Abstract
Chryseobacterium gleum (previously included in the Flavobacterium IIb species) is a gram-negative aerobe that is a source of nosocomial infections. An Ambler class B β-lactamase gene was cloned and expressed in Escherichia coli from reference strain C. gleum CIP 103039 that had reduced susceptibility to expanded-spectrum cephalosporins and carbapenems. The purified β-lactamase, CGB-1, with a pI value of 8.6 and a determined relative molecular mass of ca. 26 kDa, hydrolyzed penicillins; narrow- and expanded-spectrum cephalosporins; and carbapenems. CGB-1 was a novel member of the molecular subclass B1 of metallo-enzymes. It had 83 and 42% amino acid identity with IND-1 from Chryseobacterium indologenes and BlaB from C. meningosepticum, respectively. Thus, in addition to the previously characterized clavulanic acid-inhibited extended-spectrum β-lactamase CGA-1 of Ambler class A, C. gleum produces a very likely chromosome-borne class B β-lactamase.
Metallo-β-lactamases of Ambler class B are zinc-dependent enzymes that possess the property of hydrolyzing most β-lactam antibiotics, including carbapenems (7). Some of these enzymes are emerging worldwide in gram-negative pathogens as a result of plasmid- and integron-location of their genes that are of the blaIMP and blaVIM series (13, 14, 20, 25). However, several metallo-β-lactamases have been reported as a source of intrinsic resistance to carbapenems in bacterial species less frequently isolated in clinical microbiology, such as L-1 from Stenotrophomonas maltophilia (27), Bc-II from Bacillus cereus 569/H (11), CcrA from Bacteroides fragilis (21), CphA from Aeromonas hydrophila (15), FEZ-1 from Legionella gormanii, (6), and THIN-B from Janthinobacterium lividum (22).
Flavobacteria species are mostly a source of nosocomial infections (26). Metallo-β-lactamases have been identified so far in two Flavobacterium species, Chryseobacterium meningosepticum and Chryseobacterium indologenes (1, 2, 4, 23). C. meningosepticum usually expresses two unrelated metallo-enzymes of the BlaB and GOB-like series that share less than 12% amino acid identity (1). C. indologenes expresses IND-like enzymes that share less than 45 and 15% amino acid identity with BlaB and GOB-like enzymes, respectively (2, 4).
A search for a potential natural reservoir of acquired carbapenem-hydrolyzing β-lactamases is interesting, particularly in understanding the mechanism(s) of integration of these genes in an integron. Thus, we proceeded to study the β-lactamase content of another Flavobacterium species, Chryseobacterium gleum that is a phylogenetically related species to C. indologenes (10, 12, 28).
From C. gleum strain CIP 103039, we have reported a clavulanic-acid inhibited Ambler class A expanded-spectrum β-lactamase (ESβL), CGA-1 (3). However, since C. gleum CIP 103039 had also reduced susceptibility to carbapenems and CGA-1 hydrolyzes weakly these β-lactam group, the aim of our study was to analyze the mechanism sustaining carbapenem resistance in C. gleum. We have cloned and identified genetically and biochemically a novel metallo-β-lactamase that is closely related to metallo-enzymes of C. indologenes.
MATERIALS AND METHODS
Bacterial strains.
C. gleum reference strain CIP 103039 was from the Institut Pasteur Collection (3). Escherichia coli DH10B and nalidixic acid-resistant E. coli JM109 were used for cloning and conjugation assays, respectively. All strains were stored at −70°C in Trypticase soy (TS) broth (Becton Dickinson, Le Pont-de-Claix, France) supplemented with 15% glycerol until testing.
Antimicrobial agents and MIC determinations.
The antimicrobial agents were obtained in the form of standard laboratory powders and were used immediately after their solubilization. The agents and their sources have been described previously (19). Antibiotic disks were used for routine antibiograms (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France).
MICs of selected β-lactams were determined by an agar dilution technique on Mueller-Hinton plates (Sanofi Diagnostics Pasteur) with an inoculum of 104 CFU per spot, as described previously (17).
Cloning and analysis of recombinant plasmids.
Whole-cell DNA of C. gleum CIP103039 was extracted as described previously (19). All enzymes used in cloning experiments were from Amersham Pharmacia Biotech (Orsay, France). Fragments from whole-cell DNA partially digested with Sau3AI were ligated into BamHI-restricted phagemid pBK-CMV (Stratagene, Amsterdam, The Netherlands), as previously reported (24). Ligations were performed at a 1:5 vector/insert ratio, in 30 μl of a ligation mixture containing 5 U of T4 DNA ligase at 4°C incubated overnight. Recombinant plasmids were transformed by electroporation (Gene Pulser II; Bio-Rad, Ivry-sur-Seine, France) into E. coli DH10B electrocompetent cells (Gibco BRL, Life Technologies, Cergy Pontoise, France). Antibiotic-resistant colonies were selected onto amoxicillin (30 μg/ml)- and kanamycin (30 μg/ml)-containing TS agar plates, and their antibiotic resistance patterns were analyzed according to the results of antibiogram performed by disk diffusion.
Recombinant plasmid DNAs were obtained from 100-ml TS broth cultures grown overnight in the presence of amoxicillin (30 μg/ml) at 37°C. Plasmid DNAs were recovered by using Qiagen (Courtaboeuf, France) columns. Plasmid mapping were performed after double-restriction analysis (24). Fragment sizes were estimated by comparison with the 1-kb DNA ladder (Amersham Pharmacia Biotech). One recombinant E. coli strain that had reduced susceptibility to imipenem and harbored recombinant plasmid pCGB-1 with the smallest 2-kb insert was retained for further analysis.
Plasmid content, conjugation assay, and Southern hybridization.
Extraction of plasmid DNA from C. gleum CIP 106039 and direct transfer by conjugation of any β-lactam resistance marker into in vitro-obtained nalidixic acid-resistant E. coli JM109 were attempted as previously described (5). Southern hybridization was performed as previously described (24) with whole-cell DNA of C. gleum CIP 103039 by using the enhanced chemiluminescence nonradioactive labeling and detection kit (Amersham Pharmacia Biotech) with a 628-bp PCR-obtained probe with primers internal to blaCGB-1 (primer 1: 5′-GCAAACGCCCGGATACAACAG-3′; primer 2; 5′-TTCCATTCATCATGTCCGGG-3′)
β-Lactamase purification.
A culture of E. coli DH10B harboring recombinant plasmid pCGB-1 was grown overnight at 37°C in 4 liters of TS broth containing amoxicillin (30 μg/ml). Bacterial suspensions were pelleted, resuspended in 60 ml of 30 mM Tris-HCl buffer (pH 8.2), disrupted by sonification (three times at 30 W for 30 s with a Vibra Cell 75022 Phospholyser [Bioblock, Illkirch, France]), and centrifuged for 1 h at 48,000 × g at 4°C. the supernatant ultracentrifuged at 100,000 × g for 1 h at 4°C and the supernatant was dialyzed overnight against 30 mM Tris-HCl buffer (pH 8.2).
This extract was loaded onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech). The enzyme recovered in the flowthrough and determined qualitatively by nitrocefin hydrolysis (Oxoid, Dardilly, France) was dialyzed overnight at 4°C against 50 mM sodium phosphate buffer (pH 7.0). The β-lactamase extract was then loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech) preequilibrated with the same buffer. The enzyme was eluted by a 100-ml linear NaCl gradient (0 to 500 mM) in sodium phosphate buffer (pH 7.0). The β-lactamase was eluted at a concentration of 260 mM NaCl. The fractions containing the highest β-lactamase activities were pooled, dialyzed overnight against 50 mM sodium phosphate buffer (pH 7.0) containing 50 μM ZnCl2 and stored at −80°C. Purity of the enzyme was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (24).
IEF analysis and N-terminal sequencing.
Purified enzyme preparation from culture of E. coli DH10B harboring pCGB-1 and β-lactamase crude extracts from 100 ml-culture of C. gleum CIP 103039 were subjected to analytical isoelectric focusing (IEF) on a pH 3.5 to 9.5 ampholine polyacrylamide gel (Ampholin PAG plate; Amersham Pharmacia Biotech) as described previously (4).
In order to determine the cleavage site of the CGB-1 mature protein, the purified enzyme was subjected to an Edman analysis (9) at the laboratory for protein microsequencing at the Pasteur Institute, Paris, France. Purified enzyme and marker proteins were subjected to SDS-PAGE (25 mA, 4 h, room temperature). Proteins were then electrotransferred onto a polyvinyl difluoride membrane (Immobilon-P; Millipore) by using the Mini Protean II transfer cell (8 by 7.3 cm; Bio-Rad) in 50 mM Tris-borate buffer (pH 8.7) at room temperature (3.5 V/cm, overnight). The membrane was then rinsed in distilled water and stained with a solution made of 0.1% Coomassie brilliant blue R-250 in methanol and water (50:40 [vol/vol]). The protein band was then excised with a razor blade and allowed to air dry. The amino-terminal sequence of the mature β-lactamase was determined with an automated Edman sequencer on a 473A model gas phase sequencer (Applied Biosystems).
Kinetic measurements of β-lactamase CGB-1 and induction study.
C. gleum CIP103039 crude extract was used to detect imipenem hydrolysis in preliminary experiments. Purified metallo-β-lactamase CGB-1 was used for kinetic measurements performed at 30°C in 100 mM sodium phosphate (pH 7.0) containing 50 μM ZnCl2. Determinations of specific activities of purified enzyme and of β-lactamase extract from C. gleum CIP 103039 were performed with 100 μM imipenem as a substrate as described previously (1). The total protein content was measured with bovine albumin as the standard (Bio-Rad DC protein assay kit). Km and kcat values were determined with a spectrophotometer ULTROSPEC 2000 (Amersham Pharmacia Biotech) by analyzing the β-lactam hydrolysis under initial rate conditions by using the Eadie-Hofstee linearization of the Michaelis-Menten equation as previously described previously (1).
Various concentrations of EDTA and clavulanic acid were preincubated with the enzyme for 3 min at 30°C before testing the rate of imipenem hydrolysis. Fifty percent inhibitory concentrations (IC50s) were determined for EDTA and clavulanic acid, and results were expressed in micromolar units. Induction experiments were performed with 1 and 4 μg of cefoxitin per ml and 0.1 μg of imipenem per ml as inducer with cultures of C. gleum CIP 103039 and E. coli DH10B(pCGB-1), as previously described (1).
DNA sequencing and protein analysis.
The cloned DNA fragment of recombinant plasmids pCGB-1 was sequenced on both strands, with an Applied Biosystems sequencer (ABI 377). The nucleotide and the deduced protein sequences were analyzed with software available over the Internet as reported previously (3). The relative molecular mass of the β-lactamase expressed by a culture of E. coli DH10B(pCGB-1) was estimated by SDS-PAGE analysis, as previously described (5).
Nucleotide sequence accession number.
The nucleotide sequence and deduced β-lactamase amino acid sequence reported in this work have been assigned to the GenBank and EMBL databases under the accession no. AF339734.
RESULTS AND DISCUSSION
Cloning experiments.
Preliminary experiments of imipenem hydrolysis with a β-lactamase extract of C. gleum CIP 103039 suggested the presence of a carbapenem-hydrolyzing β-lactamase in addition to a previously characterized class A β-lactamase (3). Forty recombinant E. coli DH10B clones harboring plasmids with inserts that varied in size (2.0 to 10 kb) were obtained after shotgun cloning of Sau3AI-restricted DNA of C. gleum CIP 103039. Among them, 28 clones had decreased susceptibility to ceftazidime and a β-lactam resistance phenotype that was susceptible to clavulanic acid inhibition that was consistent with the presence of the previously characterized class A enzyme CGA-1 (3). Twelve other recombinant E. coli clones had a slight decrease of susceptibility to carbapenems. Among them, E. coli DH10B(pCGB-1) harboring a recombinant plasmid which possessed the smallest 2.0-kb insert was retained.
DNA and protein sequence analysis of CGB-1.
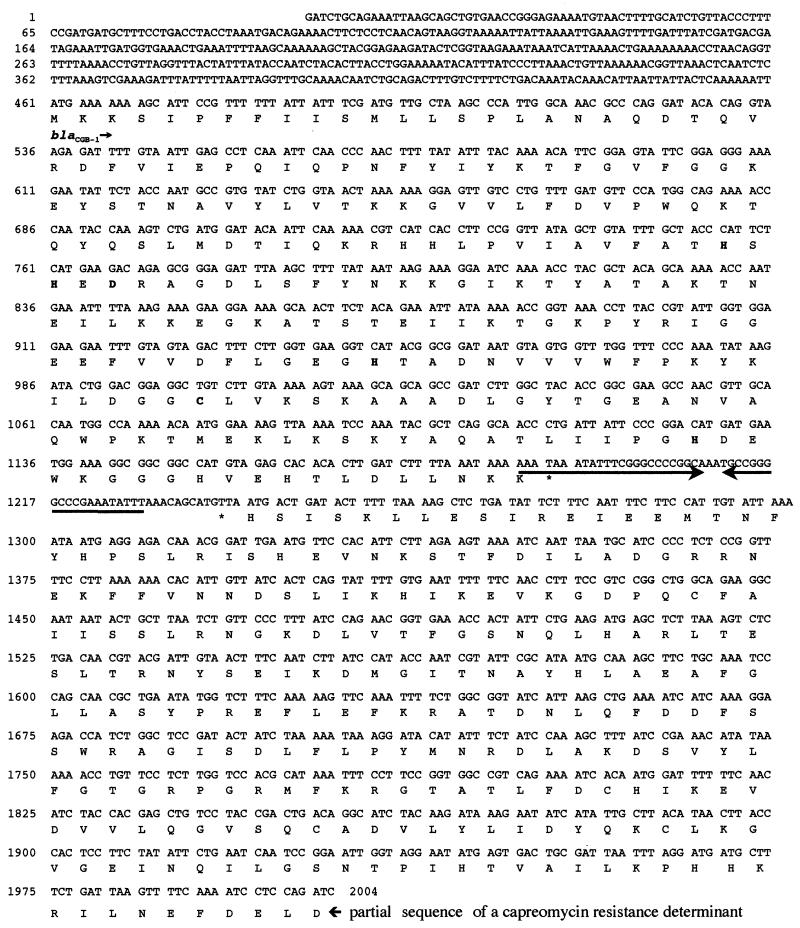
DNA sequence analysis of the 2,004-bp insert of pCGB-1 revealed an open reading frame (ORF) (named blaCGB-1) of 729 bp (from nucleotide 461 to 1,189) encoding a 243-amino-acid preprotein (Fig. 1). A putative cleavage site was found by computer analysis after the Ala-Asn-Ala motif (18). N-terminal sequence analysis of the protein confirmed that the first amino acids residues of the mature protein were QDTQ (Fig. 1). The resulting mature protein contained 223 amino acid residues. The overall G+C content of this ORF was 36%, which lies within the expected range of G+C ratio of the Chryseobacterium genes (36 to 38%) (10).
FIG. 1.
Nucleotide sequence of the cloned 2,004-bp fragment of recombinant plasmid pCGB-1 containing the blaCGB-1 coding region and a truncated ORF encoding a putative capreomycin resistance determinant. The deduced amino acid sequence is designated in the single-letter code below the nucleotide sequence. The putative terminator sequence is indicated by two inverted arrows, and stop codons are indicated by stars.
Another truncated ORF was detected on this same insert (nucleotide 1,240 to 2,004) of recombinant plasmid pCGB-1 located downstream and in opposite orientation of blaCGB-1 (Fig. 1). It encoded 254 amino acids of the carboxyl end of a putative protein that possessed 31.5% amino acid identity with the capreomycin acetyltransferase from Streptomyces capreolus (accession number no. JC4557). A similar capreomycin resistance gene had been identified downstream and in opposite orientation of the class B β-lactamase gene blaIND-1 from C. indologenes 001 (unpublished data) and in Mycobacterium tuberculosis genome (accession no. NC 000962). Thus, the blaCGB-1 gene may be part of a cluster of antibiotic resistance genes. A putative DNA sequence, located from nucleotides 1,187 to 1,229 between the stop codon of blaCGB-1 and the stop codon of the putative capreomycin acetyltransferase gene, might play a role as a transcription terminator (Fig. 1).
Genetic location of blaCGB-1.
No plasmid was detected in C. gleum CIP 103039, and direct conjugation experiments failed to transfer any β-lactam resistance marker from C. gleum CIP 103039 to nalidixic acid-resistant E. coli JM109. Using an internal probe for blaCGB-1, Southern hybridization was positive at the chromosomal position of migration of whole-cell DNA of C. gleum CIP 103039 (data not shown)
Susceptibility testing.
MICs of β-lactams for C. gleum CIP 103039 showed that it was resistant to amino- and carboxy-penicillins, narrow-spectrum cephalosporins, cefotaxime, aztreonam, had intermediate susceptibility to carbapenems and remained susceptible to piperacillin (Table 1). E. coli DH10B(pCGB-1) was resistant to amoxicillin, ticarcillin, some restricted-spectrum cephalosporins, and had a reduced susceptibility to piperacillin, ceftazidime and carbapenems compared to that of E. coli DH10B. MICs of β-lactams for E. coli DH10B(pCGB-1) were not lowered by addition of clavulanic acid and tazobactam (Table 1). Resistance to aztreonam in C. gleum CIP103039 as found in other flavobacterial species (1-5) may be partially explained by expression of the previously characterized clavulanic acid-inhibited CGA-1 enzyme (3). Other resistance mechanisms such as low outer membrane permeability, efflux, and penicillin binding protein affinity may explain also resistance to this compound.
TABLE 1.
MICs of β-lactams for C. gleum CIP 103039, E. coli DH10B(pCGB-1) expressing CGB-1, E. coli DH10B(pSO-1) expressing IND-1 from C. indologenes OO1, and reference strain E. coli DH10B
| β-Lactam(s)a | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
| C. gleum CIP 103039 | E. coli DH10B (pCGB-1) | E. coli DH10B (pSO-1)b | E. coli DH10B | |
| Amoxicillin | 256 | 512 | >512 | 2 |
| Amoxicillin-CLA | 32 | 256 | 2 | |
| Ticarcillin | 256 | >512 | >512 | 2 |
| Ticarcillin-CLA | 256 | >512 | 2 | |
| Piperacillin | 2 | 8 | 64 | 1 |
| Piperacillin-TZB | 2 | 8 | 1 | |
| Cephalothin | 256 | 32 | 128 | 2 |
| Cefuroxime | >512 | 256 | 4 | |
| Cefoperazone | 4 | 0.5 | 0.12 | |
| Ceftazidime | 4 | 0.5 | 8 | 0.06 |
| Cefotaxime | 32 | 1 | 16 | 0.06 |
| Cefepime | 1 | 0.06 | 0.06 | 0.06 |
| Cefpirome | 2 | 0.06 | 0.06 | |
| Ceftriaxone | 16 | 0.12 | 0.06 | |
| Cefoxitin | 8 | 4 | 16 | 4 |
| Moxalactam | 16 | 0.12 | 0.12 | 0.12 |
| Aztreonam | >512 | 0.06 | 0.12 | 0.06 |
| Imipenem | 2 | 0.25 | 2 | 0.06 |
| Meropenem | 8 | 0.25 | 0.5 | 0.06 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
Data are from reference 4.
Once cloned onto a plasmid vector and expressed in E. coli, both enzymes, CGB-1 and IND-1, provided a similar pattern of decreased susceptibility to β-lactams, although in the latter case the degree of resistance was more important (Table 1). The overall susceptibility of C. gleum to β-lactams was similar to that reported for C. meningosepticum (1, 5). As for C. meningosepticum that possesses a class A β-lactamase (CME) and two class B β-lactamases (BlaB and GOB) (1, 5, 23), C. gleum possesses, in addition to the class A β-lactamase CGA-1, another very likely chromosomal-encoded class B enzyme CGB-1.
Biochemical properties of CGB-1.
IEF analysis revealed that cultures of E. coli DH10B(pCGB-1) produced a β-lactamase activity with a pI value of 8.6. However, only one β-lactamase activity was detected from crude extracts of a culture of C. gleum CIP103039, at a pI of 8.9 that corresponded to the previously identified class A β-lactamase CGA-1 (3). Thus, expression of CGB-1 from C. gleum CIP 103039 might be weak. The specific activity of the purified CGB-1 enzyme was 8.3 μmol·min−1·mg of protein−1, determined with 100 μM imipenem as a substrate with a 174-fold purification coefficient. Its purity was estimated to be 95% by SDS-PAGE (data not shown).
The mature protein (named CGB-1 for C. gleum class B β-lactamase) expressed in E. coli DH10B had a relative molecular mass determined experimentally to be ca. 26 kDa (data not shown).
Kinetic parameters of CGB-1 revealed a strong activity against amino- and carboxy-penicillins and restricted-spectrum cephalosporins (Table 2). Surprisingly, cefotaxime was a good substrate for CGB-1 due to a low Km, whereas ceftazidime that possesses a Km superior to 1 mM was a very poor substrate. This difference in hydrolysis activity mirrored the MICs for both β-lactams for C. gleum CIP 103039 (but not for E. coli DH10B(pCGB-1) (Table 1). CGB-1 β-lactamase had poor affinity for imipenem (Km, >1,000 μM), resulting in a weak catalytic efficacy of imipenem (Table 2). Therefore, its kcat/Km value for imipenem was at least 10-fold lower than that of IND-2, whereas these values for meropenem and for benzylpenicillin were only twofold lower (Table 2). These results were consistent with the low level of resistance to imipenem of C. gleum CIP 103039 (Table 1). Hydrolysis of aztreonam was not detectable, as found for all metallo-enzymes (20). Catalytic efficacy of CGB-1 was lower than that of GOB-1 from C. meningosepticum for all β-lactams except for benzylpenicillin which were similar (1). CGB-1 had kcat/Km values which were 4-fold lower for benzylpenicillin, 10-fold lower for cephaloridine and for cefoxitin, 60-fold lower for imipenem, and 3-fold higher for cefotaxime, compared to those of BlaB from C. meningosepticum (1). The hydrolytic activity of CGB-1 was inhibited by EDTA (IC50, 37 μM) but not by class A β-lactamase inhibitors such as clavulanic acid (IC50 > 1 mM). These kinetic parameters lead to include CGB-1 in the biochemical group 3a of the Bush β-lactamase classification for metallo-enzymes (7). This group includes all characterized metallo-β-lactamases with a very broad substrate hydrolysis profile except FEZ-1 and Cph-A-like enzymes from L. gormanii and Aeromonas sp., respectively.
TABLE 2.
Kinetic parameters of purified CGB-1 from cultures of E. coli DH10B(pCGB-1)a
| β-Lactam | kcat(s−1) | Km (μM) | kcat/Km(s−1 · mM−1) |
|---|---|---|---|
| Benzylpenicillin | 45 | 20 | 2,200 |
| Amoxicillin | 145 | 220 | 660 |
| Ticarcillin | 60 | 107 | 570 |
| Piperacillin | NDb | >1,000 | ND |
| Cephalothin | 20 | 40 | 430 |
| Cephaloridine | 10 | 180 | 50 |
| Cefuroxime | 10 | 9 | 890 |
| Cefoperazone | 10 | 115 | 90 |
| Ceftriaxone | 3 | 20 | 150 |
| Ceftazidime | 2 | >1,000 | <2 |
| Cefotaxime | 10 | 19 | 610 |
| Cefepime | 0.2 | >1,000 | <0.2 |
| Cefpirome | 2 | >1,000 | 2 |
| Cefoxitin | 5 | 160 | 25 |
| Meropenem | 90 | >1,000 | >90 |
| Imipenem | 53 | >1,000 | <50 |
| Aztreonam | <0.1 | ND | ND |
Values are geometric means of three independent measures (standard deviations were within 15%).
ND, not determinable.
Induction experiments.
Induction studies with cefoxitin and imipenem as β-lactam inducers failed to detect induction of β-lactamase expression with cultures of C. gleum CIP 103039 and of E. coli(pCGB-1) with imipenem as substrate. These results are consistent with the absence of any sequence for regulatory gene, such as LysR-type regulator gene upstream of blaCGB-1 (16).
Amino acid sequence analysis.
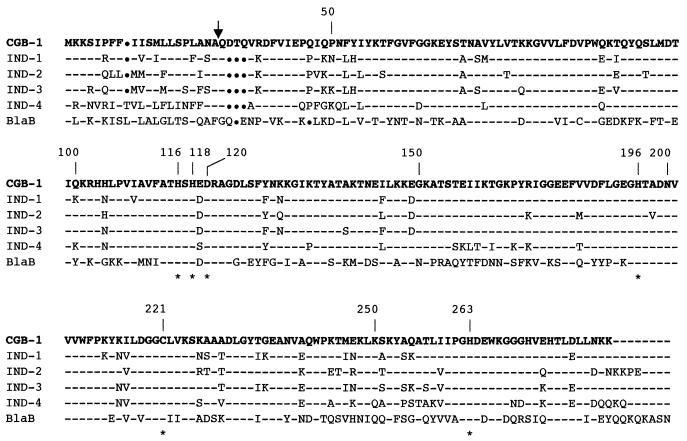
The comparison of amino acid sequence of CGB-1 with those of other class B β-lactamases revealed 83% identity with IND-1, 82% identity with IND-2 and IND-3, and 73% identity with IND-4 from C. indologenes, and only 42 and 12% identity with BlaB and GOB-1 from C. meningosepticum, respectively (1-4, 23). Thus, the metallo-enzyme of C. gleum was most closely related to those of C. indologenes thus paralleling the taxonomic position of both Chryseobacterium species (28). The six conserved amino acid residues implicated in the Zn2+ or water molecule binding (His116, His118, Asp120, His196, Cys221, and His 220) (8) of metallo-β-lactamases were found in the CGB-1 sequence (Fig. 2). However, an insertion of three amino acids (Asp, Thr, and Gln) was detected at the second, third and fourth position of the N-terminal sequence of the mature protein CGB-1 (positions 22, 23, and 24) compared to IND-like (Fig. 2). β-Lactamase CGB-1 may be classified in the molecular subclass B1 of the structural classification that groups IND-like, Bc-II, CfiA, BlaB, IMP, and VIM β-lactamases (1, 2, 20). A phylogenic analysis of CGB-1 from C. gleum, BlaB variants from C. meningosepticum (and not GOB variants from C. meningosepticum also), and IND variants from C. indologenes, showed that they belong to a same phylogenic lineage (data not shown). Comparison of amino acid sequence of CGB-1 with those of IND-1 and IND-2 did not provide evidence of any particular amino acid residue that may explain a lower affinity of CGB-1 for carbapenems.
FIG. 2.
Comparison of the amino acid sequence of β-lactamase CGB-1 with those of IND-1, IND-2, IND-3, and IND-4 from C. indologenes (4) and BlaB from C. meningosepticum (23). The BBL numbering scheme is indicated above the sequences (8). Broken lines and dots indicated identical and deleted amino acid residues, respectively. The vertical arrow indicates the putative cleavage site for the leader peptide of CGB-1. Amino acids that may be involved in binding of Zn2+ or/and water are indicated by a star.
In conclusion, this report identified a novel bacterial species for which β-lactam resistance is at least mediated by an Ambler class A expanded-spectrum β-lactamase and an Ambler class B metallo-enzyme, as found in S. maltophilia and C. meningosepticum.
Acknowledgments
This work was financed by a grant from the Ministères de l'Education Nationale et de la Recherche (grant UPRES-EA), Université Paris XI, Paris, France.
We thank C. Bizet for the gift of C. gleum reference strain.
REFERENCES
- 1.Bellais, S., D. Aubert, T. Naas, and P. Nordmann. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing beta-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellais, S., S. Léotard, L. Poirel, T. Naas, and P. Nordmann. 1999. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol. Lett. 171:127-132. [DOI] [PubMed] [Google Scholar]
- 3.Bellais, S., T. Naas, and P. Nordmann. 2002. Molecular and biochemical characterization of Ambler class A CGA-1, an extended-spectrum β-lactamase from Chryseobacterium gleum. Antimicrob. Agents Chemother. 46:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellais, S., L. Poirel, S. Léotard, T. Naas, and P. Nordmann. 2000. Genetic diversity of carbapenem-hydrolyzing metallo-beta-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob. Agents Chemother. 44:3028-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellais, S., L. Poirel, T. Naas, D. Girlich, and P. Nordmann. 2000. Genetic-biochemical analysis and distribution of the Ambler class A β-lactamase CME-2 responsible for extended-spectrum cephalosporin resistance in Chryseobacterium (Flavobacterium) meningosepticum. Antimicrob. Agents Chemother. 44:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschi, L., P. S. Mercuri, M. L. Riccio, G. Amicosante, M. Galleni, J.-M. Frère, and G. M. Rossolini. 2000. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the high divergent lineage of molecular subclass B3 β-lactamases. Antimicrob. Agents Chemother. 44:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush, K. 1998. Metallo-enzymes: a class apart. Clin. Infect. Dis. 27(Suppl. 1):S48-S53. [DOI] [PubMed] [Google Scholar]
- 8.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J.-M. Frère. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewick, R. M., M. W. Hunkapiller, D. Le Hoo, and W. J. Dreyer. 1981. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 256:7990-7997. [PubMed] [Google Scholar]
- 10.Holmes, B., R. J. Owen, A. G. Steigerwalt, and D. J. Brenner. 1984. Flavobacterium gleum, a new species found in human clinical specimens. Int. J. Syst. Bacteriol. 34:21-25. [Google Scholar]
- 11.Hussain, M., A. Carlino, M. J. Madonna, and J. O. Lampen. 1985. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J. Bacteriol. 164:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jooste, P. J., and C. J. Hugo. 1999. The taxonomy, ecology and cultivation of bacterial genera belonging to the familly Flavobacteriaceae. Int. J. Food Microbiol. 53:81-94. [DOI] [PubMed] [Google Scholar]
- 13.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore, D. M., and N. Woodford. 2000. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 3:489-495. [DOI] [PubMed] [Google Scholar]
- 15.Massidda, O., G. M. Rossolini, and G. Satta. 1991. The Aeromonas hydrophila cphA gene: molecular heterogeneity among metallo-β-lactamases. J. Bacteriol. 173:4611-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naas, T., and P. Nordmann. 1994. Analysis of a carbapenem-hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its Lys-R type regulatory protein. Proc. Natl. Acad. Sci. USA 91:7693-7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nielsen, H., J. Engelbrecht, S.Brunak, and G. Von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 19.Nordmann, P., and T. Naas. 1994. Sequence analysis of PER-1 extended-spectrum β-lactamase from Pseudomonas aeruginosa and comparison with class A beta-lactamases. Antimicrob. Agents Chemother. 38:104-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen, B. A., Y. Gluzman, and F. P. Tally. 1990. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob. Agents Chemother. 34:1590-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossolini, G. M., M. A. Condemi, F. Pantanella, J. D. Docquier, G. Amicosante, and M. C. Thaller. 2001. Metallo-β-lactamase producers in environnemental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossolini, G. M., N. Franceschini, M. L. Riccio, P. S. Mercuri, M. Perilli, M. Galleni, J.-M. Frère, and G. Amicosante. 1998. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem. J. 332:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Senda, K., Y. Arakawa, K. Nakashima, H. Ito, S. Ichiyama, K. Shimokata, N. Kato, and M. Ohta. 1996. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob. Agents Chemother. 40:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegman-Igra, Y., D. Schwartz, G. Soferman, and N. Konforti. 1987. Flavobacterium group IIb bacteremia: report of a case and review of Flavobacterium infections. Med. Microbiol. Immunol. 176:103-111. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, T. R., L. Hall, S. J. Assinder, W. W. Nichols, S. J. Cartwright, A. P. MacGowan, and P. M. Bennett. 1994. Sequence analysis of the L-1 metallo-β-lactamase from Xanthomonas maltophilia. Biochem. Biophys. Acta 1218:199-201. [DOI] [PubMed] [Google Scholar]
- 28.Yabuuchi, E., Y. Hashimoto, T. Ezaki, Y. Ido, and N. Takeuchi. 1990. Genotypic and phenotypic differentiation of Flavobacterium indologenes (Yabuuchi et al. 1983) from Flavobacterium gleum (Holmes et al. 1984). Microbiol. Immunol. 34:73-76. [DOI] [PubMed] [Google Scholar]