Abstract
A sample of 526 Y chromosomes representing six Middle Eastern populations (Ashkenazi, Sephardic, and Kurdish Jews from Israel; Muslim Kurds; Muslim Arabs from Israel and the Palestinian Authority Area; and Bedouin from the Negev) was analyzed for 13 binary polymorphisms and six microsatellite loci. The investigation of the genetic relationship among three Jewish communities revealed that Kurdish and Sephardic Jews were indistinguishable from one another, whereas both differed slightly, yet significantly, from Ashkenazi Jews. The differences among Ashkenazim may be a result of low-level gene flow from European populations and/or genetic drift during isolation. Admixture between Kurdish Jews and their former Muslim host population in Kurdistan appeared to be negligible. In comparison with data available from other relevant populations in the region, Jews were found to be more closely related to groups in the north of the Fertile Crescent (Kurds, Turks, and Armenians) than to their Arab neighbors. The two haplogroups Eu 9 and Eu 10 constitute a major part of the Y chromosome pool in the analyzed sample. Our data suggest that Eu 9 originated in the northern part, and Eu 10 in the southern part of the Fertile Crescent. Genetic dating yielded estimates of the expansion of both haplogroups that cover the Neolithic period in the region. Palestinian Arabs and Bedouin differed from the other Middle Eastern populations studied here, mainly in specific high-frequency Eu 10 haplotypes not found in the non-Arab groups. These chromosomes might have been introduced through migrations from the Arabian Peninsula during the last two millennia. The present study contributes to the elucidation of the complex demographic history that shaped the present-day genetic landscape in the region.
Introduction
The Middle East played a crucial role in early human history. Its strategic location at the crossroads of three continents facilitated the movements of peoples and the spread of novel technologies and ideas. At the beginning of the Neolithic period (∼10,500 years ago), the Fertile Crescent of the Middle East was one of the few centers in which the transition from hunting-gathering to permanent settlement and farming took place (Bar-Yosef 1995). Previous genetic studies suggested that demic diffusion of Neolithic farmers, rather than cultural transmission, was responsible for the dispersal of domesticates and technological innovations from the Middle East to Europe, North Africa, and southwest Asia (Cavalli-Sforza et al. 1994; Richards et al. 2000; Semino et al. 2000; Quintana-Murci et al. 2001).
Polymorphisms on the nonrecombining part of the Y chromosome have become powerful tools for the investigation of genetic diversity in males, complementing the information from the maternally inherited mtDNA. Y chromosome variants were shown to be much more localized geographically than mtDNA or autosomal polymorphisms (Seielstad et al. 1998). The study of population history has gained enormous momentum with the recent introduction of a large number of new binary or biallelic Y chromosome polymorphic markers (Underhill et al. 2000). The typing of these markers in a set of worldwide populations led to a comprehensive and detailed Y chromosome phylogeny.
When microsatellites are analyzed within a haplogroup classified by binary polymorphisms, a measure of diversity is added that allows evaluation of interpopulation affinities at fine resolution (Hurles et al. 1999; Helgason et al. 2000; Nebel et al. 2000; Thomas et al. 2000; Kayser et al. 2001). In addition, microsatellite variation provides an estimation of time depth and, hence, facilitates the investigation of the origin and spread of haplogroups and permits placement of the genealogical projections in the context of known or surmised historical events (Zerjal et al. 1997; Hurles et al. 1998; Quintana-Murci et al. 2001). Particular high-frequency modal haplotypes have been shown to be associated with genealogies defined by religious status (Thomas et al. 1998) or surnames that are transmitted along male lines (Sykes and Irven 2000). The Cohen modal haplotype (CMH) has been described as the signature haplotype of the paternally inherited Jewish priesthood (Thomas et al. 1998).
Previous investigations based on binary Y chromosome polymorphisms suggested a common origin for Jewish and non-Jewish populations living in the Middle East (Santachiara-Benerecetti et al. 1993; Hammer et al. 2000). Our recent study of high-resolution microsatellite haplotypes demonstrated that a substantial portion of Y chromosomes of Jews (70%) and of Palestinian Muslim Arabs (82%) belonged to the same chromosome pool (Nebel et al. 2000). Of those Palestinian chromosomes, approximately one-third formed a group of very closely related haplotypes that were only rarely found in Jews. Altogether, the findings indicated a remarkable degree of genetic continuity in both Jews and Arabs, despite their long separation and the wide geographic dispersal of Jews.
In the present study, we examined the genetic relationship among three Jewish communities, the Ashkenazi, Sephardic, and Kurdish Jews, who were geographically separated from each other for many centuries. By comparing data from these groups with data from other relevant populations, we looked for information about how the Y chromosomes of Jews fit into the genetic landscape of the Middle East.
Material and Methods
Study Populations
Ashkenazi Jews
DNA samples were extracted from mouth swabs collected from 79 paternally unrelated, otherwise randomly selected, self-designated Ashkenazim in Israel. The Ashkenazi subjects' paternal families came from various parts of Europe, spanning areas from Germany, in the west, to Russia, in the east.
Sephardic Jews
DNA samples were extracted from mouth swabs collected from 78 paternally unrelated, otherwise randomly selected, self-designated Sephardic Jews in Israel. Here the term “Sephardic Jews” refers to Jews from Mediterranean and Middle Eastern countries. Our sample consisted of two groups. The first, designated the “North African sample” (55 subjects), comprised 37 individuals from North African countries (primarily Morocco), 13 from Turkey, 3 from the Iberian Peninsula, and 2 from Bulgaria. The second group was designated the “Iraqi sample” (23 subjects), and it contained 20 Jews from Iraq and 3 from Syria.
Kurdish Jews
Y chromosomes of 99 Kurdish Jews from all over Israel, unrelated at the paternal great-grandfather level, were analyzed. Most of the DNA samples (79) were anonymously obtained from the DNA collection established in our laboratory for the study of hematological disorders. The remainder (20 samples) were collected, by mouth swab, from randomly selected volunteers of self-identified Kurdish Jewish descent. The paternal ancestors of the majority of the subjects had lived in northern Iraq.
Muslim Kurds
Ninety-five DNA samples of Muslim Kurds were obtained, as described elsewhere (Brinkmann et al. 1999). The large majority of the subjects originated from northern Iraq.
Palestinian Arabs
Data on 17 Y chromosome polymorphisms of 143 Muslim Arabs residing in Israel and the Palestinian Authority Area (designated in other reports as “Israeli & Palestinian Arabs [I & P Arabs]”) were as reported elsewhere (Nebel et al. 2000, 2001). The same specimens were typed, in the present study, for two additional markers (p12f2 and M172).
Bedouin
Anonymous DNA samples of 30 male Bedouin from the Negev were obtained from the National Laboratory for the Genetics of Israeli Populations, Tel Aviv University. Two additional samples were from our DNA depository.
Other populations
Data on 122 Russians, 112 Poles, 41 Byelorussians, 167 Turks, 89 Armenians, 126 Spaniards, 385 Portuguese, and 129 North Africans (Berbers and Arabs described by Rosser et al. [2000]), along with data on 89 Syrians, 31 Jordanians, and 30 Lebanese (R. Villems and S. Rootsi, unpublished data) were used for comparison at the haplogroup level.
The volunteers gave written informed consent before samples were collected. The study was approved by the Hebrew University committee for ethics in research.
Typing of Y Chromosome DNA Polymorphisms
DNA samples were typed for 11 binary Y chromosome polymorphisms (YAP [DYS287], 92r7, SRY4064, SRY+465, sY81 [DYS271], Tat, M9, M13, M17, M20, and SRY10831) and for 6 microsatellite loci (DYS19, DYS388, DYS390, DYS391, DYS392, and DYS393), as described by Thomas et al. (1999). Haplogroup definitions, based on the allelic state at the binary markers, are presented in table 1.
Table 1.
Y Chromosome Haplogroup Distribution
|
Haplogroup Frequencies in |
|||||||
| Numbera | Definitionb | MuslimKurds(n = 95) | KurdishJews(n = 99) | SephardicJews(n = 78) | AshkenaziJews(n = 79) | PalestinianArabs(n = 143) | Bedouin(n = 32) |
| 1 | NTGCATGGG+AG | .168 | .202 | .295 | .114 | .084 | 0 |
| 2 | NCGCATCGG+AG NT | .168 | .061 | .115 | .063 | .063 | .063 |
| 3 (Eu 19) | NTGCATGGG−AA | .116 | .040 | .039 | .127 | .014 | .094 |
| 7 | NCGCATCCG+AA | 0 | 0 | 0 | 0 | .014 | 0 |
| 9 (Eu 9) | NCGCATCGG+AG PG | .284 | .152 | .154 | .240 | .168 | .031 |
| 9 (Eu 10) | NCGCATCGG+AG PT | .116 | .222 | .128 | .190 | .384 | .625 |
| 21 | PCACATCGG+AG | .074 | .121 | .192 | .228 | .203 | .187 |
| 26 | NCGCATGGG+AG | .042 | .192 | .077 | .038 | .070 | 0 |
| 28 | NCGCATGGG+GG | .032 | .010 | 0 | 0 | 0 | 0 |
Haplogroups are defined by the allele status at 13 binary markers in the following order: YAP, 92r7, SRY4064, SRY+465, sY81, Tat, M9, M13, M17, M20, SRY10831, p12f2 and M172. P = YAP insert/p12f2 deletion present (8-kb allele); N = no YAP insert/p12f2 deletion (10-kb allele); G+ = guanine present; G− = guanine deleted.
Chromosomes classified on the basis of analysis of the above 11 binary polymorphisms as belonging to haplogroup 2+ (Hg 2+) were further analyzed for p12f2 [DYS11] and M172, in a duplex PCR assay. The marker p12f2 distinguishes Hg 9 from Hg 2 (Bosch et al. 1999; Rosser et al. 2000), and M172 (Underhill et al. 2000) subdivides Hg 9 into the two lineages Eu 9 and Eu 10 (Semino et al. 2000). Typing of p12f2 was based on the absence (Hg 9) or presence (Hg 2) of an 88-bp PCR product (Rosser et al. 2000). As an internal control, a 148-bp product encompassing the M172 polymorphism was coamplified using the primers M172-F 5′-atcccccaaacccattttgatgcat-3′ and M172-R 5′-ggatccatcttcactcaatgttg-3′. PCR amplification was performed in 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM of each dNTP, 0.2 μM of each p12f2 primer, 0.3 μM of each M172 primer, and 0.2 U of AmpliTaq Gold (Perkin-Elmer, Roche Molecular Systems). Cycling conditions were as follows: initial denaturation and AmpliTaq Gold activation at 94°C for 10 min; 30–35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 45 s, and extension at 72°C for 45 s. The final cycle ended with an additional extension of 10 min at 72°C. A subsequent restriction digest of the PCR products with NlaIII (New England Biolabs) allowed us to differentiate between the M172 T allele (not cut) and the G allele (cut into a 122-bp and a 26-bp fragment). NlaIII digestion of p12f2 resulted in a 57-bp and a 31-bp fragment in all the samples. The digestion products were visualized on 3.5% NuSieve (FMC Bioproducts) agarose gels.
Statistical and Genealogical Analyses
Haplotype diversity (h) and its sampling variance (v) were estimated as described by Nei (1987). The relationships among populations were studied by constructing unrooted neighbor-joining (NJ) trees that were based on the genetic distance (DA) (Nei 1987), using Y chromosome haplogroup frequencies. The software package DISPAN was used to calculate the DA matrix and phylogenetic analyses. The software package Arlequin (version 1.1) was used to compute FST values and to perform population differentiation tests (Raymond and Rousset 1995) and analyses of molecular variance (AMOVA) (Excoffier et al. 1992). At the haplogroup level, AMOVA was applied to estimate variance components and Φ statistics, by taking into account both frequency and molecular content of haplogroups at different levels of hierarchical subdivision (among population groups, among populations within groups, and within populations). For this purpose, we generated a distance matrix that was based on the number of mutation steps between all haplogroup pairs and on the known Y chromosome haplogroup genealogy (fig. 1). At the microsatellite haplotype level, ΦST values between population pairs were calculated using the sum of squared allele-size differences (RST) as a measure of haplotype distance (Michalakis and Excoffier 1996). Microsat (version 1.5d) was applied to compute the genetic distance measure “average square distance” (ASD) (Goldstein et al. 1995). NJ haplotype trees on ASD were drawn with the Phylogeny Inference Package PHYLIP (version 3.5c).
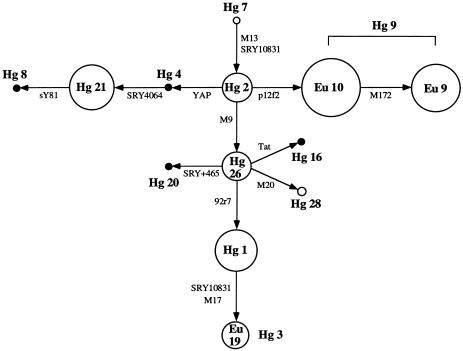
Figure 1.
Network of Y chromosome haplogroups (Hgs) based on the 13 binary polymorphisms analyzed. Unblackened circles represent haplogroups observed in the six Middle Eastern populations. The area of each circle is proportional to the frequency of the haplogroup in the total sample. Small blackened circles denote unobserved haplogroups. Arrows indicate the direction of the defining mutation. The haplogroup classification follows Rosser et al. (2000), except for the “Eu” designations, which are classified according to Semino et al. (2000). Hg 7 is the root. Eu 10 and Eu 9 are sublineages of Hg 9 that are distinguished by the mutation at M172. Hg 3 and Eu 19 appear phylogenetically equivalent in our sample. Hg 3 is defined by the reverse mutation at SRY10831 and Eu 19 by the polymorphism at M17.
Genealogical relationships among microsatellite haplotypes within haplogroups Eu 9 and Eu 10 were reconstructed using the program Network 2.0d (Bandelt et al. 1995; Bandelt et al. 1999). To adequately deal with the fast-evolving microsatellite loci, the reduced median (RM) (r=2) and median-joining (MJ) (ε=0) algorithms were applied sequentially (Forster et al. 2000). Moreover, a weighting scheme was used to compensate for the widely differing mutation rates at the six microsatellites. On the basis of locus diversity, the following weights were assigned: DYS388 and DYS390 = 1, DYS391 = 2, DYS393 = 3, and DYS19 and DYS392 = 4. According to Forster et al. (2000), length variation of different segments within one compound microsatellite locus can lead to artifacts in the network construction. Therefore, the complex DYS390 repeat region was sequenced in two Ashkenazi and three Sephardic Jews. In all samples, only one component, the n segment, was found to be variable.
The start of rapid expansion of Eu 9 and Eu 10 chromosomes within the populations studied here was estimated by use of the mean variance of microsatellite repeats (Slatkin 1995; Kittles et al. 1998) averaged across the six loci analyzed. The generation time was set at 25 years (as a mean of the commonly used estimates) and at 35 years (as suggested by Tremblay and Vézina [2000]). The average microsatellite-mutation rate for the six loci was set at 1.8 × 10−3 (95% confidence interval [CI] 9.8 × 10−4 to 3.1 × 10−3) (Quintana-Murci et al. 2001).
Results
Genetic Relationships among the Six Middle Eastern Populations
The genetic affinity among the six populations—Ashkenazi, Sephardic and Kurdish Jews, Muslim Kurds, Palestinian Arabs, and Bedouin—was first assessed at the level of the 13 binary polymorphisms. The 526 Y chromosomes were classified into nine haplogroups (table 1). The genealogical relationship of the haplogroups is shown in figure 1. In Sephardic Jewish communities from both North Africa and Iraq, the haplogroup distribution was very similar (Population Differentiation Test; P>.05). Therefore, in subsequent analyses, the two groups were pooled as Sephardic Jews. The pairwise FST values were statistically significant for all population pairs, except for Kurdish and Sephardic Jews (table 2). Ashkenazi Jews differed slightly from the other two Jewish communities. Kurdish and Sephardic Jews also clustered together on an unrooted NJ tree (fig. 2). Interestingly, the position of the Muslim Kurds on this tree is between Kurdish and Sephardic Jews on the one hand and Ashkenazim on the other. The most-distant group from the four non-Arab populations were the Bedouin.
Table 2.
Analysis of Genetic Differentiation: Pairwise FST Values between Populations[Note]
| Population | MuslimKurds | KurdishJews | SephardicJews | AshkenaziJews | PalestinianArabs |
| Kurdish Jews | .032a | … | … | … | … |
| Sephardic Jews | .023b | .012c | … | … | … |
| Ashkenazi Jews | .016b | .024b | .021b | … | … |
| Palestinian Arabs | .069a | .030d | .059a | .026d | … |
| Bedouin | .183a | .129a | .178a | .125a | .043b |
Note.— Analysis is based on frequencies of haplogroups defined by 13 binary Y chromosome polymorphisms.
P<.001.
.05>P>.005.
P>.05.
.005>P>.001.
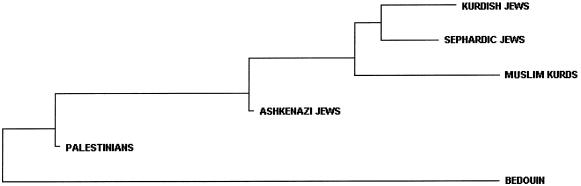
Figure 2.
Unrooted NJ tree depicting relationships among the six Middle Eastern populations, based on DA distances. The distance matrix is calculated using frequencies of haplogroups defined by 13 binary Y chromosome polymorphisms.
We further examined the relationship among the populations by incorporating microsatellites in the construction of haplotypes. The six microsatellite loci defined 250 different haplotypes within the nine haplogroups (Appendix). Haplotype diversity was high (h>.970) in all populations, except for the Bedouin, who exhibited a low value (h=.923) (Appendix). Microsatellite haplotype variance among the populations was assessed by use of AMOVA in a pairwise manner. The pattern obtained was similar to that observed at the haplogroup level (not shown). Notably, we found that the variance between the North African and Iraqi Sephardic samples was insignificant (ΦST=.013; P>.05), thereby justifying the pooling of the two data sets.
The relationship of the five modal haplotypes that were found in the six populations is presented in figure 3. The most-frequent haplotype in all three Jewish groups (the CMH [haplotype 159 in the Appendix]) segregated on a Eu 10 background, together with the three modal haplotypes in Palestinians and Bedouin (haplotypes 144, 151, and 166). The dominant haplotype of the Muslim Kurds (haplotype 114) was only one microsatellite-mutation step apart from the CMH and the modal haplotype of the Bedouin, but it belonged to haplogroup Eu 9. The three modal haplotypes in the Palestinians and Bedouin were entirely restricted to the two Arab populations. On the other hand, chromosomes with the modal haplotypes of the Jews and of the Muslim Kurds were observed in all the populations except the Bedouin. The three Jewish communities had many additional haplotypes in common with Muslim Kurds (table 3). They shared more haplotypes and chromosomes with Muslim Kurds than with either Palestinians or Bedouin.
Figure 3.
Simplified network relating the five modal haplotypes found in the six Middle Eastern populations. The haplotypes are defined by alleles at six microsatellite loci in the order DYS19, DYS388, DYS390, DYS391, DYS392, and DYS393. Lines between the haplotypes represent single-microsatellite mutation steps. The frequency of the modal haplotypes in each population is shown. AJ = Ashkenazi Jews; SJ = Sephardic Jews; KJ = Kurdish Jews; PA = Palestinian Arabs; B = Bedouin; MK = Muslim Kurds; MH = modal haplotype; and CMH = Cohen modal haplotype. Note that the CMH and the MH of the Muslim Kurds are only one microsatellite mutation step apart but occur on different haplogroup backgrounds (Eu 10 and Eu 9, respectively).
Table 3.
Proportions of Haplotypes (ht) and Chromosomes (ch) Shared by Population Pairs
| Population | MuslimKurds | KurdishJews | SephardicJews | AshkenaziJews | PalestinianArabs |
| Kurdish Jews | |||||
| ht | .213 | … | … | … | … |
| ch | .314 | ||||
| Sephardic Jews | |||||
| ht | .208 | .296 | … | … | … |
| ch | .289 | .390 | |||
| Ashkenazi Jews | |||||
| ht | .263 | .214 | .296 | … | … |
| ch | .345 | .292 | .382 | ||
| Palestinian Arabs | |||||
| ht | .133 | .142 | .153 | .198 | … |
| ch | .160 | .198 | .167 | .225 | |
| Bedouin | |||||
| ht | .024 | .027 | .053 | .082 | .157 |
| ch | .016 | .017 | .055 | .099 | .217 |
Genetic Relationships of Jews with Non-Jewish Populations
To place the six populations studied here in a broader geographical context, we expanded the analysis to include eight additional populations in the region (Rosser et al. 2000; R. Villems and S. Rootsi, unpublished data). AMOVA was performed on data of haplogroups defined by nine binary polymorphisms (data on M13, M17, M20, and M172 were not available for all populations). To compute variance components at three different levels of hierarchical subdivision (within populations, among populations within population groups, and among population groups), the 14 populations were classified into four groups according to ethnic affiliation (Jews: Ashkenazi, Sephardic, and Kurdish Jews; Arabs: Palestinians, Syrians, Jordanians, Lebanese, and Bedouin) or geographic proximity (Transcaucasians: Muslim Kurds, Armenians, and Turks; Eastern Europeans: Russians, Byelorussians, and Poles). We estimated that 80.8% (P<.001) of the total genetic variance resulted from differences within the 14 populations and that only 1.2% (P<.003) was partitioned among the populations within the groups, indicating that the criteria applied for the grouping were justified. Of the total variance, 18% was attributable to differences among the four groups (P<.001). The interpopulation ΦST variances within Transcaucasians and Jews were negligible (table 4, diagonal). Pairwise comparisons yielded an insignificant ΦCT value only for Jews and Transcaucasians (table 4, below diagonal). In contrast, Arabs showed small, yet significant, variance components with both Jews and Transcaucasians. Eastern Europeans represented an outgroup in this comparison, yielding high ΦCT values with all three Middle Eastern groups.
Table 4.
Genetic Variance within and between Population Groups[Note]
|
Jewsa |
Trans-caucasiansb |
Arabsc |
EasternEuropeansd |
|||||
| Population | Φ | P | Φ | P | Φ | P | Φ | P |
| Jews | .011 | .092 | … | … | … | … | … | … |
| Transcaucasians | .006 | .089 | .000 | .411 | … | … | … | … |
| Arabs | .062 | <.001 | .079 | <.001 | .021 | .018 | … | … |
| Eastern Europeans | .215 | <.001 | .222 | <.001 | .396 | <.001 | .030 | .006 |
Note.— The analysis is based on data of haplogroups defined by nine Y chromosome binary polymorphisms (without M13, M17, M20, and M172). Variance component is calculated within (ΦST, diagonal) and between (ΦCT, below diagonal) population groups.
Ashkenazi, Sephardic, and Kurdish Jews.
Palestinians, Jordanians, Syrians, Lebanese, and Bedouin.
Muslim Kurds, Armenians, and Turks.
Russians, Byelorussians, and Poles.
Further analysis, which included three additional populations, was performed by constructing an unrooted NJ tree (fig. 4). For this purpose, the Sephardic Jews were divided into a North African and an Iraqi sample. The Arab populations (including the North Africans) formed a distinct cluster. The Europeans and the Armenians made up another cluster at the opposite end of the tree. The four Jewish communities grouped closely with Muslim Kurds and Turks. Neither Ashkenazi Jews nor the two Sephardic samples clustered with their former host populations (non-Jewish Eastern European, Iberian, and North African populations). This finding is supported by highly significant FST values (all FSTs >.12; P<.001) between Jews and their respective host populations (not shown).
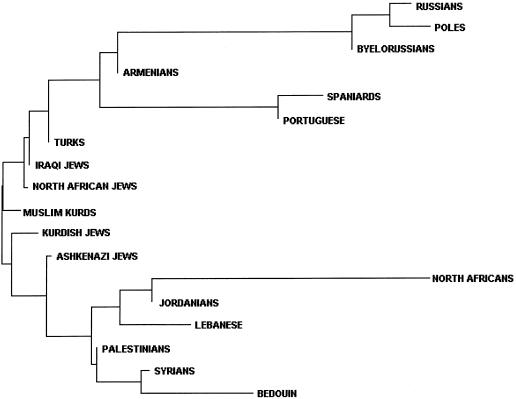
Figure 4.
Unrooted NJ tree depicting relationships among 18 populations based on DA. The distance matrix is calculated using frequencies of haplogroups defined by nine binary Y chromosome polymorphisms.
Haplogroups Eu 9 and Eu 10
According to Underhill et al. (2000), Eu 9 (H58) evolved from Eu 10 (H71) through a T→G transversion at M172. Eu 9 and Eu 10 together make up the Middle Eastern/Mediterranean Hg 9 (Semino et al. 2000). In the total sample analyzed here, nine instances of homoplasy were observed between the two closely related lineages. For instance, the microsatellite constellation of the CMH was also detected in Eu 9 (haplotype 108); the constellation of the modal haplotype of the Muslim Kurds was also found in Eu 10 (haplotype 167). Interestingly, the microsatellite constellation of the dominant haplotype in Iranians (Quintana-Murci et al. 2001) is identical to that in Kurds and also segregates on a Hg 9 background (M172 was not assayed in that study). In addition, both Eu 9 and Eu 10 were characterized by a very high frequency of alleles with ⩾15 repeats at DYS388 (83% for Eu 9 and 93% for Eu 10, respectively). These alleles with high numbers of repeats were found to be essentially restricted to the two haplogroups.
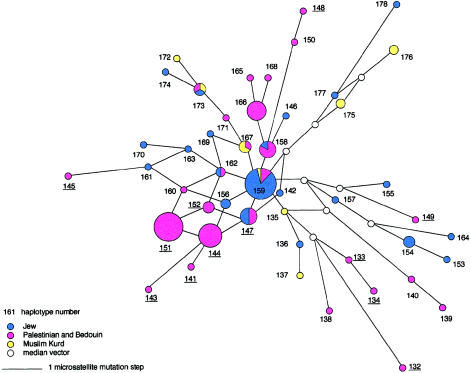
Figure 5 displays an MJ network relating all 47 haplotypes of Eu 10, representing 133 chromosomes of the sample. The CMH occupies the central position in the network and is connected to eight haplotypes, suggesting that it was at the core of expansion of the Eu 10 lineages in the populations studied. The two modal haplotypes in Palestinian Arabs, on the other hand, lie at the periphery of the network and have fewer links to neighbors. In the MJ network of Eu 9, the modal haplotype of the Muslim Kurds (haplotype 114) is in the center with nine links radiating from it (not shown).
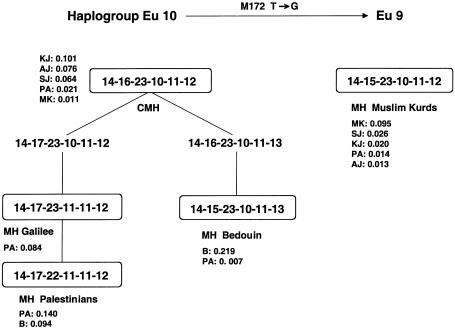
Figure 5.
MJ network of Eu 10. The network shows the genealogical relationships of the 47 microsatellite haplotypes found in 133 Eu 10 Y chromosomes from six Middle Eastern populations. The areas of the circles are proportional to the haplotype frequencies. Branch lengths are proportional to the number of mutational steps, and parallel links in a reticulation represent the same mutational changes. “Median vector” stands for a presumed haplotype not observed in the sample. The various populations are represented by different colors, as designated. The number next to the circle refers to the haplotype number in the Appendix. Haplotype 159, at the center of the network, is the CMH. The Arab clade haplotypes that have been defined elsewhere are underlined.
Eu 10 was the most frequent haplogroup among Palestinian Arabs and Bedouin (table 1), with a low haplotype diversity (h=.82) in both populations. Forty-two percent of the haplotypes and 47% of the chromosomes in Eu 10 were only observed in the two Arab populations. Palestinians had ∼42% of their Eu 10 chromosomes in common with Bedouin but had only 11% in common with the other four populations. The commonalities with the other four populations resulted from the sharing of low-frequency haplotypes. In contrast, in all the other haplogroups (except Hg 7, which was observed in only two Palestinian individuals in the present study), Palestinians shared 20%–46% of their chromosomes with the four non-Arab populations. Thus, the genetic distinctiveness of the Palestinian Arabs is mainly seen in the presence of specific high-frequency Eu 10 haplotypes not found in non-Arab groups.
Elsewhere, 12 haplotypes of the Palestinian Arabs, including their two modal haplotypes, were reported to form a clade on an NJ tree (designated as “Arab clade”), with a moderate bootstrap value (Nebel et al. 2000). The tree was constructed with haplotypes of Arabs, Jews, and Welsh that belonged to a haplogroup defined by only six binary markers. It actually represented a compound of the six haplogroups Hg 2, Hg 7, Hg 26, Hg 28, Eu 9, and Eu 10. In the present study, the Arab clade haplotypes were all found to be part of Eu 10, confirming their genetic clustering. However, as shown in figure 5, the Arab clade haplotypes are dispersed throughout the Eu 10 network, indicating that they do not represent a separate genealogical lineage but are an integral part of this haplogroup. On an NJ tree of Eu 10 haplotypes, nine of these haplotypes grouped in two neighboring branches but without any bootstrap support (not shown).
We have performed genetic dating on the basis of microsatellite variation (Slatkin 1995; Kittles et al. 1998). For a generation time of 25 years, the estimate for the start of the expansion of Eu 9 was 7,038 years ago (95% CI 12,900–4,100 years ago), and that of Eu 10 was 6,426 years ago (95% CI 11,800–3,700 years ago). The expansion of Hg 9, which includes both Eu 9 and Eu 10, was dated to 7,492 years ago (95% CI 13,760–4,350 years ago). For a generation time of 35 years, the date for Eu 9 was 9,854 years ago (95% CI 18,095–5,705 years ago), and the date for Eu 10 was 8,997 years ago (95% CI 16,520–5,215 years ago). The expansion of Hg 9 was estimated to have begun 10,488 years ago (95% CI 19,265–6,090 years ago).
Discussion
Genetic Relationships among Jewish Communities
It is believed that the majority of contemporary Jews descended from the ancient Israelites that had lived in the historic land of Israel until ∼2000 years ago. Many of the Jewish diaspora communities were separated from each other for hundreds of years. Therefore, some divergence due to genetic drift and/or admixture could be expected. However, although Ashkenazi Jews were found to differ slightly from Sephardic and Kurdish Jews, it is noteworthy that there is, overall, a high degree of genetic affinity among the three Jewish communities. Moreover, neither Ashkenazi nor Sephardic Jews cluster adjacent to their former host populations, a finding that argues against substantial admixture of males. These findings are in accordance with those described by Hammer et al. (2000).
Ashkenazi Jews
Ashkenazi Jews consolidated into a distinct ethnicity in Germany during the Middle Ages and spread eastwards to Poland and Russia in the 13th century (Ben-Sasson 1976). Previous studies of Y chromosome polymorphisms reported a small European contribution to the Ashkenazi paternal gene pool (Santachiara-Benerecetti et al. 1993; Hammer et al. 2000). In our sample, this low-level gene flow may be reflected in the Eu 19 chromosomes, which are found at elevated frequency (12.7%) in Ashkenazi Jews and which are very frequent in Eastern Europeans (54%–60%; Semino et al. 2000). Alternatively, it is attractive to hypothesize that Ashkenazim with Eu 19 chromosomes represent descendents of the Khazars, originally a Turkic tribe from Central Asia, who settled in southern Russia and eastern Ukraine and converted en masse to Judaism in the ninth century of the present era, as described by Yehuda Ha-Levi in 1140 a.d. (Dunlop 1954).
Kurdish Jews
The Jews of Kurdistan lived—until their immigration to Israel in the early 1950s—as a closed ethnic isolate, mostly in northern Iraq and Iran and in eastern Turkey. According to an old tradition, the Jews of Kurdistan are descendents of the Ten Tribes from the time of the Assyrian exile in 723 b.c. (Roth 1972). Genetically, Kurdish Jews are not closer to Muslim Kurds than are Sephardim or Ashkenazim, suggesting that reciprocal male gene flow between Jews in Kurdistan and their Muslim host population was below the detectable level. The acceptance of Judaism by the rulers and inhabitants of the Kurdish Kingdom of Adiabene in the first century of the Common Era resulted in the assimilation of non-Jews into the community (Brauer 1993). This recorded conversion does not appear to have had a considerable effect on the Y chromosome pool of the Kurdish Jews.
Sephardic Jews
Iraqi and North African Jews are both considered to belong to the ethnically heterogeneous group of Sephardim, although the two communities were probably separated for 1,000 years. The Jewish community in Iraq was formed by deportees during the Assyrian and Babylonian exiles (723 and 586 b.c.) and by waves of immigrants in subsequent centuries. Communities in various North African countries and in the Iberian Peninsula were established primarily in the course of the Muslim conquest in the seventh and eighth centuries. After their expulsion from Spain in 1492 a.d., Jews were dispersed in North Africa and Southern Europe (Ben-Sasson 1976). The two Sephardic communities and Kurdish Jews are very closely related to each other. Thus, these populations seem to have preserved, to a large extent, their original Y chromosome pools.
Genetic Relationships among Middle Eastern Populations
In a report published elsewhere, we recently showed that Jews and Palestinian Arabs share a large portion of their Y chromosomes, suggesting a common ancestry (Nebel et al. 2000). Surprisingly, in the present study, Jews were found to be even closer to populations in the northern part of the Middle East than to several Arab populations. It is worth mentioning that, on the basis of protein polymorphisms, most Jewish populations cluster very closely with Iraqis (Livshits et al. 1991) and that the latter, in turn, cluster very closely with Kurds (Cavalli-Sforza et al. 1994). These findings are consistent with known cultural links that existed among populations in the Fertile Crescent in early history.
Muslim Kurds
The Kurds are considered an ancient autochthonous population (Kinnane 1970; Pelletiere 1984) who may even be the descendants of the shepherds who first populated the highlands during the Neolithic period (Comas et al. 2000). Although Kurdistan came under the successive dominion of various conquerors, including the Armenians, Romans, Byzantines, Arabs, Ottoman Turks, and Iraqis (Kinnane 1970), they may be the only western Asian group that remained relatively unmixed by the influx of invaders, because of their protected and inhospitable mountainous homeland (Pelletiere 1984). The Y chromosome variation of Muslim Kurds falls within the spectrum observed in other populations (Turks and Armenians) living in the same region. The three populations are closer to Jews and Arabs than to Europeans. This is in good agreement with data on classical markers (Cavalli-Sforza et al. 1994). However, on the basis of mtDNA polymorphisms, Kurds were reported to be more closely related to Europeans than to Middle Easterners (Comas et al. 2000).
Palestinian Arabs and Bedouin
Bedouin are largely nomadic Arab herders, with a tribal organization. They live in all Arab countries, constituting about one tenth of the population (Cavalli-Sforza et al. 1994). The Bedouin population of the Negev desert was found to be most distant from Jews and Muslim Kurds and to be closely related only to Palestinians. Both these Arab populations differ from the other Middle Eastern groups sampled for the present study, mainly in having a higher frequency of Eu 10 chromosomes, the majority of which they share with each other. Traditional marriage practices—such as male polygamy, a high rate of consanguineous marriages, and patrilocality—may have enhanced the low haplogroup and haplotype diversity of the Negev Bedouin, as was suggested elsewhere for the Bedouin tribes in the Sinai Peninsula (Salem et al. 1996).
We propose that the Y chromosomes in Palestinian Arabs and Bedouin represent, to a large extent, early lineages derived from the Neolithic inhabitants of the area and additional lineages from more-recent population movements. The early lineages are part of the common chromosome pool shared with Jews (Nebel et al. 2000). According to our working model, the more-recent migrations were mostly from the Arabian Peninsula, as is seen in the Arab-specific Eu 10 chromosomes that include the modal haplotypes observed in Palestinians and Bedouin. These haplotypes and their one-step microsatellite neighbors constitute a substantial portion of the total Palestinian (29%) and Bedouin (37.5%) Y chromosome pools and were not found in any of the non-Arab populations in the present study. The peripheral position of the modal haplotypes, with few links in the network (fig. 5), suggests that the Arab-specific chromosomes are a result of recent gene flow. Historical records describe tribal migrations from Arabia to the southern Levant in the Byzantine period, migrations that reached their climax with the Muslim conquest 633–640 a.d.; Patrich 1995). Indeed, Arab-specific haplotypes have been observed at significant frequencies in Muslim Arabs from Sena (56%) and the Hadramaut (16%) in the Yemen (Thomas et al. 2000). Thus, although Y chromosome data of Arabian populations are limited, it seems very likely that populations from the Arabian Peninsula were the source of these chromosomes. The genetic closeness, in classical protein markers, of Bedouin to Yemenis and Saudis (Cavalli-Sforza et al. 1994) supports an Arabian origin of the Bedouin. The alternative explanation for the distribution of the Arab-specific haplotypes (i.e., random genetic drift) is unlikely. It is difficult to imagine that the different populations in the Yemen and the southern Levant, in which Arab-specific chromosomes have been detected at moderate-to-high frequencies, would have drifted in the same direction.
Linguistics
The high degree of genetic similarity of the Middle Eastern populations studied here is not reflected in linguistic affinity. The Kurdish language is related to Persian and belongs to the Indo-European family, which sets the Kurds apart from the Semitic-speaking Jews and Arabs and from the Turkic speakers in Turkey (Pelletiere 1984). Like Kurdish, the Armenian language is also of Indo-European origin, but it forms a separate branch within the western group of this family. The high genetic affinity across major language divisions therefore suggests that the Y chromosome pool of Middle Eastern populations is ancient and predates the emergence or introduction of different languages into the region.
Geographical and Historical Origins of Eu 9 and Eu 10
Eu 9 and Eu 10 make up the Middle Eastern/Mediterranean Hg 9. They are characterized by a very high frequency of DYS388 alleles with ⩾15 repeats, which are restricted to these two haplogroups. This finding confirms our previous hypotheses, which postulated that low- and high-repeat number DYS388 alleles segregate in distinct haplogroups, that high-score repeats are the hallmark of Hg 9 and, hence, that DYS388 is a useful Middle Eastern–specific marker (Nebel et al. 2001).
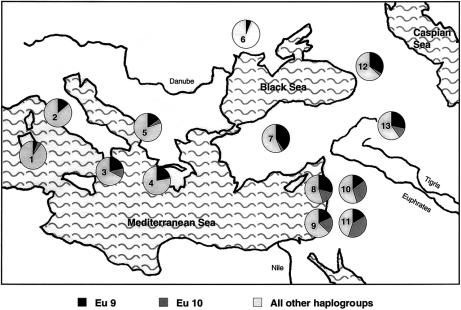
Hg 9 was shown to have spread from the Zagros Mountains in northwestern Iran (Quintana-Murci et al. 2001). Our data confirm the Middle Eastern origin of Hg 9. Its two sublineages (Eu 9 and Eu 10) show clear opposite clines, with a north-south frequency gradient in the Middle East that decreases for Eu 9 and increases for Eu 10 (fig. 6). Thus, the possible place of origin for Eu 9 could be in the northern part of the Fertile Crescent and that for Eu 10 in the southern part. However, since data for some relevant populations are not available, this interpretation should be regarded with caution.
Figure 6.
Geographical distribution of Eu 9 and Eu 10. The haplogroups are represented by different shades of grey as designated. The number in each pie indicates the population analyzed. The data are from Semino et al. (2000) for the following populations: 1 = Sardinians; 2 = central-northern Italians; 3 = Calabrians; 4 = Greeks; 5 = Macedonians; 6 = Ukrainians; 7 = Turks; 8 = Lebanese; 10 = Syrians; and 12 = Georgians. Data from the present study are from the following populations: 9 = Jews; 11 = Palestinian Arabs; and 13 = Muslim Kurds.
The dates for the start of expansion of Hg 9 found here (∼10,500 and 7,500 years ago, based on generation times of 35 or 25 years, respectively) are in good agreement with estimates published elsewhere (Quintana-Murci et al. 2001). This range of dates and the ones obtained for Eu 9 (∼9,800–7,000 years ago) and Eu 10 (∼9,000–6,400 years ago) cover the Neolithic period and the beginning of the Chalcolithic period in the region (Bar-Yosef 1995; Levy 1995). These estimates and the geographic distribution of both Eu 9 and Eu 10 support the model of demic diffusion from the Middle East during the Neolithic period (Semino et al. 2000).
The p12f2 deletion defining Hg 9 has been dated to 14,800±9,700 years ago (Hammer et al. 2000). The discrepancy between the older mutation age obtained by coalescence dating (Hammer et al. 2000) and the estimates based on microsatellite variation (Quintana-Murci et al. 2001 and the present study) may be the result of any of the following: (a) a lag between the mutation event proper and the expansion of the lineage it defines, (b) microsatellite saturation, (c) overestimation of the microsatellite-mutation rate, or (d) sampling bias (Bosch et al. 1999).
In the present sample, the modal haplotype of the Muslim Kurds is at the core of Eu 9, and the CMH is at the center of Eu 10, attesting to the antiquity of both haplotypes. Their close microsatellite constellations (they differ by only one microsatellite mutation) suggest that the expansion and genetic divergence of the two haplogroups followed closely the T→G transversion that split Eu 9 from Eu 10. The high degree of homoplasy between Eu 9 and Eu 10, together with their very similar microsatellite allele spectra, corroborates this hypothesis.
In conclusion, the present study shows that the Middle Eastern populations we analyzed are closely related and that their Y chromosome pool is distinct from that of Europeans. Genetic dating performed in the present study, together with age estimates reported elsewhere (reviewed by Bosch et al. 1999), suggests that the major haplogroups observed in our sample are much older than the populations in which they are found. Thus, the common genetic Middle Eastern background predates the ethnogenesis in the region. The study demonstrates that the Y chromosome pool of Jews is an integral part of the genetic landscape of the region and, in particular, that Jews exhibit a high degree of genetic affinity to populations living in the north of the Fertile Crescent.
Acknowledgments
We wish to thank Professor Richard Villems and Siiri Rootsi (Estonian Biocentre, Tartu, Estonia) for sharing their typing data before publication. We are grateful to Dr. Mark Thomas (Department of Biology, University College London, United Kingdom) for inviting A.N. to type the Kurdish samples in his laboratory. We thank Professor Israel Eph'al (Department of History of the Jewish People, Hebrew University of Jerusalem, Israel), and Professor Sergio della Pergola (A. Harman Institute of Contemporary Jewry, Hebrew University of Jerusalem, Israel) for historical insights. We would like to acknowledge Dr. Peter Forster (McDonald Institute for Archaeological Research, University of Cambridge, United Kingdom) for helpful suggestions regarding the use of the Network program. Special thanks to Dr. Mira Korner (The National Genome Center at the Hebrew University of Jerusalem, Israel) for assistance and advice in DNA typing. This work was supported by a research grant from the Israeli Ministry of Science, Culture and Sport.
Appendix
Table A1.
Distribution of Y Chromosome Haplotypes in Six Middle Eastern Populations
|
Allele Status at |
Populationa |
||||||||||||
| HaplotypeNumber | DYS19 | DYS388 | DYS390 | DYS391 | DYS392 | DYS393 | AJ | SJ | KJ | MK | PA | B | Total |
| Hg 1 | |||||||||||||
| 1 | 16 | 13 | 23 | 10 | 13 | 13 | 1Iq | 1 | |||||
| 2 | 16 | 13 | 23 | 10 | 10 | 14 | 1NA | 1 | |||||
| 3 | 16 | 12 | 23 | 10 | 10 | 14 | 1Iq | 1 | 2 | ||||
| 4 | 15 | 12 | 24 | 11 | 13 | 13 | 1 | 1 | 2 | ||||
| 5 | 15 | 12 | 24 | 10 | 13 | 13 | 2 | 2 | |||||
| 6 | 15 | 12 | 24 | 10 | 13 | 12 | 1 | 1 | |||||
| 7 | 15 | 12 | 23 | 11 | 13 | 13 | 3 | 3 | |||||
| 8 | 15 | 12 | 23 | 10 | 10 | 14 | 1Iq | 1 | |||||
| 9 | 15 | 12 | 22 | 10 | 15 | 13 | 1NA | 1 | |||||
| 10 | 14 | 12 | 26 | 11 | 13 | 13 | 1Ib | 1 | |||||
| 11 | 14 | 12 | 25 | 11 | 13 | 13 | 1Iq | 1 | |||||
| 12 | 14 | 12 | 25 | 11 | 13 | 12 | 1 | 1 | |||||
| 13 | 14 | 12 | 25 | 10 | 14 | 12 | 1 | 1 | |||||
| 14 | 14 | 12 | 25 | 10 | 13 | 13 | 1 | 1 | |||||
| 15 | 14 | 12 | 24 | 11 | 14 | 12 | 1 | 1Iq | 2 | 2 | 1 | 7 | |
| 16 | 14 | 12 | 24 | 11 | 13 | 13 | 1 | 1 | |||||
| 17 | 14 | 12 | 24 | 11 | 13 | 12 | 1 | 4 | 2 | 3 | 10 | ||
| 18 | 14 | 12 | 24 | 10 | 14 | 12 | 2 | 3NA | 1 | 6 | |||
| 19 | 14 | 12 | 24 | 10 | 13 | 13 | 2 | 2 | |||||
| 20 | 14 | 12 | 24 | 10 | 13 | 12 | 1NA | 1 | |||||
| 21 | 14 | 12 | 24 | 10 | 12 | 12 | 1NA | 1 | |||||
| 22 | 14 | 12 | 23 | 11 | 14 | 12 | 3 | 3 | |||||
| 23 | 14 | 12 | 23 | 11 | 13 | 12 | 1 | 1 | |||||
| 24 | 14 | 12 | 23 | 10 | 13 | 13 | 1 | 1 | 2 | ||||
| 25 | 14 | 12 | 23 | 10 | 10 | 14 | 2T, 2Iq | 4 | 8 | ||||
| 26 | 14 | 12 | 23 | 10 | 10 | 12 | 1 | 1 | |||||
| 27 | 14 | 12 | 22 | 10 | 16 | 13 | 1NA | 1 | |||||
| 28 | 14 | 12 | 22 | 10 | 15 | 13 | 2NA | 1 | 3 | ||||
| 29 | 14 | 11 | 23 | 10 | 13 | 13 | 1 | 1 | |||||
| 30 | 13 | 13 | 24 | 11 | 13 | 12 | 1 | 1 | |||||
| 31 | 13 | 12 | 24 | 10 | 14 | 13 | 1 | 1 | |||||
| 32 | 13 | 12 | 23 | 11 | 13 | 13 | 1 | 1 | |||||
| 33 | 13 | 12 | 23 | 10 | 15 | 14 | 1 | 1 | |||||
| 34 | 13 | 12 | 22 | 10 | 15 | 14 | 1 | 1 | |||||
| 35 | 13 | 12 | 22 | 10 | 15 | 13 | 2 | 2NA, 1Ib | 1 | 1 | 7 | ||
| 36 | 13 | 12 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| Hg 2 | |||||||||||||
| 37 | 17 | 12 | 23 | 11 | 11 | 11 | 1Bl | 1 | |||||
| 38 | 16 | 13 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| 39 | 16 | 13 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 40 | 16 | 13 | 23 | 10 | 12 | 14 | 1 | 1 | |||||
| 41 | 16 | 12 | 22 | 10 | 11 | 14 | 1T | 2 | 3 | ||||
| 42 | 16 | 12 | 22 | 10 | 10 | 14 | 1 | 1 | |||||
| 43 | 16 | 12 | 20 | 9 | 11 | 15 | 1 | 1 | |||||
| 44 | 16 | 10 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 45 | 15 | 13 | 26 | 10 | 11 | 14 | 2 | 2 | |||||
| 46 | 15 | 13 | 22 | 10 | 11 | 12 | 1 | 1 | |||||
| 47 | 15 | 12 | 23 | 11 | 11 | 13 | 1Iq | 1 | |||||
| 48 | 15 | 12 | 23 | 10 | 13 | 13 | 1 | 1 | |||||
| 49 | 15 | 12 | 23 | 10 | 12 | 14 | 1 | 1 | |||||
| 50 | 15 | 12 | 23 | 10 | 11 | 14 | 1NA | 1 | |||||
| 51 | 15 | 12 | 23 | 10 | 11 | 13 | 1 | 3 | 4 | ||||
| 52 | 15 | 12 | 23 | 10 | 10 | 14 | 1 | 1 | |||||
| 53 | 15 | 12 | 22 | 11 | 11 | 14 | 2 | 2 | |||||
| 54 | 15 | 12 | 22 | 11 | 11 | 13 | 2Iq | 2 | |||||
| 55 | 15 | 12 | 22 | 10 | 11 | 13 | 1NA | 1 | |||||
| 56 | 15 | 12 | 22 | 10 | 10 | 14 | 1 | 1 | |||||
| 57 | 15 | 12 | 21 | 11 | 12 | 15 | 1NA | 1 | |||||
| 58 | 15 | 12 | 21 | 10 | 11 | 14 | 5 | 5 | |||||
| 59 | 15 | 10 | 23 | 10 | 12 | 13 | 2 | 2 | |||||
| 60 | 14 | 12 | 24 | 10 | 12 | 13 | 1 | 1 | |||||
| 61 | 14 | 12 | 23 | 10 | 12 | 14 | 1 | 1 | |||||
| 62 | 14 | 12 | 23 | 10 | 11 | 14 | 1 | 1 | |||||
| 63 | 14 | 12 | 22 | 11 | 12 | 13 | 2 | 2 | |||||
| 64 | 14 | 12 | 22 | 11 | 11 | 14 | 1 | 1 | |||||
| 65 | 14 | 12 | 22 | 10 | 12 | 13 | 1 | 1 | |||||
| 66 | 14 | 12 | 22 | 10 | 11 | 14 | 1NA | 1 | 2 | ||||
| 67 | 14 | 10 | 23 | 11 | 11 | 15 | 1 | 1 | |||||
| 68 | 13 | 12 | 25 | 9 | 11 | 13 | 1 | 1 | |||||
| Hg 3 (Eu 19) | |||||||||||||
| 69 | 17 | 12 | 25 | 11 | 11 | 13 | 1T | 2 | 3 | 6 | |||
| 70 | 17 | 12 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| 71 | 16 | 12 | 25 | 11 | 11 | 13 | 1 | 2 | 1 | 4 | |||
| 72 | 16 | 12 | 25 | 10 | 11 | 13 | 5 | 1Iq | 1 | 7 | |||
| 73 | 16 | 12 | 24 | 11 | 11 | 13 | 1 | 1 | 2 | ||||
| 74 | 16 | 12 | 24 | 10 | 11 | 14 | 1 | 1 | |||||
| 75 | 16 | 12 | 24 | 10 | 11 | 13 | 1 | 1NA | 2 | ||||
| 76 | 16 | 12 | 23 | 10 | 11 | 15 | 1 | 1 | |||||
| 77 | 15 | 12 | 25 | 11 | 11 | 14 | 1 | 1 | |||||
| 78 | 15 | 12 | 25 | 11 | 11 | 12 | 1 | 3 | 4 | ||||
| 79 | 15 | 12 | 25 | 10 | 11 | 13 | 2 | 2 | |||||
| 80 | 15 | 12 | 24 | 11 | 11 | 14 | 1 | 1 | |||||
| 81 | 15 | 12 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| Hg 7 | |||||||||||||
| 82 | 15 | 11 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| 83 | 14 | 15 | 23 | 9 | 11 | 12 | 1 | 1 | |||||
| Hg 9 (Eu 9) | |||||||||||||
| 84 | 16 | 16 | 23 | 10 | 11 | 13 | 1 | 1 | |||||
| 85 | 16 | 16 | 23 | 9 | 11 | 12 | 1 | 1 | |||||
| 86 | 16 | 15 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 87 | 16 | 15 | 22 | 11 | 11 | 12 | 1 | 1 | |||||
| 88 | 16 | 14 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 89 | 16 | 12 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 90 | 15 | 16 | 24 | 10 | 11 | 12 | 1 | 1 | 2 | ||||
| 91 | 15 | 16 | 23 | 9 | 11 | 12 | 3 | 3 | |||||
| 92 | 15 | 16 | 22 | 9 | 11 | 12 | 1 | 1 | |||||
| 93 | 15 | 15 | 25 | 11 | 11 | 13 | 1T | 1 | |||||
| 94 | 15 | 15 | 24 | 10 | 11 | 12 | 2 | 5 | 7 | ||||
| 95 | 15 | 15 | 23 | 11 | 11 | 12 | 1 | 1 | |||||
| 96 | 15 | 15 | 23 | 10 | 11 | 12 | 1 | 1NA | 1 | 1 | 4 | ||
| 97 | 15 | 15 | 23 | 9 | 11 | 13 | 1NA | 1 | |||||
| 98 | 15 | 15 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| 99 | 15 | 15 | 22 | 10 | 11 | 12 | 2 | 1 | 3 | ||||
| 100 | 15 | 15 | 22 | 10 | 8 | 12 | 1 | 1 | |||||
| 101 | 15 | 14 | 23 | 9 | 11 | 13 | 1 | 1 | |||||
| 102 | 15 | 13 | 25 | 10 | 11 | 12 | 1 | 1 | |||||
| 103 | 15 | 12 | 24 | 11 | 11 | 12 | 1NA | 1 | |||||
| 104 | 14 | 18 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 105 | 14 | 17 | 23 | 10 | 11 | 12 | 1Iq | 1 | 2 | 1 | 5 | ||
| 106 | 14 | 17 | 22 | 9 | 10 | 12 | 1 | 1 | |||||
| 107 | 14 | 16 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 108 | 14 | 16 | 23 | 10 | 11 | 12 | 3 | 1 | 4 | ||||
| 109 | 14 | 16 | 23 | 10 | 11 | 11 | 2 | 2 | |||||
| 110 | 14 | 16 | 23 | 9 | 11 | 16 | 1 | 1 | |||||
| 111 | 14 | 15 | 26 | 10 | 11 | 12 | 2 | 2 | |||||
| 112 | 14 | 15 | 25 | 10 | 11 | 12 | 1 | 1 | |||||
| 113 | 14 | 15 | 23 | 10 | 11 | 13 | 1 | 1 | |||||
| 114 | 14 | 15 | 23 | 10 | 11 | 12 | 1 | 2NA | 2 | 9 | 2 | 16 | |
| 115 | 14 | 15 | 23 | 10 | 11 | 10 | 1 | 1 | |||||
| 116 | 14 | 15 | 22 | 11 | 11 | 13 | 2 | 2 | |||||
| 117 | 14 | 15 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| 118 | 14 | 15 | 22 | 10 | 11 | 12 | 1 | 1NA | 2 | 4 | |||
| 119 | 14 | 15 | 22 | 10 | 11 | 11 | 1T | 1 | |||||
| 120 | 14 | 15 | 22 | 9 | 11 | 13 | 1Iq | 2 | 3 | ||||
| 121 | 14 | 15 | 22 | 9 | 11 | 12 | 1 | 1 | 2 | ||||
| 122 | 14 | 14 | 25 | 11 | 11 | 12 | 1 | 1 | |||||
| 123 | 14 | 14 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 124 | 14 | 14 | 24 | 10 | 11 | 12 | 4 | 1 | 5 | ||||
| 125 | 14 | 14 | 23 | 11 | 11 | 12 | 1 | 1 | |||||
| 126 | 14 | 14 | 22 | 10 | 11 | 12 | 1 | 1 | |||||
| 127 | 14 | 13 | 24 | 10 | 11 | 12 | 1NA | 1 | 2 | ||||
| 128 | 13 | 17 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 129 | 13 | 15 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 130 | 13 | 15 | 23 | 10 | 11 | 12 | 1Iq | 1 | |||||
| 131 | 13 | 11 | 22 | 10 | 11 | 12 | 1 | 1 | |||||
| Hg 9 (Eu 10) | |||||||||||||
| 132 | 15 | 18 | 23 | 12 | 11 | 13 | 1 | 1 | |||||
| 133 | 15 | 17 | 23 | 11 | 11 | 12 | 1 | 1 | |||||
| 134 | 15 | 17 | 22 | 11 | 11 | 12 | 1 | 1 | |||||
| 135 | 15 | 16 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 136 | 15 | 16 | 23 | 10 | 11 | 11 | 1 | 1 | |||||
| 137 | 15 | 16 | 22 | 10 | 11 | 11 | 1 | 1 | |||||
| 138 | 15 | 15 | 24 | 11 | 11 | 12 | 1 | 1 | |||||
| 139 | 15 | 13 | 23 | 10 | 12 | 13 | 1 | 1 | |||||
| 140 | 15 | 13 | 23 | 10 | 12 | 12 | 1 | 1 | |||||
| 141 | 14 | 17 | 24 | 11 | 11 | 12 | 1 | 1 | |||||
| 142 | 14 | 17 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 143 | 14 | 17 | 23 | 13 | 11 | 12 | 1 | 1 | |||||
| 144 | 14 | 17 | 23 | 11 | 11 | 12 | 12 | 12 | |||||
| 145 | 14 | 17 | 23 | 11 | 11 | 11 | 1 | 1 | |||||
| 146 | 14 | 17 | 23 | 10 | 11 | 13 | 1T | 1 | |||||
| 147 | 14 | 17 | 23 | 10 | 11 | 12 | 1 | 1NA, 1Iq | 2 | 1 | 6 | ||
| 148 | 14 | 17 | 22 | 12 | 11 | 13 | 1 | 1 | |||||
| 149 | 14 | 17 | 22 | 11 | 12 | 12 | 1 | 1 | |||||
| 150 | 14 | 17 | 22 | 11 | 11 | 13 | 1 | 1 | |||||
| 151 | 14 | 17 | 22 | 11 | 11 | 12 | 20 | 3 | 23 | ||||
| 152 | 14 | 17 | 22 | 10 | 11 | 12 | 3 | 3 | |||||
| 153 | 14 | 16 | 26 | 10 | 13 | 12 | 1 | 1 | |||||
| 154 | 14 | 16 | 25 | 10 | 13 | 12 | 3 | 3 | |||||
| 155 | 14 | 16 | 23 | 11 | 12 | 13 | 1 | 1 | |||||
| 156 | 14 | 16 | 23 | 11 | 11 | 12 | 1 | 1 | 2 | ||||
| 157 | 14 | 16 | 23 | 10 | 13 | 12 | 1 | 1 | |||||
| 158 | 14 | 16 | 23 | 10 | 11 | 13 | 1 | 5 | 6 | ||||
| 159 | 14 | 16 | 23 | 10 | 11 | 12 | 6 | 2NA, 2S, 1Ib | 10 | 1 | 3 | 25 | |
| 160 | 14 | 16 | 22 | 11 | 11 | 12 | 1 | 1 | |||||
| 161 | 14 | 16 | 22 | 11 | 11 | 11 | 1 | 1 | |||||
| 162 | 14 | 16 | 22 | 10 | 11 | 12 | 1 | 1 | 2 | ||||
| 163 | 14 | 16 | 22 | 10 | 11 | 11 | 1 | 1 | |||||
| 164 | 14 | 15 | 24 | 10 | 14 | 12 | 1T | 1 | |||||
| 165 | 14 | 15 | 23 | 10 | 11 | 14 | 1 | 1 | |||||
| 166 | 14 | 15 | 23 | 10 | 11 | 13 | 1 | 7 | 8 | ||||
| 167 | 14 | 15 | 23 | 10 | 11 | 12 | 1 | 2 | 3 | ||||
| 168 | 14 | 15 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| 169 | 14 | 15 | 22 | 10 | 11 | 12 | 1NA | 1 | |||||
| 170 | 14 | 15 | 22 | 10 | 11 | 11 | 1 | 1 | |||||
| 171 | 14 | 14 | 23 | 10 | 11 | 12 | 1 | 1 | |||||
| 172 | 14 | 13 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 173 | 14 | 13 | 23 | 10 | 11 | 12 | 1 | 1 | 1 | 3 | |||
| 174 | 14 | 13 | 22 | 10 | 11 | 12 | 1 | 1 | |||||
| 175 | 13 | 16 | 25 | 10 | 11 | 12 | 2 | 2 | |||||
| 176 | 13 | 15 | 25 | 11 | 11 | 12 | 2 | 2 | |||||
| 177 | 13 | 15 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 178 | 13 | 13 | 24 | 10 | 10 | 12 | 1 | 1 | |||||
| Hg 21 | |||||||||||||
| 179 | 16 | 12 | 25 | 10 | 11 | 14 | 1 | 1 | |||||
| 180 | 16 | 12 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 181 | 16 | 12 | 23 | 10 | 11 | 13 | 1 | 1 | |||||
| 182 | 16 | 12 | 22 | 9 | 12 | 13 | 1Iq | 1 | 2 | ||||
| 183 | 15 | 14 | 23 | 11 | 11 | 12 | 1NA | 1 | |||||
| 184 | 15 | 13 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| 185 | 15 | 12 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| 186 | 15 | 12 | 24 | 10 | 11 | 14 | 1 | 1 | |||||
| 187 | 15 | 12 | 24 | 10 | 11 | 13 | 1 | 1NA | 2 | 4 | |||
| 188 | 15 | 12 | 24 | 9 | 11 | 13 | 1 | 1 | |||||
| 189 | 15 | 12 | 23 | 10 | 11 | 13 | 1 | 1 | |||||
| 190 | 15 | 12 | 22 | 10 | 11 | 13 | 1 | 1 | |||||
| 191 | 14 | 15 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 192 | 14 | 13 | 25 | 10 | 13 | 13 | 1 | 1 | |||||
| 193 | 14 | 13 | 24 | 10 | 11 | 13 | 1 | 1 | |||||
| 194 | 14 | 13 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 195 | 14 | 12 | 26 | 10 | 11 | 13 | 1 | 1 | |||||
| 196 | 14 | 12 | 25 | 10 | 11 | 14 | 2 | 2 | |||||
| 197 | 14 | 12 | 25 | 10 | 11 | 13 | 1 | 1 | 2 | ||||
| 198 | 14 | 12 | 25 | 9 | 11 | 13 | 1 | 1 | |||||
| 199 | 14 | 12 | 24 | 11 | 11 | 13 | 1 | 1 | |||||
| 200 | 14 | 12 | 24 | 10 | 11 | 14 | 1 | 1S | 2 | 4 | |||
| 201 | 14 | 12 | 24 | 10 | 11 | 13 | 1 | 1 | 1 | 2 | 5 | ||
| 202 | 14 | 12 | 24 | 10 | 11 | 12 | 1 | 1 | |||||
| 203 | 14 | 12 | 23 | 10 | 11 | 13 | 1Iq | 3 | 4 | ||||
| 204 | 14 | 12 | 22 | 11 | 11 | 13 | 1NA | 1 | |||||
| 205 | 13 | 13 | 26 | 10 | 11 | 14 | 1 | 1 | |||||
| 206 | 13 | 13 | 24 | 11 | 12 | 13 | 1 | 1 | |||||
| 207 | 13 | 12 | 26 | 10 | 11 | 14 | 4 | 4 | |||||
| 208 | 13 | 12 | 25 | 11 | 11 | 13 | 1T | 1 | |||||
| 209 | 13 | 12 | 25 | 10 | 12 | 13 | 1 | 1 | |||||
| 210 | 13 | 12 | 25 | 10 | 11 | 14 | 1 | 1 | |||||
| 211 | 13 | 12 | 25 | 10 | 11 | 13 | 3 | 1Bl | 3 | 7 | |||
| 212 | 13 | 12 | 25 | 9 | 11 | 14 | 2 | 2 | |||||
| 213 | 13 | 12 | 24 | 11 | 12 | 13 | 1 | 1 | |||||
| 214 | 13 | 12 | 24 | 10 | 11 | 14 | 1 | 1 | |||||
| 215 | 13 | 12 | 24 | 10 | 11 | 13 | 3 | 1NA, 1T | 5 | ||||
| 216 | 13 | 12 | 24 | 10 | 11 | 12 | 2 | 2 | |||||
| 217 | 13 | 12 | 24 | 10 | 11 | 11 | 1 | 1 | |||||
| 218 | 13 | 12 | 24 | 10 | 10 | 13 | 1 | 1 | |||||
| 219 | 13 | 12 | 24 | 9 | 11 | 14 | 1 | 1 | |||||
| 220 | 13 | 12 | 24 | 9 | 11 | 13 | 1 | 1T | 1 | 3 | |||
| 221 | 13 | 12 | 23 | 11 | 11 | 13 | 1 | 1 | |||||
| 222 | 13 | 12 | 23 | 10 | 11 | 13 | 1 | 1NA | 1 | 1 | 1 | 5 | |
| 223 | 13 | 12 | 23 | 10 | 10 | 13 | 1T | 1 | |||||
| 224 | 13 | 12 | 23 | 9 | 11 | 13 | 1 | 1 | |||||
| 225 | 13 | 12 | 22 | 10 | 11 | 13 | 2Iq | 1 | 3 | ||||
| 226 | 11 | 12 | 23 | 10 | 12 | 13 | 1 | 1 | |||||
| Hg 26 | |||||||||||||
| 227 | 15 | 12 | 24 | 10 | 14 | 13 | 2NA | 1 | 3 | ||||
| 228 | 15 | 12 | 23 | 11 | 13 | 15 | 1 | 1 | |||||
| 229 | 15 | 12 | 23 | 10 | 14 | 13 | 1 | 1 | |||||
| 230 | 15 | 12 | 23 | 10 | 13 | 13 | 1 | 1 | |||||
| 231 | 15 | 12 | 23 | 9 | 13 | 13 | 1 | 1 | |||||
| 232 | 14 | 14 | 26 | 10 | 13 | 13 | 1 | 1 | |||||
| 233 | 14 | 12 | 24 | 11 | 13 | 13 | 1 | 1 | |||||
| 234 | 14 | 12 | 24 | 10 | 13 | 13 | 1 | 1 | |||||
| 235 | 14 | 12 | 24 | 10 | 13 | 12 | 1 | 1 | |||||
| 236 | 14 | 12 | 23 | 11 | 13 | 13 | 1NA | 1 | |||||
| 237 | 14 | 12 | 23 | 10 | 15 | 13 | 2 | 2 | |||||
| 238 | 14 | 12 | 23 | 10 | 14 | 13 | 6 | 1 | 7 | ||||
| 239 | 14 | 12 | 23 | 10 | 14 | 12 | 1 | 1 | |||||
| 240 | 14 | 12 | 23 | 10 | 13 | 13 | 1 | 1Iq | 6 | 3 | 11 | ||
| 241 | 14 | 12 | 22 | 11 | 13 | 12 | 1 | 1 | |||||
| 242 | 14 | 12 | 22 | 10 | 14 | 11 | 1 | 1 | |||||
| 243 | 14 | 12 | 22 | 10 | 13 | 13 | 1 | 1 | |||||
| 244 | 14 | 12 | 22 | 10 | 13 | 12 | 2 | 2 | |||||
| 245 | 13 | 12 | 24 | 10 | 13 | 13 | 2 | 2 | |||||
| 246 | 13 | 12 | 23 | 10 | 13 | 13 | 1NA, 1T | 2 | |||||
| Hg 28 | |||||||||||||
| 247 | 15 | 12 | 23 | 10 | 14 | 12 | 1 | 1 | |||||
| 248 | 14 | 12 | 23 | 10 | 13 | 11 | 1 | 1 | |||||
| 249 | 14 | 12 | 22 | 10 | 14 | 11 | 1 | 1 | |||||
| 250 | 13 | 12 | 22 | 11 | 16 | 11 | 1 | 1 | |||||
| Total | 79 | 78 | 99 | 95 | 143 | 32 | 526 | ||||||
| h b | .986 | .990 | .977 | .985 | .971 | .923 | |||||||
| v c | .005 | .004 | .006 | .006 | .008 | .029 | |||||||
AJ = Ashkenazi Jew; SJ = Sephardic Jew; KJ = Kurdish Jew; MK = Muslim Kurd; PA = Palestinian Arab; B = Bedouin; NA = North Africa; Iq = Iraq; T = Turkey; S = Syria; Ib = Iberian Peninsula; Bl = Bulgaria.
h = Haplotype diversity.
v = Sampling variance.
Electronic-Database Information
URLs for software mentioned in this article are as follows:
References
- Bandelt H-J, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48 [DOI] [PubMed] [Google Scholar]
- Bandelt H-J, Forster P, Sykes B, Richards M (1995) Mitochondrial portraits of human populations using median networks. Genetics 141:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yosef O (1995) Earliest food producers: pre-pottery Neolithic. In: Levy T (ed) The archaeology of society in the Holy Land. Leicester University Press, London, pp 190–204 [Google Scholar]
- Ben-Sasson H (1976) A history of the Jewish people. Harvard University Press, Cambridge [Google Scholar]
- Bosch E, Calafell F, Santos FR, Pérez-Lezaun A, Comas D, Benchemsi N, Tyler-Smith C, Bertranpetit J (1999) Variation in short tandem repeats is deeply structured by genetic background on the Y chromosome. Am J Hum Genet 65:1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer E (1993) The Jews of Kurdistan. Wayne State University Press, Detroit [Google Scholar]
- Brinkmann C, Forster P, Schürenkamp M, Horst J, Rolf B, Brinkmann B (1999) Human Y-chromosomal STR haplotypes in a Kurdish population sample. Int J Legal Med 112:181–183 [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L, Menozzi P, Piazza A (1994) The history and geography of human genes. Princeton University Press, Princeton, NJ [Google Scholar]
- Comas D, Calafell F, Bendukidze N, Fañanás L, Bertranpetit J (2000) Georgian and Kurd mtDNA sequence analysis shows a lack of correlation between languages and female genetic lineages. Am J Phys Anthropol 112:5–16 [DOI] [PubMed] [Google Scholar]
- Dunlop D (1954) The history of the Jewish Khazars. Princeton University Press, Princeton, NJ [Google Scholar]
- Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P, Röhl A, Lünnemann P, Brinkmann C, Zerjal T, Tyler-Smith C, Brinkmann B (2000) A short tandem repeat–based phylogeny for the human Y chromosome. Am J Hum Genet 67:182–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D, Ruiz-Linares A, Cavalli-Sforza LL, Feldman M (1995) An evaluation of genetic distances for use with microsatellite loci. Genetics 139:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MF, Redd AJ, Wood ET, Bonner MR, Jarjanazi H, Karafet T, Santachiara-Benerecetti SA, Oppenheim A, Jobling MA, Jenkins T, Ostrer H, Bonné-Tamir B (2000) Jewish and Middle Eastern non-Jewish populations share a common pool of Y-chromosome biallelic haplotypes. Proc Natl Acad Sci USA 97:6769–6774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason A, Sigurdardóttir S, Nicholson J, Sykes B, Hill EW, Bradely DG, Bosnes V, Gulcher JR, Ward R, Stefánsson K (2000) Estimating Scandinavian and Gaelic ancestry in the male settlers of Iceland. Am J Hum Genet 67:697–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurles ME, Irven C, Nicholson J, Taylor PG, Santos FR, Loughlin J, Jobling MA, Sykes BC (1998) European Y-chromosomal lineages in Polynesians: a contrast to the population structure revealed by mtDNA. Am J Hum Genet 63:1793–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurles ME, Veitia R, Arroyo E, Armenteros M, Bertranpetit J, Peréz-Lezaun, Bosch E, Shlumukova M, Cabon-Thomsen A, McElreavey K, López de Munain L, Röhl A, Wilson IJ, Singh L, Pandya A, Santos FR, Tyler-Smith C, Jobling MA (1999) Recent male-mediated gene flow over a linguistic barrier in Iberia, suggested by analysis of a Y-chromosomal DNA polymorphism. Am J Hum Genet 65:1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M, Brauer S, Weiss G, Schiefenhövel W, Underhill P, Stoneking M (2001) Independent histories of human Y chromosomes from Melanesia and Australia. Am J Hum Genet 68:173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnane D (1970) The Kurds and Kurdistan. Oxford University Press, London [Google Scholar]
- Kittles R, Perola M, Peltonen L, Bergen AW, Aragon RA, Virkkunen M, Linnoila M, Goldman D, Long JC (1998) Dual origins of Finns revealed by Y chromosome haplotype variation. Am J Hum Genet 62:1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy T (1995) Cult, metallurgy and rank societies: the Chalcolithic period (ca. 4500–3500 BCE). In: Levy T (ed) The archaeology of society in the Holy Land. Leicester University Press, London, pp 226–244 [Google Scholar]
- Livshits G, Sokal R, Kobyliansky E (1991) Genetic affinities of Jewish populations. Am J Hum Genet 49:131–146 [PMC free article] [PubMed] [Google Scholar]
- Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics 142:1061–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel A, Filon D, Hohoff C, Faerman M, Brinkmann B, Oppenheim A (2001) Haplogroup-specific deviation from the stepwise mutation model at the microsatellite loci DYS388 and DYS392. Eur J Hum Genet 9:22–26 [DOI] [PubMed] [Google Scholar]
- Nebel A, Filon D, Weiss D, Weale M, Faerman M, Oppenheim A, Thomas M (2000) High-resolution Y chromosome haplotypes of Israeli and Palestinian Arabs reveal geographic substructure and substantial overlap with haplotypes of Jews. Hum Genet 107:630–641 [DOI] [PubMed] [Google Scholar]
- Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York [Google Scholar]
- Patrich J (1995) Church, state and the transformation of Palestine: the Byzantine period (324–640 CE). In: Levy T (ed) The archaeology of society in the Holy Land. Leicester University Press, London, pp 470–487 [Google Scholar]
- Pelletiere S (1984) The Kurds: an unstable element in the Gulf. Westview Press, Boulder, CO [Google Scholar]
- Quintana-Murci L, Krausz C, Zerjal T, Sayar H, Hammer MF, Mehdi SQ, Ayub Q, Qamar R, Mohyuddin A, Radhakrishna U, Jobling MA, Tyler-Smith C, McElreavey K (2001) Y-chromosome lineages trace diffusion of people and languages in southwestern Asia. Am J Hum Genet 68:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283 [DOI] [PubMed] [Google Scholar]
- Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, Rengo C et al (2000) Tracing European founder lineages in the Near Eastern mtDNA pool. Am J Hum Genet 67:1251–1276 [PMC free article] [PubMed] [Google Scholar]
- Rosser Z, Zerjal T, Hurles M, Adojaan M, Alavantic D, Amorim A, Amos W, et al (2000) Y-chromosomal diversity in Europe is clinal and influenced by geography, rather than by language. Am J Hum Genet 67:1526–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C (1972) Encyclopedia Judaica. Keter, Jerusalem, pp 1296–1299 [Google Scholar]
- Salem A-H, Badr F, Gaballah M, Pääbo S (1996) The genetics of traditional living: Y-chromosomal and mitochondrial lineages in the Sinai Peninsula. Am J Hum Genet 59:741–743 [PMC free article] [PubMed] [Google Scholar]
- Santachiara-Benerecetti A, Semino O, Passarino G, Torroni A, Brdicka R, Fellous M, Modiano G (1993) The common Near Eastern origin of Ashkenazi and Sephardi Jews supported by Y-chromosome similarity. Ann Hum Genet 57:55–64 [DOI] [PubMed] [Google Scholar]
- Seielstad M, Minch E, Cavalli-Sforza L (1998) Genetic evidence for a higher female migration rate in humans. Nat Genet 20:278–280 [DOI] [PubMed] [Google Scholar]
- Semino O, Passarino G, Oefner PJ, Lin AA, Arbuzova S, Beckman LE, De Benedictis G, Francalacci P, Kouvatsi A, Limborska S, Marcikiae M, Mika A, Mika B, Primorac D, Santachiara-Benerecetti AS, Cavalli-Sforza LL, Underhill, PA (2000) The genetic legacy of Paleolithic Homo sapiens sapiens in extant Europeans: a Y chromosome perspective. Science 290:1155–1159 [DOI] [PubMed] [Google Scholar]
- Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B, Irven C (2000) Surnames and the Y chromosome. Am J Hum Genet 66:1417–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MG, Bradman N, Flinn H (1999) High throughput analysis of 10 microsatellite and 11 diallelic polymorphisms on the human Y chromosome. Hum Genet 105:577–581 [DOI] [PubMed] [Google Scholar]
- Thomas MG, Parfitt T, Weiss DA, Skorecki K, Wilson JF, le Roux M, Bradman N, Goldstein DB (2000) Y chromosomes traveling south: the Cohen modal haplotype and the origins of the Lemba—the “Black Jews of Southern Africa.” Am J Hum Genet 66:674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MG, Skorecki K, Ben-Ami H, Parfitt T, Bradman N, Goldstein DB (1998) Origins of Old Testament priests. Nature 394:138–140 [DOI] [PubMed] [Google Scholar]
- Tremblay M, Vézina H (2000) New estimates of intergenerational time intervals for the calculation of age and origin of mutations. Am J Hum Genet 66:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill PA, Shen P, Jin L, Lin AA, Jin L, Passarino G, Yang WH, Kauffman E, Bonne-Tamir B, Bertranpetit J, Francalacci P, Ibrahim M, Jenkins T, Kidd JR, Mehdi SQ, Seielstad MT, Wells RS, Piazza A, Davis RW, Feldman MW, Cavalli-Sforza LL, Oefner P (2000) Y chromosome sequence variation and the history of human populations. Nat Genet 26:358–361 [DOI] [PubMed] [Google Scholar]
- Zerjal T, Dashnyam B, Pandya A, Kayser M, Roewer L, Santos FR, Schiefenhövel W, Fretwell N, Jobling MA, Harihara S, Shimizu K, Semjidmaa D, Sajantila A, Salo P, Crawford MH, Ginter EK, Evgrafov OV, Tyler-Smith C (1997) Genetic relationships of Asians and Northern Europeans, revealed by Y-chromosomal DNA analysis. Am J Hum Genet 60:1174–1183 [PMC free article] [PubMed] [Google Scholar]