Abstract
The in vitro and in vivo activities of DW286, a novel fluoronaphthyridone with potent antibacterial activity, were compared with those of ciprofloxacin, gemifloxacin, sparfloxacin, and trovafloxacin. Against gram-positive bacteria, such as Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Enterococcus faecalis, the in vitro activity of DW286 was stronger than that of any other reference antibiotic. Against gram-negative bacteria, the activity of DW286 was similar to those of trovafloxacin and gemifloxacin but was weaker than that of ciprofloxacin. In a mouse systemic infection caused by three S. aureus strains, including methicillin-resistant S. aureus and quinolone-resistant S. aureus (QRSA), DW286 demonstrated the most potent activity, as found in vitro. Specially, DW286 is ≥8-fold more active against QRSA than the other fluoroquinolones. And the 50% protective doses for DW286 were correspondent with the in vitro activities.
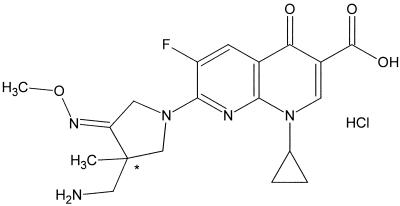
The quinolones have evolved from agents used solely for the treatment of urinary tract infections to molecules with potent activity against a wide spectrum of significant bacterial pathogens. Progressive modifications in their molecular configurations have improved both the spectrum and potency of their in vitro activities (2). These compounds have been successfully used in clinics for a decade. The targets in fluoroquinolone research during the last few years include improving the pharmacokinetic properties, increasing the activity against gram-positive cocci and anaerobes and against fluoroquinolone-resistant strains, and improving activity against nonfermentative gram-negative species (1, 4, 5, 6). In addition, the increasing pathogen resistance to antimicrobial agents is a cause of concern. This increase in the resistance to fluoroquinolone emphasizes the importance of the continued development of new structural candidates (3, 4, 5, 6, 7). The use of newer fluoroquinolone derivatives will potentially contribute to decreasing the spread of resistance to other antimicrobial agents by reducing the selective pressure on other antibiotic groups (5). New candidates have been developed in an attempt to help improve this situation. DW286, 7-[3-(aminomethyl)-4-(methoxyimino)-3-methyltetrahydro-1H-1-pyrrolyl]-1-cyclopropyl-6-fluoro-4-oxo-1, 4-dihydro[1,8]naphthyridine-3-carboxylic acid hydrochloric acid salt, is a novel fluoronaphthyridoneantibacterial agent that was synthesized for this purpose (Fig. 1). In this study, we determined the in vitro activity of DW286 in several groups of clinical isolates and compared it with those of ciprofloxacin, gemifloxacin, sparfloxacin, and trovafloxacin. We also compared the in vivo protective efficacy of DW286 with those of the fluoroquinolones against a systemic infection in mice.
FIG. 1.
Chemical structure of DW286.
The studied compounds were obtained as follows: DW286, ciprofloxacin, sparfloxacin, and gemifloxacin were synthesized at the R & D Center, Dong Wha Pharmaceutical Company, Anyang City, Korea. Trovafloxacin was provided by Pfizer Pharmaceuticals, New York, N.Y. Clinical isolates were obtained from hospitals in Seoul, Korea, between 1996 and 2000. The challenge organisms used in the mouse systemic infections were as follows: Streptococcus pyogenes ATCC 8668 was obtained from the American Type Culture Collection (Rockville, Md.), and Staphylococcus aureus Smith was kindly provided by S. Goto of Toho University, Tokyo, Japan. Escherichia coli MB4-01, Klebsiella pneumoniae MB4-02, Streptococcus pneumoniae MB4-21, Pseudomonas aeruginosa MB4-16, methicillin-resistant S. aureus (MRSA) MB4-19, and quinolone-resistant S. aureus (QRSA) MB4-20 were selected through the pathogenicity screening of clinical isolates (3). MICs were determined by an agar dilution method with Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.) following the National Committee for Clinical Laboratory Standards procedure (10). Haemophilus influenzae, S. pyogenes, and S. pneumoniae were grown on brain heart infusion (Difco) agar supplemented with 5% defibrinated sheep blood (Difco) at 37 °C.
The in vitro antibacterial activity of DW286 against the clinical isolates is presented in Table 1. Its antibacterial activity was superior to those of ciprofloxacin, gemifloxacin, sparfloxacin, and trovafloxacin against quinolone-sensitive S. aureus (DW286, MIC at which 50% of the isolates tested are inhibited [MIC50] = 0.008 μg/ml; MIC90 = 0.016 μg/ml) and QRSA (DW286, MIC50 = 0.5 μg/ml; MIC90 = 8 μg/ml). DW286 was also more active than ciprofloxacin and sparfloxacin and was slightly superior to trovafloxacin and gemifloxacin against Staphylococcus epidermidis (DW286, MIC50 = 0.031 μg/ml; MIC90 = 0.25 μg/ml), S. pyogenes (DW286, MIC50 = 0.016 μg/ml; MIC90 = 0.031 μg/ml), and Enterococcus spp. (DW286, MIC50 = 1 to 4 μg/ml; MIC90 = 4 to 8 μg/ml). Its antibacterial activity against S. pneumoniae was fourfold stronger than those of gemifloxacin and trovafloxacin (MIC90 = 0.125 μg/ml for DW286 and 0.5 μg/ml for gemifloxacin and trovafloxacin). In summary, DW286 exhibited potent in vitro antibacterial activities against gram-positive organisms. Specially, the improved antibacterial efficacy of DW286 against pneumococci was the most prominent difference. Against gram-negative bacteria, including members of the family Enterobacteriaceae, DW286 was less active than ciprofloxacin, but it was as active as trovafloxacin and gemifloxacin. The MIC50 and MIC90 for clinical strains of E. coli, K. pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, Enterobacter aerogenes, and Morganella morganii were 0.016 to 0.25 μg/ml and 0.125 to 0.5 μg/ml, respectively. Its activity against P. aeruginosa (MIC50 = 4 μg/ml; MIC90 = 32 μg/ml) was inferior to that of ciprofloxacin (MIC50 = 0.25 μg/ml; MIC90 = 2 μg/ml) and was slightly inferior to those of sparfloxacin, gemifloxacin, and trovafloxacin. Against Serratia marcescens, Xanthomonas maltophilia, Acinetobacter calcoaceticus, and Proteus spp., DW286 was less active than the other antibiotics. Its activity against gram-negative organisms was comparable to those of trovafloxacin and gemifloxacin but was less than that of ciprofloxacin.
TABLE 1.
In vitro antibacterial activities of DW286 and quinolones against clinical isolates
| Microorganism(s) (no. of isolates) and compound | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| QSSA (112)a | |||
| DW286 | ≤0.004-0.25 | 0.008 | 0.016 |
| Ciprofloxacin | 0.063-2 | 0.25 | 0.5 |
| Gemifloxacin | 0.008-0.125 | 0.031 | 0.063 |
| Sparfloxacin | 0.016-0.5 | 0.063 | 0.125 |
| Trovafloxacin | 0.016-2 | 0.063 | 0.125 |
| QRSA (128)a | |||
| DW286 | 0.063-32 | 0.5 | 8 |
| Ciprofloxacin | 4->64 | 64 | >64 |
| Gemifloxacin | 0.25->64 | 4 | 64 |
| Sparfloxacin | 1->64 | 16 | >64 |
| Trovafloxacin | 0.25-64 | 8 | 64 |
| S. epidermidis (53) | |||
| DW286 | 0.008-0.5 | 0.031 | 0.25 |
| Ciprofloxacin | 0.016->64 | 0.25 | 16 |
| Gemifloxacin | 0.016-4 | 0.063 | 2 |
| Sparfloxacin | 0.031-32 | 0.125 | 8 |
| Trovafloxacin | 0.031-4 | 0.063 | 1 |
| S. pyogenes (34) | |||
| DW286 | ≤0.004-0.25 | 0.016 | 0.031 |
| Ciprofloxacin | ≤0.004-4 | 1 | 2 |
| Gemifloxacin | ≤0.004-1 | 0.063 | 0.25 |
| Sparfloxacin | ≤0.004-2 | 0.25 | 0.5 |
| Trovafloxacin | ≤0.004-0.5 | 0.125 | 0.25 |
| Enterococcus faecalis (62) | |||
| DW286 | 0.031-16 | 1 | 4 |
| Ciprofloxacin | 0.25->64 | 4 | >64 |
| Gemifloxacin | 0.125-64 | 4 | 32 |
| Sparfloxacin | 0.5->64 | 32 | >64 |
| Trovafloxacin | 0.063-64 | 8 | 16 |
| Enterococcus faecium (54) | |||
| DW286 | 0.063-16 | 4 | 8 |
| Ciprofloxacin | 0.5->64 | 16 | >64 |
| Gemifloxacin | 0.063->64 | 8 | 16 |
| Sparfloxacin | 1->64 | 8 | >64 |
| Trovafloxacin | 0.25-64 | 8 | 32 |
| S. pneumoniae (30) | |||
| DW286 | ≤0.004-2 | 0.016 | 0.125 |
| Ciprofloxacin | 0.031-32 | 1 | 4 |
| Gemifloxacin | ≤0.004-8 | 0.063 | 0.5 |
| Sparfloxacin | 0.016-16 | 0.5 | 2 |
| Trovafloxacin | 0.031-4 | 0.25 | 0.5 |
| E. coli (97) | |||
| DW286 | 0.016-16 | 0.063 | 0.5 |
| Ciprofloxacin | 0.008-8 | 0.031 | 0.5 |
| Gemifloxacin | ≤0.004-16 | 0.031 | 1 |
| Sparfloxacin | ≤0.004-32 | 0.016 | 0.5 |
| Trovafloxacin | ≤0.004-32 | 0.016 | 0.25 |
| K. pneumoniae (78) | |||
| DW286 | ≤0.004-1 | 0.063 | 0.5 |
| Ciprofloxacin | ≤0.004-4 | 0.063 | 0.5 |
| Gemifloxacin | ≤0.004-2 | 0.063 | 1 |
| Sparfloxacin | ≤0.004-1 | 0.031 | 0.25 |
| Trovafloxacin | ≤0.004-0.5 | 0.063 | 0.25 |
| K. axytoca (50) | |||
| DW286 | ≤0.004-0.25 | 0.016 | 0.125 |
| Ciprofloxacin | 0.016-0.25 | 0.031 | 0.125 |
| Gemifloxacin | 0.031-0.5 | 0.125 | 0.25 |
| Sparfloxacin | 0.016-0.5 | 0.031 | 0.125 |
| Trovafloxacin | 0.016-0.25 | 0.031 | 0.125 |
| Citrobacter freundii (45) | |||
| DW286 | 0.016-16 | 1 | 2 |
| Ciprofloxacin | ≤0.004-4 | 0.125 | 0.5 |
| Gemifloxacin | 0.031-8 | 0.5 | 2 |
| Sparfloxacin | 0.031-8 | 0.5 | 2 |
| Trovafloxacin | 0.016-8 | 0.25 | 1 |
| E. cloacae (65) | |||
| DW286 | 0.008-4 | 0.031 | 0.5 |
| Ciprofloxacin | ≤0.004-2 | 0.063 | 0.5 |
| Gemifloxacin | 0.031-4 | 0.25 | 2 |
| Sparfloxacin | 0.016-8 | 0.125 | 1 |
| Trovafloxacin | 0.016-8 | 0.125 | 2 |
| E. aerogenes (30) | |||
| DW286 | 0.063-0.25 | 0.125 | 0.25 |
| Ciprofloxacin | 0.016-1 | 0.063 | 0.5 |
| Gemifloxacin | 0.063-0.25 | 0.063 | 0.25 |
| Sparfloxacin | 0.016-0.5 | 0.063 | 0.125 |
| Trovafloxacin | 0.031-0.5 | 0.125 | 0.25 |
| Proteus mirabilis (45) | |||
| DW286 | 0.008-64 | 0.25 | 2 |
| Ciprofloxacin | ≤0.004-8 | 0.016 | 4 |
| Gemifloxacin | 0.016-64 | 0.125 | 0.5 |
| Sparfloxacin | ≤0.004->64 | 0.25 | 2 |
| Trovafloxacin | 0.008-32 | 0.125 | 0.5 |
| Proteus vulgaris (13) | |||
| DW286 | ≤0.004-8 | 0.25 | 1 |
| Ciprofloxacin | 0.016-64 | 0.063 | 2 |
| Gemifloxacin | ≤0.004-64 | 0.063 | 0.25 |
| Sparfloxacin | 0.125->64 | 0.25 | 2 |
| Trovafloxacin | 0.063->64 | 0.125 | 2 |
| Proteus rettgeri (11) | |||
| DW286 | 0.008-0.25 | 0.031 | 0.125 |
| Ciprofloxacin | ≤0.004-0.063 | 0.016 | 0.063 |
| Gemifloxacin | 0.016-0.125 | 0.031 | 0.063 |
| Sparfloxacin | 0.008-0.25 | 0.031 | 0.125 |
| Trovafloxacin | 0.008-0.125 | 0.031 | 0.063 |
| Providencia stuartii (17) | |||
| DW286 | 0.125-2 | 0.25 | 1 |
| Ciprofloxacin | 0.031-1 | 0.125 | 1 |
| Gemifloxacin | 0.125-1 | 0.25 | 0.5 |
| Sparfloxacin | 0.063-1 | 0.125 | 0.5 |
| Trovafloxacin | 0.125-0.5 | 0.125 | 0.5 |
| M. morganii (28) | |||
| DW286 | 0.031-16 | 0.25 | 0.5 |
| Ciprofloxacin | 0.008-4 | 0.016 | 0.5 |
| Gemifloxacin | 0.016-2 | 0.125 | 0.25 |
| Sparfloxacin | 0.008-2 | 0.25 | 0.5 |
| Trovafloxacin | 0.008-4 | 0.25 | 0.5 |
| S. marcescens (81) | |||
| DW286 | 0.25-32 | 2 | 16 |
| Ciprofloxacin | ≤0.004-16 | 0.25 | 4 |
| Gemifloxacin | ≤0.004->64 | 0.5 | 8 |
| Sparfloxacin | 0.063->64 | 1 | 16 |
| Trovafloxacin | 0.063->64 | 0.5 | 4 |
| Salmonella enterica serovar Typhimurium (41) | |||
| DW286 | 0.016-0.5 | 0.125 | 0.25 |
| Ciprofloxacin | 0.008-0.25 | 0.031 | 0.063 |
| Gemifloxacin | ≤0.004-0.5 | 0.063 | 0.125 |
| Sparfloxacin | 0.008-1 | 0.031 | 0.063 |
| Trovafloxacin | 0.008-0.125 | 0.063 | 0.125 |
| S. enterica serovar Enteritidis (20) | |||
| DW286 | ≤0.004-1 | 0.125 | 0.25 |
| Ciprofloxacin | ≤0.004-0.125 | 0.031 | 0.063 |
| Gemifloxacin | ≤0.004-0.5 | 0.063 | 0.5 |
| Sparfloxacin | ≤0.004-0.5 | 0.063 | 0.125 |
| Trovafloxacin | ≤0.004-0.125 | 0.031 | 0.063 |
| Shigella spp. (60) | |||
| DW286 | ≤0.004-4 | 0.016 | 0.125 |
| Ciprofloxacin | ≤0.004-1 | 0.016 | 0.031 |
| Gemifloxacin | ≤0.004-2 | ≤0.004 | 0.031 |
| Sparfloxacin | ≤0.004-1 | 0.016 | 0.063 |
| Trovafloxacin | ≤0.004-0.5 | 0.008 | 0.031 |
| X. maltophilia (35) | |||
| DW286 | 0.25-8 | 1 | 4 |
| Ciprofloxacin | 0.5-8 | 2 | 8 |
| Gemifloxacin | 0.5-2 | 1 | 1 |
| Sparfloxacin | 1-4 | 1 | 2 |
| Trovafloxacin | 0.5-2 | 0.5 | 2 |
| A. calcoaceticus (35) | |||
| DW286 | 0.063-8 | 0.25 | 2 |
| Ciprofloxacin | 0.016-16 | 0.5 | 1 |
| Gemifloxacin | 0.031-8 | 0.25 | 4 |
| Sparfloxacin | 0.016-4 | 0.25 | 2 |
| Trovafloxacin | 0.031-8 | 0.25 | 2 |
| H. influenzae (20) | |||
| DW286 | ≤0.004-0.063 | 0.004 | 0.008 |
| Ciprofloxacin | ≤0.004-0.5 | 0.004 | 0.016 |
| Gemifloxacin | ≤0.004-0.031 | 0.004 | 0.016 |
| Sparfloxacin | ≤0.004-0.063 | 0.004 | 0.063 |
| Trovafloxacin | ≤0.004-0.031 | 0.004 | 0.031 |
| P. aeruginosa (70) | |||
| DW286 | ≤0.004->64 | 4 | 32 |
| Ciprofloxacin | ≤0.004->64 | 0.25 | 2 |
| Gemifloxacin | ≤0.004->64 | 2 | 8 |
| Sparfloxacin | ≤0.004->64 | 1 | 8 |
| Trovafloxacin | ≤0.004->64 | 0.5 | 4 |
QSSA, quinolone-susceptible S. aureus (ciprofloxacin MIC ≤ 2 μg/ml). For QRSA, ciprofloxacin MIC ≥ 4 μg/ml.
The effects of serum on the antibacterial activity of DW286 against S. aureus ATCC 25923, Bacillus subtilis ATCC 6633, and E. coli ATCC 25922 were also evaluated. Heat-inactivated horse serum (Gibco BRL, Gaithersburg, Md.) to a final concentration of 10 or 25% (vol/vol) had no significant effect on the activities of DW286 and the other quinolones tested (data not shown).
The protective efficacy of the fluoroquinolones in a systemic infection model in mice was determined. The organisms used were S. aureus Smith, S. pyogenes ATCC 8668, E. coli MB4-01, K. pneumoniae MB4-02, P. aeruginosa MB4-16, S. pneumoniae MB4-21, MRSA MB4-19, and QRSA MB4-20. The organisms were cultured overnight in brain heart infusion broth at 37°C. Male ICR mice (body weight, 18 to 22 g; age, 4 weeks) were inoculated intraperitoneally with 0.3 ml of a bacterial suspension adjusted with 3% gastric mucin (ICN Biomedicals, Columbus, Ohio) in saline solution at 100 times the minimal lethal dose. The challenge inoculum was sufficient to kill 100% of the untreated control mice, which died within 48 h postinfection. Each test compound was administered once orally to mice immediately after infection. Five groups of seven mice each were treated with different doses of each antibacterial agent. The 50% protective dose (PD50) was calculated by the method of Litchfield and Wilcoxon (8, 9) from the survival rates on day 7 after infection. The protective effects of DW286 against systemic infections were compared with those of ciprofloxacin, sparfloxacin, trovafloxacin, and gemifloxacin (Table 2). In this mouse infection model, DW286 showed good in vivo efficacies against gram-positive organisms such as S. aureus, including MRSA and QRSA, S. pyogenes, and S. pneumoniae. In infections caused by E. coli, K. pneumoniae, and P. aeruginosa, its activity was inferior to that of ciprofloxacin. However, its in vivo efficacies were comparable to those of trovafloxacin and gemifloxacin. The PD50s for DW286 correlated with the in vitro MICs. Based on its in vitro activity and in vivo efficacy, DW286 seems to be a promising antibiotic with a broad spectrum of antimicrobial activity, including potent activity against S. aureus and S. pneumoniae.
TABLE 2.
Protective efficacy of DW286 on systemic infections in mice
| Microorganism [challenge dose (CFU/ml)] and compounda | MIC (μg/ml) | PD50b (mg/kg) | 95% Confidence limits |
|---|---|---|---|
| S. aureus Smith [1.2 × 107] | |||
| DW286 | 0.004 | 0.12 | 0.08-0.17 |
| Ciprofloxacin | 0.5 | 3.43 | 2.40-4.89 |
| Gemifloxacin | 0.031 | 0.77 | 0.39-1.51 |
| Sparfloxacin | 0.063 | 0.54 | 0.33-0.89 |
| Trovafloxacin | 0.016 | 0.32 | 0.16-0.64 |
| MRSA MB4-19 [3.1 × 108] | |||
| DW286 | 0.004 | 0.12 | 0.07-0.20 |
| Ciprofloxacin | 0.25 | 3.99 | 2.12-7.53 |
| Gemifloxacin | 0.016 | 0.70 | 0.39-1.26 |
| Sparfloxacin | 0.031 | 0.22 | 0.11-0.42 |
| Trovafloxacin | 0.016 | 0.17 | 0.10-0.28 |
| QRSA MB4-20 [5.0 × 108] | |||
| DW286 | 0.25 | 5.83 | 3.13-10.87 |
| Ciprofloxacin | 32 | >40 | |
| Gemifloxacin | 2 | >40 | |
| Sparfloxacin | 32 | >40 | |
| Trovafloxacin | 8 | >40 | |
| S. pyogenes ATCC 8668 [1.5 × 107] | |||
| DW286 | 0.008 | 0.12 | 0.07-0.20 |
| Ciprofloxacin | 0.25 | 15.00 | 7.82-28.77 |
| Gemifloxacin | 0.016 | 1.02 | 0.68-1.54 |
| Sparfloxacin | 0.25 | 13.36 | 5.14-34.71 |
| Trovafloxacin | 0.063 | 1.71 | 0.85-3.44 |
| S. pneumoniae MB4-21 [1.1 × 105] | |||
| DW286 | 0.008 | 0.19 | 0.10-0.38 |
| Ciprofloxacin | 0.5 | 10.05 | 5.31-19.04 |
| Gemifloxacin | 0.016 | 0.33 | 0.21-0.51 |
| Sparfloxacin | 0.125 | 1.80 | 0.99-3.28 |
| Trovafloxacin | 0.031 | 2.32 | 1.26-4.29 |
| E. coli MB4-01 [6.6 × 107] | |||
| DW286 | 0.008 | 0.37 | 0.12-1.09 |
| Ciprofloxacin | 0.016 | 0.32 | 0.11-0.88 |
| Gemifloxacin | 0.008 | 0.26 | 0.10-0.71 |
| Sparfloxacin | 0.008 | 0.52 | 0.32-0.82 |
| Trovafloxacin | 0.008 | 0.84 | 0.47-1.51 |
| P. aeruginosa MB4-16 [9.8 × 104] | |||
| DW286 | 1 | 6.29 | 3.23-12.24 |
| Ciprofloxacin | 0.125 | 0.94 | 0.46-1.93 |
| Gemifloxacin | 0.25 | 4.47 | 2.49-8.05 |
| Sparfloxacin | 0.25 | 2.56 | 1.19-5.50 |
| Trovafloxacin | 0.5 | 3.15 | 1.61-6.15 |
| K. pneumoniae MB4-02 [1.3 × 105] | |||
| DW286 | 0.125 | 0.50 | 0.28-0.90 |
| Ciprofloxacin | 0.031 | 0.19 | 0.10-0.37 |
| Gemifloxacin | 0.125 | 0.55 | 0.31-0.98 |
| Sparfloxacin | 0.125 | 0.17 | 0.11-0.28 |
| Trovafloxacin | 0.125 | 0.26 | 0.16-0.42 |
Each test compound was administered once orally to mice immediately postinfection.
The number of survivors on day 7 postinfection was used to calculate the PD50 by Litchfield and Wilcoxon's method (P < 0.05).
The pharmacokinetic studies of DW286 were performed after oral administration in mice, rats, and dogs (C. Lee, Y. Jung, J. Ryu, H. Kwon, and J. Lee, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-555, 2001). The mean maximum concentrations (in micrograms per milliliter) of DW286 in serum in mice were 1.06, 8.55, 24.28, and 96.54, and the total areas under the concentration-time curve (expressed in micrograms · hours/milliliter) were 3.06, 30.56, 110.47, and 783.90 at each dose of 10, 50, 250, and 1,000 mg/kg of body weight, respectively. In a single oral administration in rats (40 mg/kg) and dogs (10 mg/kg), the maximum concentrations of DW286 in serum were 9.06 μg/ml (2.0 h) in rats and 3.95 μg/ml (3.33 h) in dogs. The elimination half-life and the total area under the concentration-time curve were 4.5 h and 90.82 μg · h/ml in rats and 5.37 h and 34.65 μg · h/ml in female dogs. Hence, the pharmacokinetics of DW286 seem to be superior to those of ciprofloxacin. The new fluoronaphthyridone antibiotic, DW286, was absorbed efficiently from the gastrointestinal tract, regardless of the animal species. The pharmacokinetic data explain the correlation between the in vitro activity and the in vivo efficacy. Our findings need to be confirmed in further preclinical and clinical studies of this new fluoronaphthyridone antibiotic.
Acknowledgments
This work was supported by grants 00-PJ1-PG1-CH15-0002 and HMP-98-D-7-0008 from the Korean Ministry of Health & Welfare, the Basic Research Program of the Korea Science & Engineering Foundation (grant no. R01-1999-00110), and the 2001 BK21 project for Medicine, Dentistry, and Pharmacy.
We are grateful to Pfizer Pharmaceuticals for supplying trovafloxacin.
REFERENCES
- 1.Appelbaum, P. C., and P. A. Hunter. 2000. The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Agents 16:5-15. [DOI] [PubMed] [Google Scholar]
- 2.Ball, P. 2000. Quinolone generations: natural history or natural selection? J. Antimicrob. Chemother. 46(Suppl. T1):17-24. [DOI] [PubMed] [Google Scholar]
- 3.Choi, K. H., J. S. Hong, S. K. Kim, D. K. Lee, S. J. Yoon, and E. C. Choi. 1997. In-vitro and in-vivo activities of DW-116, a new fluoroquinolone. J. Antimicrob. Chemother. 39:509-514. [DOI] [PubMed] [Google Scholar]
- 4.Fung-Tomc, J., B. Minassian, B. Kolek, T. Washo, E. Huczko, and D. Bonner. 2000. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J. Antimicrob. Chemother. 45:437-446. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Garrote, F., E. Cercenado, J. Martin-Pedroviejo, O. Cuevas, and E. Bouza. 2001. Comparative in vitro activity of the new quinolone gemifloxacin (SB-265805) with other fluoroquinolones against respiratory tract pathogens. J. Antimicrob. Chemother. 47:681-684. [DOI] [PubMed] [Google Scholar]
- 6.Kim, J. H., J. A. Kang, Y. G. Kim, J. W. Kim, J. H. Lee, E. C. Choi, and B. K. Kim. 1997. In vitro and in vivo antibacterial efficacies of CFC-222, a new fluoroquinolone. Antimicrob. Agents Chemother. 41:2209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klepser, M. E., E. J. Ernst, C. R. Petzold, P. Rhomberg, and G. V. Doern. 2001. Comparative bactericidal activities of ciprofloxacin, clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin against Streptococcus pneumoniae in a dynamic in vitro model. Antimicrob. Agents Chemother. 45:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litchfield, J. T., and F. Wilcoxon. 1949. Simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99-113. [PubMed] [Google Scholar]
- 9.Lorian, V. 1996. Antibiotics in laboratory medicine, 4th ed., p. 635-636. Williams & Wilkins, Baltimore, Md.
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.