Abstract
The antiherpesvirus agent penciclovir (PCV) shares an identical activation pathway and a similar mode of action with acyclovir (ACV). However, since PCV represents a relatively recent treatment option, the clinical resistance profile to PCV is less well known. A susceptibility program was established to assess the resistance profile for serial herpes simplex virus isolates from immunocompetent patients with recurrent herpes labialis obtained throughout a 4-day period of treatment with topical PCV (1% cream) or a placebo. Two isolates (2 of 1,035 [0.19%]), representing 0.34% of the patients (2 of 585), were confirmed to be PCV-resistant (Pcvr) herpes simplex virus type 1 by a plaque reduction assay in MRC-5 cells. These two viruses were highly resistant to PCV (50% inhibitory concentrations [IC50s], >55 μg/ml) and were isolated less than 17 h after the start of patient-initiated treatment. However, subsequent isolates on days 2 and 3 from these patients were completely susceptible to PCV (IC50s, <2.0 μg/ml). Thus, it is not clear whether the resistance to PCV for these two early-treatment isolates was directly associated with the 17 h of PCV treatment; several possible explanations are discussed. In an analysis of the distribution of IC50 differences between the first and last isolates, there were three patients with minor IC50 increases in the PCV-treated population and one in the placebo-treated group, although statistically, only the latter was an outlier. No patients were found to have Pcvr virus at the end of acute treatment, regardless of treatment group. Overall, the prevalence of Pcvr was found to be similar to the 0.3% Acvr reported for immunocompetent, untreated populations.
The use of acyclovir (ACV) and penciclovir (PCV) in treating herpesvirus infections has increased significantly since the introduction of ACV 2 decades ago. These nucleoside analogues of guanine are preferentially phosphorylated to monophosphate by the viral thymidine kinase (TK) and subsequently converted to ACV triphosphate by cellular enzymes. The activated triphosphate inhibits viral DNA polymerase activity and prevents viral DNA elongation (9). Herpes simplex virus (HSV) resistance to these agents typically develops by mutations in the TK gene, although mutations within the viral DNA polymerase can also confer ACV resistance (2-5, 14, 28).
ACV-resistant (Acvr) HSV variants have been readily isolated in culture after sequential passages in the presence of increasing concentrations of ACV (reviewed in reference 19). However, several studies suggest that clinical use of ACV has not been associated with an increased emergence of drug-resistant virus. Sensitivity monitoring surveys have revealed that since the introduction of ACV, the prevalence of resistance in the general population has remained unchanged (1, 2), and little if any impact on the prevalence of resistant virus in the immunocompetent population has been shown (6, 13). Clinically significant resistance to ACV has been limited almost exclusively to the immunocompromised population (7, 10, 11, 21, 30), in which approximately 4 to 10% of patients develop resistance during antiviral treatment (2, 31).
To discern the prevalence of PCV resistance in the immunocompetent population, susceptibility assays were performed on virus isolates from patients participating in two placebo-controlled trials for evaluation of the efficacy of topical PCV for recurrent herpes labialis (29; unpublished data).
MATERIALS AND METHODS
Isolate sampling and resistance breakpoint.
Topical PCV cream (1%) was evaluated as a treatment for recurrent herpes labialis in two phase III studies conducted according to the same protocol (29). Patients were instructed to apply PCV or placebo cream nine times per day for 4 days within 1 h of the first sign or symptom of a recurrence. Although treatment was initiated by the patient, the patients visited the study center daily for evaluation and virus isolation. Additionally, each patient kept a daily record of treatment time and compliance. To determine the pattern of virus susceptibility to PCV, antiviral assays were performed on the first and last virus isolates obtained from the patients. In some instances, additional isolates obtained at intervals between the samplings for the first and last isolates were obtained and also tested. The first isolate was defined as the virus isolated within 24 h of the start of topical PCV treatment. The last isolate was defined as the final virus-positive culture obtained. In practice these were obtained during the treatment period or after cessation of treatment. Virus isolates were taken during the vesicle to soft-crust stage. The mean interval between first and last isolates was 1.8 days for the PCV group and 1.9 days for the placebo group. In order to determine the pattern of virus susceptibility, antiviral assays were performed on a random, blinded sample of isolates from each study. An in vitro breakpoint for defining ACV resistance has been set at 2.0 μg/ml, since 50% inhibitory concentrations (IC50s) for isolates from individuals failing to respond to drug therapy were generally above this level (22). Breakpoints are typically established through a consensus of researchers in the field based on the correlation between clinical treatment failure and in vitro IC50s. Because there is no recognized in vitro breakpoint for defining resistance to PCV, provisional guidelines were set for defining resistance based on the susceptibility of the sensitive control virus strain to PCV, which was determined in each assay. This takes into account the known variation in IC50s between laboratories. Resistance was defined as an IC50 of ≥2.0 μg/ml or an IC50 >10-fold higher than the IC50 for the wild-type sensitive control virus within that particular assay (24). Virological work was performed at two College of American Pathology-approved centers (the clinical virology laboratories of the Children's Hospital of Philadelphia and the University of San Francisco General Hospital), and each center used wild-type HSV type 1 (HSV-1) as a standard virus for susceptibility assays (either strain SC16 or strain F, depending on the center).
Statistical analysis.
Analysis of covariance was used to determine whether HSV isolates from PCV-treated patients and placebo-treated patients had different last IC50s after comparison with their respective first IC50s. The analysis was performed using natural logarithms of the IC50s, which provides statistical stringency. It was assumed that within each treatment group, the average difference between last and first isolates was zero, and significant deviation from zero would indicate a trend. Statistically significant differences in the change from the first to the last IC50 between PCV-treated and placebo-treated groups would be indicated by a P value of less than 0.05. Similarly, it was assumed that between the two treatment groups, the average difference between first isolates was zero and the average difference between last isolates was zero; a P value below 0.05 would suggest a trend.
Virus culture.
Lesions were swabbed daily throughout the course of the recurrence, during treatment, and often after treatment had ceased, to maximize the chance of identifying Pcvr isolates. Briefly, Dacron swabs moistened with Viral-Chlamydial transport medium (Carr-Scarborough Microbiologicals, Decatur, Ga.) were used to swab the lesions. Swabs were placed in transport medium and either processed immediately for virus isolation or stored at 4°C for no more than 48 h. Human diploid fibroblast cells in shell vials were inoculated with the transport medium and incubated overnight at 37°C. Resistant virus isolates were typed by staining of viral antigens with type-specific monoclonal antibodies (Dako) and were confirmed to be HSV-1. All other virus isolates were presumed to be HSV-1, although not directly typed.
Plaque reduction assay.
Testing of susceptibility to PCV was performed by the plaque reduction assay method in human diploid fibroblast cells (MRC-5) between passages 12 and 20. Briefly, cells were seeded into 12-well microtiter plates at approximately 3 × 105/well in 1.0 ml of Eagle's minimum essential medium containing 10% fetal calf serum or into 24-well plates with approximately one-half the number of cells. Cells were inoculated with 10-fold dilutions (102 to 104) of the viral isolate for 1 h at 37°C in a final volume of 0.5 ml of Hanks buffered salt solution. Testing was performed in triplicate in MRC-5 cells by using a series of PCV concentrations over 10 or 11 serial dilutions to provide at least two data points on either side of the IC50. After virus adsorption, the drug, 2× Eagle's minimum essential medium, and 0.8% (wt/vol) SeaPlaque agarose (FMC Bioproducts) were mixed, and 3.0-ml volumes were added to each well of a 12-well plate. After 3 days at 37°C, plates were fixed with 1.0 ml of 10% formaldehyde solution for 1 h at room temperature. Cell monolayers were stained with crystal violet after removal of the agarose plugs. Plaque numbers were counted, and IC50s were calculated.
Plaque reduction assays on the resistant isolates were performed in D21 cells, a line derived from BUHK-TK cells which constitutively expresses an HSV TK gene, as described in reference 26. These cells were a kind gift from H. Field (University of Cambridge, Cambridge, United Kingdom). These transformed cells were maintained in modified Eagle's medium with 10% calf serum and HAT supplement (hypoxanthine, aminopterin, and thymidine). For resistant samples, susceptibilities to ACV and foscarnet were also determined by the plaque reduction assay, with compounds obtained from Sigma Chemical Co. (St. Louis, Mo.).
TK assay.
Viral TK activity was determined by a modification of the method described by Coen and Schaffer (5). Human 143 TK-negative cells seeded in duplicate 100-mm dishes were infected at 5 PFU/cell with the HSV preparation in a Beckman G6-CR tabletop centrifuge, in 4.0 ml of serum-free medium. Parallel cell monolayers were mock infected. One hour postinfection, monolayers were rinsed with phosphate-buffered saline, and fresh medium was added for 8 h. Infected cells were then rinsed with phosphate-buffered saline, scraped, and centrifuged for 10 min at 1,000 rpm (4°C) and cell pellets were frozen at −80°C. Thawed pellets were resuspended in 300 μl of 10 mM sodium phosphate buffer (pH 6.0)-5 mM 2-mercaptoethanol-10% glycerol-50 μM thymidine. Extracts were sonicated on ice and centrifuged to remove cellular debris. This extract (9 μl) was added to a mixture to yield final concentrations of 100 mM sodium phosphate (pH 6.0), 10 mM ATP, 10 mM magnesium acetate, 6 μCi of [3H]thymidine (11 Ci/mmol; NEN Research Products), 50 μM TTP, 25 mM NaI, 0.67 mM dithiothreitol, and 10 μg of bovine serum albumin/ml in a final volume of 30 μl. Reaction mixtures were incubated at 30°C. At various times ranging from 0 to 180 min after addition of the cell extract, 5-μl aliquots were removed, added to 20 μl of 1 mM thymidine, and boiled for 2 min. Samples were then spotted onto Whatman DE81 circle filters. After drying, the filters were washed three times with 4 mM ammonium formate and 10 μM thymidine, once with distilled water, and twice with ethanol. Dry filters were placed in scintillation vials with Betafluor and counted. Values from duplicate samples were averaged. Radioactivity from the mock-infected control processed in parallel was used to subtract background. Data points from the linear range of thymidine phosphorylation were used. TK activity for HSV-1 SC16 was set at 100%. The limit of detection was estimated to be 0.3%, in agreement with a previous report (4).
RESULTS
PCV susceptibilities of paired HSV isolates.
Sensitivity testing was performed on a total of 1,035 isolates from 585 patients, of which 864 (83%) represented paired isolates (first and last isolate obtained from each patient). Of the paired isolates, 358 were from PCV-treated patients and 506 were from the placebo-treated group. The IC50 results, including standard deviations and ranges, are summarized in Table 1. The average IC50s for the isolates at the start of treatment were 1.1 ± 7.4 and 0.28 ± 0.28 μg of PCV/ml for the PCV- and placebo-treated groups, respectively. As discussed below, first virus isolates from two patients were resistant to PCV; subsequent isolates from these patients were sensitive to PCV. Analysis of the first-isolate data after removal of the IC50s for these two isolates (55 and 83 μg of PCV/ml) resulted in similar susceptibility profiles overall for the two treatment groups (0.29 ± 0.25 and 0.28 ± 0.28 μg of PCV/ml for the PCV- and placebo-treated groups, respectively). Lastly, similar average IC50s were reported for all last isolates regardless of treatment (0.32 ± 0.33 and 0.31 ± 0.33 μg of PCV/ml for the PCV- and placebo-treated groups, respectively).
TABLE 1.
Analysis of PCV susceptibilities of paired HSV-1 isolates
| Treatment | No. of patientsa | No. of isolates | Avg (range) PCV IC50 (μg/ml) for:
|
Avg difference (last − first) | Pb | |
|---|---|---|---|---|---|---|
| First isolates | Last isolates | |||||
| PCV | 177 | 356 | 0.29 ± 0.25 (0.07-1.10) | 0.32 ± 0.33 (0.07-1.80) | 0.028 | 0.078 |
| Placebo | 253 | 506 | 0.28 ± 0.28 (0.08-2.0)c | 0.31 ± 0.33 (0.07-3.1)d | 0.025e | 0.065 |
This analysis did not include the two patients who had resistant isolates at the start of treatment (IC50s, 83 and 55 μg/ml). If these isolates were included in the analysis, the average IC50 for first isolates would be 1.1 ± 7.4 μg/ml and the IC50 range would be 0.07 to 83.2 μg/ml.
Significance of the average difference between first and last isolates within the treatment group.
P = 0.796 for the difference between the average first IC50s for the two treatment groups.
P = 0.757 for the difference between the average last IC50s for the two treatment groups.
P = 0.791 for the comparison between groups of the average differences between first and last isolates after adjustment for any differences in the first isolates.
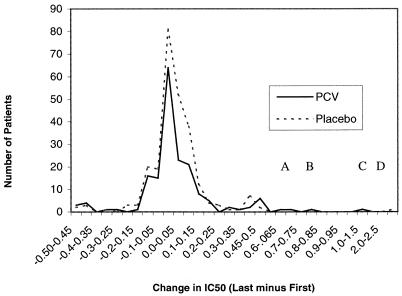
The trend analysis on data for paired isolates (first and last HSV isolates tested for PCV susceptibility) from this study indicates there are no statistically significant differences between IC50s for last isolates from PCV-treated and placebo-treated patient populations after accounting for any difference in the IC50s for first viral isolates (P = 0.791 by analysis of covariance) (Table 1). Furthermore, monitoring changes in IC50s between first and last isolates can serve as a powerful method to identify alterations in the pattern of susceptibility during therapy. The histogram in Fig. 1 illustrates the differences in IC50 (IC50 for last isolate minus IC50 for first isolate) between the paired first and last isolates for each treatment group, up to an increase of 2.5 μg of PCV/ml. Only two isolates, the two Pcvr variants, had IC50 changes greater than 2.5 μg of PCV/ml.
FIG. 1.
Changes in IC50 between first and last HSV-1 isolates. This parameter was utilized to identify alterations or trends in the pattern of susceptibility during the course of therapy. Differences between the IC50 for the last isolate and that for the first isolate are shown as ranges (in micrograms of PCV per milliliter) on the y axis. Numbers of patients analyzed are shown on the x axis. The distributions of susceptibility are similar for the two treatment groups except for four minor populations. Peaks A through C represent three minor populations in the PCV-treated group with a shift to the right in susceptibility, and peak D corresponds to one minor population in the placebo-treated group with decreased susceptibility to PCV.
Several peaks, representing subpopulations with increasing IC50s, were apparent in the PCV and placebo treatment groups (Fig. 1). Clearly, for the majority of samples evaluated, similar profiles were apparent for the two treatment groups. However, to the right of the midpoint, which corresponds to no change in the IC50 between first and last isolates, three minor peaks in the PCV-treated population and one minor peak in the placebo-treated group were present. For three PCV-treated samples, the last PCV IC50 was approximately 0.65 to 0.7, 0.75 to 0.80, or 1.0 to 1.5 μg/ml higher than the IC50 for the first isolate (Fig. 1, peaks A, B, and C, respectively). For one placebo-treated last isolate, the PCV IC50 was more than 1.5 μg/ml greater than that for the corresponding first isolate (Fig. 1, peak D). Overall, however, for the majority of isolates, the distribution profiles of the two treatment groups overlap, suggesting that no significant differences in susceptibility are present between treatment groups, although the minor increases in IC50 for four patient samples tested could indicate a trend to increasing resistance or simply represent the inherent variability within the plaque reduction assay.
PCV susceptibilities of all HSV isolates.
Table 2 summarizes the susceptibility data for all HSV isolates (paired and nonpaired) collected during the two clinical trials. Once again, average IC50s for the two groups of isolates were very similar, as measured by susceptibility to PCV in the plaque reduction assay (0.29 ± 0.28 and 0.28 ± 0.29 μg of PCV/ml for the PCV- and placebo-treated groups, respectively), when IC50s for the two resistant first isolates were excluded. Inclusion of the two Pcvr isolates in the data set results in an average IC50 of 0.60 ± 4.7 μg of PCV/ml for the PCV-treated group. Inclusion of these highly resistant isolates in the test population provides the most accurate representation of the background frequency of Pcvr, approximately 0.34% (2 of 585 patients).
TABLE 2.
Analysis of PCV susceptibilities of all HSV-1 isolates, paired and nonpaired
| Treatment | No. of patientsa | No. of isolatesa | Avg (range) PCV IC50 (μg/ml) |
|---|---|---|---|
| PCV | 255 | 437 | 0.29 ± 0.28 (0.07-1.8) |
| Placebo | 330 | 596 | 0.28 ± 0.29 (0.07-3.1) |
Values for the PCV treatment group are for 255 patients who provided 437 isolates. This analysis did not include the first isolates from the two patients possessing resistant isolates at the start of treatment (IC50s, 83 and 55 μg/ml). If these isolates were included in the analysis, the average IC50 would be 0.60 ± 4.7 μg/ml and the IC50 range would be 0.07 to 83.2 μg/ml.
PCV-resistant clinical isolates.
PCV IC50s for virus isolates taken from two patients (patients 024.028.2586 and 024.023.0495) within 17 h of the start of PCV treatment were 83 and 55 μg/ml, respectively, clearly meeting the criteria defining resistance. However, subsequent isolates from these two patients taken from the same episode were susceptible to PCV (PCV IC50s, 1.1 and 0.8 μg/ml, respectively; viruses were isolated on day 3 of treatment in both cases). No other Pcvr viruses were identified from the treatment period or after cessation of treatment with topical PCV.
Only for two other isolates were PCV IC50s equal to or greater than 2 μg/ml; both of these were from patients treated with a placebo. For one isolate, obtained on day 3 from patient 024.027.0424, the IC50 was 3.1 μg/ml, but this value was substantially below 9 μg/ml, the breakpoint defined as 10-fold above the IC50 for the wild type. When the isolate was retested against PCV, an IC50 of 0.74 μg/ml was determined, and therefore this isolate was not considered to be resistant to PCV. For another isolate (obtained on day 1 from patient 024.028.2016), the IC50 was 2 μg/ml. The IC50 for the sensitive control strain ranged between 0.9 and 1.6 μg/ml, and therefore, according to the 10-fold criterion, the test isolate was classified as sensitive; however, based on the standard breakpoint of an IC50 of ≥2.0 μg/ml, this isolate would be labeled as possibly resistant. Unfortunately, this isolate was not available for further analysis or retesting.
Molecular characterization of Pcvr isolates.
Plaque autoradiography on the two Pcvr patient samples (patients 024.028.2586 and 024.023.0495) confirmed that the resistant variants represented the majority of virus present in the virus preparation (data not shown). Plaque-purified isolates from the two patient samples which were confirmed as Pcvr (patients 024.028.2586 and 024.023.0495) were evaluated for the ability to phosphorylate 3H-labeled substrates including thymidine, ACV, and PCV according to previously described methods (23, 26). These isolates were unable to utilize ACV or PCV as a substrate, compared to phosphorylation by wild-type HSV-1 (set at 100%), and they were also highly impaired in thymidine phosphorylation, suggestive of a TK-negative or TK-partial phenotype (Table 3). Lastly, PCV IC50s were reduced to below 0.7 μg/ml upon testing in BUHK-TK cells (data not shown), confirming that impaired TK activity was responsible for conferring resistance to PCV. However, the important distinction between TK-negative and TK-partial remains difficult to make based on in vitro TK assays alone.
TABLE 3.
Biochemical characterization of Pcvr HSV-1 isolates
| Patient IDa | Phosphorylationb of substrate
|
||
|---|---|---|---|
| Thymidine | ACV | PCV | |
| 024.028.2586 | 4.0 | <0.3 | <0.3 |
| 024.023.0495 | 4.3 | <0.3 | <0.3 |
ID, identification number.
Expressed as a percentage of phosphorylation of the given substrate by wild-type HSV-1 (set at 100%).
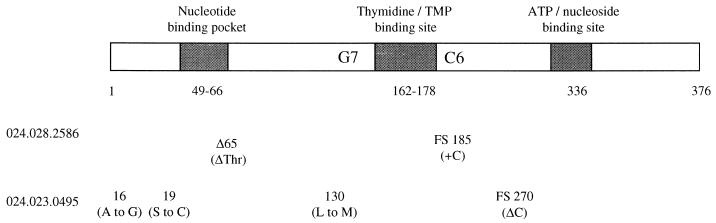
Sequence analysis of the TK coding region identified the presence of frameshift mutations, consistent with the TK-negative phenotype (Fig. 2). The isolate from patient 024.028.2586 contained two mutations, a deletion of residue 65 (threonine) and a frameshift (cytosine insertion) in the homopolymeric C run at residue 185. The isolate obtained from patient 024.023.0495 contained three nonconservative changes and a frameshift at residue 270 (cytosine deletion). It was not determined whether the nonconservative residues and/or frameshift was directly responsible for conferring drug resistance.
FIG. 2.
Alignment of mutations within the TK coding sequence. The schematic representation depicts the HSV-1 TK polypeptide and three conserved domains, the nucleotide binding pocket, the thymidine binding site, and the ATP binding site. The homopolymeric hot-spot regions (G7 and C6) are indicated. Below the diagram, the genotypic mutations and resulting residue changes or frameshifts (FS) identified in the two confirmed Pcvr isolates are shown.
DISCUSSION
This study demonstrates that there is no evidence of reduced PCV sensitivity among viral isolates obtained from patients treating a single episode of recurrent herpes labialis with topical PCV. No statistically significant difference between the PCV and placebo treatment groups was identified in the IC50s for first isolates (P = 0.796) or the IC50s for last isolates (P = 0.757) (Table 1). Furthermore, the average difference between IC50s for paired isolates in the PCV group was 0.028, and that in the placebo group was 0.025. Thus, there was no statistically significant difference between the last and first IC50s in either the PCV-treated group (P = 0.078) or the placebo-treated group (P = 0.065) (Table 1).
The plaque reduction assay data, along with the histogram analysis presented in Fig. 1, confirm that acute treatment of recurrent herpes labialis in immunocompetent patients with topical PCV did not result in an increase in the prevalence of resistant HSV. Collecting serial isolates represents a powerful method for examining trends in resistance. Since the distribution profiles of changes in IC50 after treatment significantly overlap for the two treatment groups, acute treatment with PCV cream versus a placebo does not appear to increase the likelihood of selecting for Pcvr HSV in immunocompetent patients with recurrent herpes labialis. However, studies monitoring resistance trends during episodic, prolonged treatment need to be performed in order to assess the development of resistance over time with repeated usage.
Two virus isolates for which PCV IC50s were 55 and 83 μg/ml were confirmed by biochemical and molecular analyses to be Pcvr. Both were first isolates, taken within 17 h of the start of treatment with topical PCV. Spontaneous mutations within the HSV genome are introduced by errors during DNA replication and are independent of the presence of an antiviral agent (15). This natural phenomenon results in the accumulation of 6 to 8 TK-deficient variants per 104 plaque-forming viruses in virus populations that have never been exposed to selective pressure (8, 16, 17) and can explain the coexistence of both resistant and sensitive viruses within all clinical HSV isolates. Consistent with this, there is a low prevalence of resistant HSV among individuals who have not been treated with an antiviral agent (1, 2).
Given the replication cycle kinetics of HSV and the fact that both Pcvr isolates were obtained within 17 h of initiation of treatment, it seems unlikely, although possible, that they resulted from selection pressure as the result of antiviral treatment. Since approximately 50% resistant variants are required within a virus preparation to confer such high IC50s (12, 24), a preexisting resistant variant would have to represent a substantial proportion of the virus swab and be selectively amplified immediately upon the initiation of treatment. Although the presence of these two resistant isolates from the first isolate swabs may simply reflect the natural heterogeneity of HSV populations (20, 25), isolates taken on day 2 would be expected to also be resistant, yet these IC50s were below 2.0 μg/ml. Another possible explanation is that these two isolates result from rare cases where residual topical PCV carried from the viral swab facilitated in vitro selection of resistant virus during the isolation process. If this is true, isolates obtained from these patients on days 2 and 3 would be expected to be completely susceptible to PCV. A third explanation which cannot be ruled out is preferential selection for resistant variants on day 1, followed by subsequent selection against these isolates for fitness. Lastly, patient recording of treatment times indicates that lack of compliance was not a factor in the selection for resistance (data not shown).
Genotypic characterization of the two confirmed Pcvr HSV isolates described in this report resulted in the identification of a frameshift mutation at residue 185 in one isolate, which was also found in a previously characterized Pcvr isolate (26) and is similar to the mutations routinely found within the homopolymeric region of TK (G7 and C6 hot spots) (27). Moreover, several mutations were also identified in the second isolate characterized, notably with a frameshift at residue 270, which, like the frameshift at residue 185, could be predicted to disrupt the integrity of the ATP/nucleoside binding pocket. Interestingly, although these isolates were plaque purified several times, they were not absolutely defective in the ability to phosphorylate thymidine, whereas phosphorylation of ACV and PCV was below the level of detection. It is not known whether the minor level of thymidine phosphorylation is due to contamination with wild-type virus undetectable by plaque autoradiography, to ribosomal frameshifting (18), or to other factors.
Most recently, the prevalence of ACV resistance was determined to be approximately 0.4% based on data for HSV isolates obtained from 708 immunocompetent, ACV-naïve individuals with genital herpes (1, 2). Furthermore, the proportion of ACV resistance appears to be relatively stable in immunocompetent patients, even after the increased usage of ACV over the past 2 decades. The present study indicates that the overall prevalence of PCV-resistant HSV isolates, for the immunocompetent population examined, was no greater than 0.19%, in agreement with historical data on ACV. Although PCV and ACV share an identical activation pathway and a similar mode of action, suggesting that the mechanisms of resistance are similar, the widespread usage of ACV for treatment has not increased the prevalence of PCV resistance above that reported for ACV.
In conclusion, based on the PCV susceptibilities of sequential isolates from patients with recurrent herpes labialis taken throughout the treatment period in this study, there is no reason to expect a change in the overall prevalence of resistant HSV isolates with use of topical PCV for acute treatment of recurrent herpes labialis in immunocompetent patients.
Acknowledgments
We thank Bob Gagnon, Ruth MacDonald, and Paul McAllister for performing the statistical analyses, H. Field for the BUHK-TK cell line, and S. Safrin and R. Hodinka for clinical sample testing.
REFERENCES
- 1.Boon, R. J., T. H. Bacon, H. L. Robey, T. J. Coleman, A. Connolly, P. Crosson, and S. L. Sacks. 2000. Antiviral susceptibilities of herpes simplex virus from immunocompetent subjects with recurrent herpes labialis: a UK-based survey. J. Antimicrob. Chemother. 46:323-342. [DOI] [PubMed] [Google Scholar]
- 2.Christophers, J., J. Clayton, J. Craske, R. Ward, P. Collins, M. Trowbridge, and G. Darby. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42:868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coen, D. M. 1986. General aspects of virus drug resistance with special reference to herpes simplex virus. J. Antimicrob. Chemother. 18:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coen, D. M., and P. A. Schaffer. 1980. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 77:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, P., and M. N. Ellis. 1993. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J. Med. Virol. 41(Suppl. 1):58-66. [DOI] [PubMed] [Google Scholar]
- 7.Crumpacker, C. S., L. E. Schnipper, S. I. Marlowe, P. N. Kowalsky, B. J. Hershey, and M. J. Levin. 1982. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N. Engl. J. Med. 306:343-346. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta, U. B., and W. C. Summers. 1978. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammalian cells. Proc. Natl. Acad. Sci. USA 75:2378-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elion, G. B., P. A. Furman, J. A. Fyfe, P. de Miranda, L. Beauchamp, and J. H. Schaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc. Natl. Acad. Sci. USA 74:5716-5720.202961 [Google Scholar]
- 10.Englund, J. A., M. E. Zimmerman, E. M. Swierkosz, J. L. Goodman, D. R. Scholl, and H. H. J. Balfour. 1990. Herpes simplex virus resistant to acyclovir: a study in a tertiary care center. Ann. Intern. Med. 112:416-422. [DOI] [PubMed] [Google Scholar]
- 11.Erlich, K. S., J. Mills, P. Chatis, G. J. Mertz, D. F. Busch, S. E. Follansbee, R. M. Grant, and C. S. Crumpacker. 1989. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 320:293-296. [DOI] [PubMed] [Google Scholar]
- 12.Field, H. J. 1982. Development of clinical resistance to acyclovir in herpes simplex virus-infected mice receiving oral therapy. Antimicrob. Agents Chemother. 21:744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fife, K. H., C. S. Crumpacker, G. Mertz, E. L. Hill, G. S. Boone, and the Acyclovir Study Group. 1994. Recurrence and resistance patterns of herpes simplex virus following cessation of >6 years of chronic suppression with acyclovir. J. Infect. Dis. 169:1338-1341. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreau, A., E. Hill, H. H. Balfour, A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 15.Hall, J. D., and R. E. Almy. 1982. Evidence for control of herpes simplex virus mutagenesis by the viral DNA polymerase. Virology 116:535-543. [DOI] [PubMed] [Google Scholar]
- 16.Hall, J. D., D. M. Coen, B. L. Fisher, M. Weisslitz, S. Randall, R. E. Almy, P. T. Gelep, and P. A. Schaffer. 1984. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology 132:26-37. [DOI] [PubMed] [Google Scholar]
- 17.Hwang, C. C., and H. H. Chen. 1995. An altered spectrum of herpes simplex virus mutations mediated by an antimutator DNA polymerase. Gene 152:191-193. [DOI] [PubMed] [Google Scholar]
- 18.Hwang, C. C., B. Horsburgh, E. Pelosi, S. Roberts, P. Digard, and D. M. Coen. 1994. A net +1 frameshift permits synthesis of thymidine kinase from a drug-resistant herpes simplex virus mutant. Proc. Natl. Acad. Sci. USA 91:5461-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larder, B. A., and G. Darby. 1984. Virus drug resistance: mechanisms and consequences. Antivir. Res. 4:1-42. [DOI] [PubMed] [Google Scholar]
- 20.Parris, D. S., and J. E. Harrington. 1982. Herpes simplex virus variants resistant to high concentrations of acyclovir exist in clinical isolates. Antimicrob. Agents Chemother. 22:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pottage, J. C., and H. A. Kessler. 1995. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect. Agents Dis. 4:115-124. [PubMed] [Google Scholar]
- 22.Safrin, S., T. Elbeik, L. Phan, D. Robinson, J. Rush, A. Elbaggari, and J. Mills. 1994. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 38:1246-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarisky, R. T., R. Cano, T. T. Nguyen, R. J. Wittrock, K. E. Duffy, P. E. Clark, J. O. Bartus, T. H. Bacon, L. Caspers-Velu, R. L. Hodinka, and J. J. Leary. 2001. Biochemical characterization of a virus isolate recovered from a patient with herpes keratitis that was clinically resistant to acyclovir. Clin. Infect. Dis. 33:2034-2039. [DOI] [PubMed] [Google Scholar]
- 24.Sarisky, R. T., P. M. Crosson, R. Cano, M. R. Quail, T. T. Nguyen, R. J. Wittrock, T. H. Bacon, S. L. Sacks, L. Caspers-Velu, R. L. Hodinka, and J. J. Leary. 2001. Comparison of methods for identifying resistant herpes simplex virus and measuring antiviral susceptibility. J. Clin. Virol. 23:191-200. [DOI] [PubMed] [Google Scholar]
- 25.Sarisky, R. T., T. T. Nguyen, K. E. Duffy, R. J. Wittrock, and J. J. Leary. 2000. Difference in incidence of spontaneous mutations distinct between herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 44:1524-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarisky, R. T., M. R. Quail, P. E. Clark, T. T. Nguyen, W. S. Halsey, R. J. Wittrock, J. O. Bartus, M. M. Van Horn, G. S. Sathe, S. Van Horn, M. D. Kelly, T. H. Bacon, and J. J. Leary. 2001. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J. Virol. 75:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasadeusz, J. J., F. Tufaro, S. Safrin, K. Schubert, M. M. Hubinette, P. K. Cheung, and S. L. Sacks. 1997. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 71:3872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnipper, L. E., and C. S. Crumpacker. 1980. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc. Natl. Acad. Sci. USA 77:2270-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spruance, S. L., T. L. Rea, C. Thoming, R. Tucker, R. Saltzman, and R. J. Boon. 1997. Penciclovir cream for the treatment of herpes simplex labialis. JAMA 277:1374-1379. [PubMed] [Google Scholar]
- 30.Wade, J. C., C. McLaren, and J. D. Meyers. 1983. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J. Infect. Dis. 148:1077-1082. [DOI] [PubMed] [Google Scholar]
- 31.Wade, J. C., B. Newton, C. McLaren, N. Flournoy, R. E. Keeney, and J. D. Meyers. 1983. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double blind test. Ann. Intern. Med. 96:265-269. [DOI] [PubMed] [Google Scholar]