E. Balestrieri, S. Grelli, C. Matteucci, V. Pagnini, C. D'Agostini, A. Mastino, and B. Macchi. 2001. Efficacy of 3′-azido-3′-deoxythymidine (AZT) in preventing HTLV-1 transmission to human cord blood mononuclear cells. Virus Res. 78:67-78. [DOI] [PubMed] [Google Scholar]