Abstract
The mechanisms of herpes simplex virus (HSV) inactivation by sodium lauryl sulfate (SLS) and n-lauroylsarcosine (LS), two anionic surfactants with protein denaturant potency, have been evaluated in cultured cells. Results showed that pretreatment of HSV type 1 (HSV-1) strain F and HSV-2 strain 333 with either surfactant inhibited, in a concentration- and time-dependent manner, their infectivities on Vero cells. SLS was a more potent inhibitor of HSV-2 strain 333 infectivity than LS with respect to the concentration (4.8-fold lower) and time (2.4-fold shorter) required to completely inactivate the virus. No inhibition of both herpesvirus strains infectivities was observed when Vero cells were pretreated with either surfactant. LS prevented the binding of HSV-2 strain 333 to cells without affecting the stable attachment and the rate of penetration into cells, whereas SLS exerted the opposite effect. Both SLS and LS inhibited, in a concentration-dependent manner, the HSV-2 strain 333-induced cytopathic effect, probably by affecting newly synthesized virions that come into contact with surfactant molecules present in culture medium. The pretreatment of HSV-2 strain 333 with specific combinations of SLS and LS concentrations inhibited the viral infectivity in a synergistic manner and resulted in only a small increase in their toxicities for exponentially growing Vero cells compared with that caused by each compound alone. Taken together, these results suggest that SLS and LS, alone or combined, could represent potent candidates as microbicides in topical vaginal formulations to prevent the transmission of herpes and possibly other pathogens that cause sexually transmitted diseases, including human immunodeficiency virus type 1.
The number of individuals infected with human immunodeficiency virus type 1 (HIV-1), herpesviruses and other sexually transmitted pathogens is growing dramatically worldwide. The global incidence, morbidity, and mortality of sexually transmitted diseases (STDs) are very significant. Herpes simplex virus type 1 (HSV-1) and HSV-2 are the most common causes of genital ulceration in developed countries. In the United States only, approximately 500,000 new cases of herpes are reported each year. Genital herpes is lifelong and may result in painful and recurrent lesions, systemic complications, and psychosocial morbidity (6). HSVs are transmitted by symptomatic lesions and through asymptomatic viral shedding. The high viral titers in symptomatic lesions make this mode of transmission the most efficient. However, the contribution of asymptomatic viral shedding to the genital transmission of herpes in the population may account for as much as 50 to 90% of cases (25-27, 41); the infected partner being unaware that he or she could transmit the virus. Moreover, intrapartum transmission of herpes from the mother to the neonate is also observed during vaginal delivery in 85% of cases and depends on the prevalence of viral shedding at the time of delivery. The transmission of the virus to neonates results in cutaneous, mucocutaneous, or eye infections or in some cases in the spreading of the virus to the brain or internal organs (41).
Latex condoms represent an effective barrier against HIV and other sexually transmitted pathogens, but unfortunately, their use is not generalized. More attention is now given to female-controlled methods to prevent the sexual transmission of these microorganisms, since many women are unable to negotiate condom use with their partners (9, 10, 16, 35, 37, 41). Nonoxynol-9, a nonionic surfactant with membrane-solubilizing properties, is the most currently used active ingredient in available vaginal formulations. In vitro, this compound inactivates enveloped viruses such as HIV-1 and HSV as well as other microorganisms, including Chlamydia trachomatis and Neisseria gonorrhoeae (1, 3, 5, 11, 17, 18, 23, 31), but is not effective against nonenveloped papillomaviruses (13). The activity of nonoxynol-9 is nonspecific and is often associated with adverse effects, including epithelial disruption, genital inflammation, and ulceration, as well as a reduction in the number of lactobacilli (19, 21, 28, 32, 33, 36), which may explain its lack of protection against HIV-1 and other pathogens causing STDs in clinical trials (21, 33; L. Van Damme, plenary lecture, XIII Int. AIDS Conf., 2000). There is thus an urgent need to develop new compounds which can be used as microbicides in topical vaginal formulations for women to protect themselves against sexually transmitted infections.
Sodium lauryl sulfate (SLS), an anionic surfactant with protein denaturant potency, has been demonstrated to be a potent inhibitor of the infectivities of different strains of enveloped viruses such as HSV and HIV-1 as well as nonenveloped viruses such as papillomaviruses in vitro (4, 30, 34). We have also demonstrated that n-lauroylsarcosine (LS), another anionic surfactant structurally close to SLS, inactivates HSV infectivity in vitro (34). Moreover, gel formulations containing SLS or LS were very effective in preventing HSV-2 infection following intravaginal challenge in mice. These formulations were also well tolerated following repeated intravaginal administrations to rabbits. These data suggest that SLS and LS could represent potent candidates for use in topical vaginal formulations to prevent the transmission of HSV, HIV-1, and possibly other pathogens causing STDs. In the present study, we have compared the mechanisms of HSV-1 and HSV-2 inactivation by SLS and LS in cultured cells. We have also evaluated the inactivating potency of combinations of different concentrations of both compounds on herpesvirus infectivity as well as their potential toxicities to cells.
MATERIALS AND METHODS
Materials.
SLS and LS were obtained from Sigma Chemical Co. (St. Louis, Mo.). [3H]methyl thymidine was purchased from Amersham Canada, Ltd. (Oakville, Ontario, Canada).
Cell lines.
Vero cells (African green monkey kidney cells; American Type Culture Collection, Manassas, Va.) were cultivated in Eagle's minimum essential medium (EMEM) (Canadian Life Technologies, Burlington, Ontario, Canada) supplemented with sodium bicarbonate (0.22%), penicillin-streptomycin (100 U/ml), l-glutamine (2 mM), and 5% heat-inactivated fetal bovine serum (FBS) (Canadian Life Technologies) (EMEM-5% FBS). Cultures were maintained at 37°C in a 5% CO2 atmosphere.
Virus strains.
HSV-1 strain F (American Type Culture Collection) and HSV-2 strain 333 (kindly provided by Lawrence R. Stanberry, Children's Hospital Medical Center, Cincinnati, Ohio) were propagated in Vero cells in complete EMEM containing 2% FBS (EMEM-2% FBS).
Preparation of radiolabeled virus.
HSV-2 strain 333 was labeled with [3H]methyl thymidine according to a previously described protocol with some modifications (2). In brief, Vero cells were incubated with the virus in EMEM-2% FBS at a multiplicity of infection of 0.1 for 1 h at 37°C to allow virus adsorption. The virus was removed, and cell sheets were washed twice with culture medium. Cells were then incubated in EMEM-2% FBS containing [3H]methyl thymidine (25 μCi/ml) for 2 days at 37°C. Cells and medium were collected, frozen at −80°C, and thawed at 37°C. The suspension was centrifuged (600 × g for 10 min at 4°C) to pellet cell debris, and the supernatant (10 ml) was layered over a 3-ml cushion of 15% sucrose. Samples were centrifuged (100,000 × g for 2 h at 4°C) to pellet the virus and resuspended in 1 ml of phosphate-buffered saline (PBS) (pH 7.4) overnight on ice at 4°C. The specific activity of the virus was approximately 0.33 cpm/PFU.
Virus inactivation assay.
In a first set of experiments, HSV-1 strain F or HSV-2 strain 333 was first preincubated in PBS (control) or in PBS containing increasing concentrations of SLS or LS for different periods of time (0 to 60 min) in a water bath at 37°C. Confluent Vero cells seeded in 24-well plates were then infected with pretreated viruses (approximately 50 to 100 PFU). In a second set of experiments, the virus and SLS or LS were added simultaneously to cells (i.e., in the absence of a pretreatment period). In a third set of experiments, Vero cells were preincubated in PBS (control) or in PBS containing increasing concentrations of SLS or LS for 1 h at 37°C prior to the infection with either HSV-1 strain F or HSV-2 strain 333. In all experiments, the plates were immediately centrifuged (750 × g for 45 min at 20°C) after the infection of cells to allow virus adsorption. Unbound virus was removed by aspiration, and cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose (Mandel Scientific, St-Laurent, Québec, Canada). Cells were incubated for 2 days at 37°C in a 5% CO2 atmosphere. Cells were then fixed with 10% formaldehyde in PBS for 20 min, washed with deionized water, and stained with 0.05% methylene blue. Virus inactivation was evaluated from the determination of the numbers of PFU.
Virus inactivation assay in the presence of proteins.
In a first set of experiments, HSV-2 strain 333 was preincubated in EMEM-5% FBS (control) or in EMEM-5% FBS containing increasing concentrations of SLS or LS for 1 h in a water bath at 37°C. In a second set of experiments, the virus was preincubated in EMEM containing increasing concentrations of FBS (0 to 60%; control) or with, in addition, 0.5 mM SLS or 1 mM LS for 1 h in a water bath at 37°C. In both cases, the residual viral infectivity was determined on Vero cells as described above in the virus inactivation assay.
Attachment assay.
The effect of SLS and LS on the attachment of radiolabeled HSV-2 strain 333 to Vero cells was assessed at 4°C as previously described (2). Confluent monolayers of Vero cells seeded in 96-well plates were first incubated in PBS plus 1% bovine serum albumin (BSA) for 30 min at 4°C in order to block nonspecific virus adsorption. [3H]methyl thymidine-labeled HSV-2 strain 333 was preincubated in PBS-1% BSA (control) or in PBS-1% BSA containing increasing concentrations of SLS or LS for 1 h in a water bath at 37°C. The viral suspension was cooled in a melting ice bath for a few minutes. Cells were then incubated with pretreated virus for 1 h at 4°C with gentle agitation (50 to 60 rpm). Cells were washed three times with ice-cold PBS and disrupted with lysis buffer (150 mM NaCl, 10 mM Tris [pH 7.6], 1% Igepal CA-630, 1% Na-deoxycholate). The amount of radioactivity associated with cell lysates was determined by liquid scintillation counting.
Postbinding assay.
The effect of SLS and LS on HSV-2 strain 333 stably attached to Vero cells was evaluated as previously described (24). Confluent Vero cells seeded in 24-well plates were maintained on ice for a few minutes. Cells were first incubated with HSV-2 strain 333 (approximately 50 to 100 PFU) suspended in PBS for 2 h at 4°C to allow a stable attachment of the virus without fusion with cell membrane. Unbound viruses were removed by aspiration. Cells were then washed with ice-cold PBS (control) or with ice-cold low-pH citrate buffer (Na citrate, 40 mM; KCl, 10 mM; NaCl, 135 mM, pH 3.0; positive control) or with ice-cold PBS containing increasing concentrations of SLS or LS for 1 min at 4°C. Cell sheets were washed once with ice-cold PBS and overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose. Cells were incubated for 2 days at 37°C in a 5% CO2 atmosphere. Cells were then fixed, washed, and stained as described above. The amount of virus which had penetrated into cells after the shift at 37°C was evaluated following the determination of the numbers of PFU.
Penetration assay.
The effect of SLS and LS on the rate of penetration of HSV-2 strain 333 into Vero cells was examined as previously described (24). In brief, confluent monolayers of Vero cells seeded in six-well plates were first incubated with HSV-2 strain 333 for 2 h at 4°C. After removal of unbound viruses, the plates were shifted to 37°C to allow penetration of virus into cells. At selected times after the temperature shift (0, 15, 30, and 60 min), cells were treated for 1 min with PBS (control) or with PBS containing 300 μM SLS or 500 μM LS prewarmed at 37°C. Cells were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 2 days at 37°C in a 5% CO2 atmosphere. Cells were then fixed, washed, and stained as described above. The amount of virus which had penetrated into cells was evaluated following the determination of the numbers of PFU.
Plaque reduction assay.
Confluent Vero cells seeded in 24-well plates were first incubated with HSV-2 strain 333 (approximately 50 to 100 PFU) suspended in EMEM-2% FBS for 2 h at 37°C. Cells were then overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose (control) or with, in addition, increasing concentrations of SLS or LS and incubated for 2 days at 37°C in a 5% CO2 atmosphere. Cells were then fixed, washed, and stained as described above. Virus-induced cytopathic effect was evaluated following the determination of the numbers of PFU.
Analysis of drug combination effect.
The inactivating potency of different combinations of SLS and LS on the infectivity of HSV-2 strain 333 was examined with combinations of various concentrations of the test compounds in a checkerboard design. In brief, HSV-2 strain 333 was first preincubated in PBS (control) or in PBS containing different combinations of SLS and LS concentrations for 1 h in a water bath at 37°C. The residual viral infectivity was then determined on Vero cells as described above in the virus inactivation assay. The drug combination effect was analyzed by the isobologram method as described previously (12, 38, 39). In this analysis, the 50% effective dose (ED50) was used to calculate the fractional inhibitory concentration (FIC). When the FIC index of the combined compounds (i.e., FICx + FICy) is equal to 1.0, the combination is assumed to act in an additive manner; when it is between 0.5 and 1.0, the combination acts subsynergistically; and when it is less than 0.5, it acts synergistically. On the other hand, when the FIC index is between 1.0 and 2.0, the combination is subantagonistic, and when it is greater than 2.0, the combination is antagonistic.
Cellular viability.
Vero cells seeded at midconfluency in 24-well plates were incubated in EMEM-5% FBS (control) or with EMEM-5% FBS containing different combinations of SLS and LS concentrations for 72 h at 37°C in a 5% CO2 atmosphere. Cells were washed once with complete culture medium and incubated in EMEM-5% FBS containing a 100-μg/ml concentration of a tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl]-2H-tetrazolium, inner salt [MTS]; Promega, Madison, Wis.) and phenazine methosulfate (7.5 μg/ml) for 30 min at 37°C. The tetrazolium salt is reduced by living cells to yield a formazan product that can be assayed colorimetrically (7). Reaction mixtures were transferred in a microplate, and the optical density was read at a wavelength of 492 nm.
Statistical analysis.
The significance of the differences between (i) the inactivating potency of SLS and LS on herpesviruses' infectivities for susceptible cells and (ii) the toxic effect caused by combinations of SLS and LS concentrations and each compound alone on the viability of Vero cells was evaluated by an unpaired Student t test. All statistical analyses were performed with a computer package (Statview+SE Software; Abacus Concepts, Berkeley, Calif.). A P value of less than 0.05 was considered statistically significant.
RESULTS
Viral inactivation.
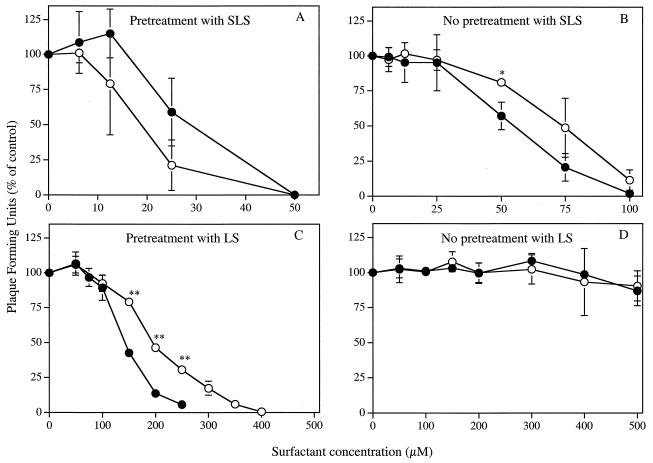
Figure 1A shows that pretreatment of HSV-1 strain F or HSV-2 strain 333 with SLS for 1 h at 37°C decreased, in a concentration-dependent manner, their infectivities for Vero cells. The concentrations of SLS which inhibit 50% (ED50) and 90% (ED90) of the infectivity of HSV-1 strain F were 17.88 ± 5.86 μM and 34.89 ± 10.77 μM, respectively, whereas the corresponding values for HSV-2 strain 333 were 32.67 ± 2.18 μM and 46.53 ± 0.44 μM, respectively (unless otherwise noted, results are presented as means ± standard deviations). In the absence of a pretreatment period with SLS (Fig. 1B), the ED50 and ED90 values increased to 70.68 ± 5.90 μM and 96.04 ± 11.20 μM for HSV-1 strain F, respectively, and to 54.94 ± 6.65 μM and 82.29 ± 6.75 μM for HSV-2 strain 333, respectively. Pretreatment of Vero cells with SLS concentrations up to 300 μM for 1 h at 37°C prior to viral infection did not cause any loss of infectivity for both strains of herpesviruses (data not shown).
FIG. 1.
Effect of SLS (A and B) and LS (C and D) on the infectivities of HSV-1 strain F (○) and HSV-2 strain 333 (•) on Vero cells. In a first set of experiments, confluent cell monolayers were inoculated with viruses pretreated with PBS (control) or with PBS containing increasing concentrations of surfactants for 1 h at 37°C (A and C). In a second set of experiments, surfactants and viruses were added simultaneously to cells without any pretreatment period (B and D). In both cases, the plates were immediately centrifuged (750 × g for 45 min at 20°C) to allow virus adsorption. Unbound viruses were removed by aspiration. Cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 48 h at 37°C. Infectivity was expressed as the percentage of PFU compared with that of control cells. Results are the means ± standard deviations (error bars) of three independent experiments.
Similarly, Fig. 1C shows that pretreatment of both strains of herpesviruses with LS reduced, in a concentration-dependent manner, their infectivities for Vero cells. The ED50 and ED90 values obtained following pretreatment of HSV-1 strain F with LS for 1 h at 37°C were 194.40 ± 1.11 μM and 329.97 ± 16.54 μM, respectively, whereas the corresponding values for HSV-2 strain 333 were 141.76 ± 4.10 μM and 225.30 ± 12.79 μM, respectively. In the absence of a pretreatment period with LS (Fig. 1D) or when Vero cells were pretreated with LS for 1 h at 37°C prior to viral infection (data not shown), no significant inactivation of both herpesvirus strains infectivities could be observed at concentrations up to 500 μM.
Time course effect.
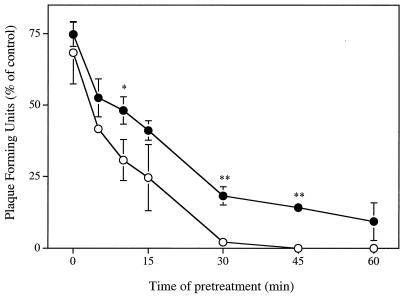
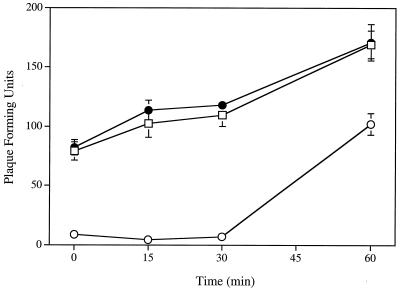
Figure 2 shows that the inactivating potencies of SLS and LS against HSV-2 strain 333 infectivity on Vero cells were directly related to the duration of the viral pretreatment period. At equipotent drug concentrations, the times of pretreatment required to inhibit 50 and 90% of viral infectivity were 3.27 ± 0.86 min and 23.10 ± 5.28 min when incubated with 50 μM SLS, respectively, whereas the corresponding values after an incubation of the virus with 250 μM LS were 7.32 ± 2.86 min and 55.41 ± 4.50 min (P < 0.01 compared with SLS), respectively.
FIG. 2.
Time course effect of SLS (○) and LS (•) on the infectivity of HSV-2 strain 333 on Vero cells. Confluent cells were inoculated with viruses pretreated with PBS (control) or with PBS containing 50 μM SLS or 250 μM LS for various periods of time (0 to 60 min). The plates were then immediately centrifuged (750 × g for 45 min at 20°C) to allow virus adsorption. Unbound viruses were removed by aspiration. Cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 48 h at 37°C. Infectivity was expressed as the percentage of PFU compared with that observed for control cells. Results are the means ± standard deviations (error bars) of three independent experiments.
Effect on binding and postbinding steps.
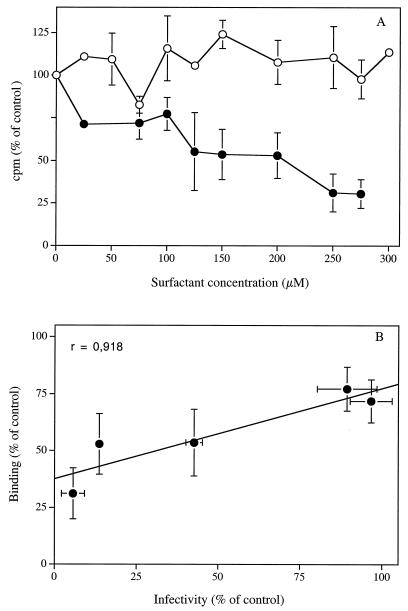
Figure 3A shows that pretreatment of [3H]methyl thymidine-labeled HSV-2 strain 333 with SLS concentrations up to 300 μM for 1 h at 37°C did not reduce its binding to Vero cells when assessed at 4°C. In contrast, pretreatment of the radiolabeled virus with LS for 1 h at 37°C decreased, in a concentration-dependent manner, the initial attachment of the virus to cell surface heparan sulfate. The 50% reduction of viral binding was observed at an LS concentration of 139.08 ± 34.10 μM, which corresponds with the ED50 obtained for the inhibition of viral infectivity (i.e., 141.76 ± 4.10 μM). We also obtained a correlation coefficient between infectivity and binding data of 0.918 (Fig. 3B), which suggests that elimination of the viral attachment step is probably the principal mode of inhibition of herpesvirus infectivities by LS.
FIG. 3.
(A) Effect of SLS (○) and LS (•) on the attachment of [3H]methyl thymidine-labeled HSV-2 strain 333 to Vero cells. Viruses were first pretreated with PBS plus 1% BSA (control) or with PBS-1% BSA containing increasing concentrations of SLS or LS for 1 h at 37°C. Confluent cells were then incubated with pretreated viruses for 1 h at 4°C. Cells were washed three times with PBS and lysed, and radioactivity associated with cell lysates was determined by liquid scintillation counting. Binding was expressed as the percentage of counts per minute in the control cell lysates. Results are the means ± standard deviations (error bars) of three independent experiments. (B) Correlation calculated between inhibition of viral infectivity (Fig. 1C) and inhibition of viral attachment (Fig. 3A) data obtained following pretreatment of HSV-2 strain 333 with increasing LS concentrations for 1 h at 37°C.
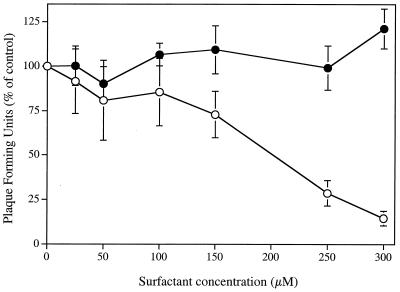
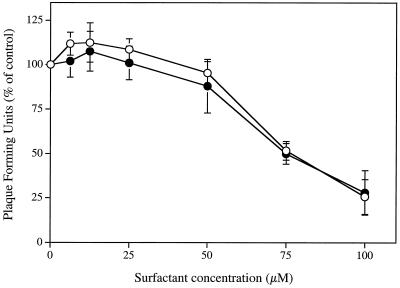
Figure 4 shows that SLS, added to cell cultures after the initial viral binding period at 4°C, was able to inhibit the infectivity of HSV-2 strain 333 stably attached to Vero cells. The ED50 and ED90 values were 204.71 ± 25.98 μM and 297.16 ± 24.66 μM, respectively. However, the concentrations of SLS required to inhibit the virus infectivity under these conditions were 3.5-fold higher than that needed to inhibit its infectivity in the absence of a viral pretreatment period (Fig. 1B). In contrast, LS concentrations up to 300 μM had no effect on viruses stably attached to cells. Treatment of HSV-2 strain 333 stably attached to cells with a low-pH citrate buffer (positive control) for 1 min at 4°C reduced to 1.87% ± 0.82% of control values the amount of virus which had penetrated into cells after the shift at 37°C (data not shown).
FIG. 4.
Effect of SLS (○) and LS (•) on HSV-2 strain 333 stably attached to Vero cells. Virus was first adsorbed to the confluent cell monolayer by a 2-h incubation period at 4°C. After removal of unbound viruses, cells were treated with ice-cold PBS (control) or with ice-cold PBS containing increasing concentrations of SLS or LS for 1 min at 4°C. Cells were washed once with ice-cold PBS, overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose, and incubated for 48 h at 37°C. Results were expressed as the percentage of PFU compared with that of control cells. Values are the means ± standard deviations (error bars) of three independent experiments.
Effect on rate of penetration into cells.
Figure 5 shows that SLS, at a concentration of 300 μM, decreases the rate of penetration of HSV-2 strain 333 in Vero cells. The inhibitory effect was more pronounced when the 1-min treatment period with SLS was made 15 or 30 min (approximately 90% inhibition) compared to 60 min (approximately 40% decrease) after the temperature shift at 37°C. In contrast, LS, at a concentration of 500 μM, had no effect on the rate of penetration of the virus into cells at any time after the temperature shift at 37°C.
FIG. 5.
Effect of SLS (○) and LS (•) on the rate of penetration of HSV-2 strain 333 in Vero cells. Virus was first adsorbed to the confluent cell monolayer after a 2-h incubation period at 4°C. After removal of unbound viruses, cells were shifted to 37°C to allow penetration into cells. At selected times after the temperature shift (0, 15, 30, and 60 min), cells were treated for 1 min with PBS (control [□]) or PBS containing 300 μM SLS or 500 μM LS prewarmed at 37°C. Cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 48 h at 37°C. Results were expressed as the number of PFU. Values are the means ± standard deviations (error bars) of three independent experiments.
Effect on virus-induced cytopathic effect.
Figure 6 shows that SLS and LS, added to Vero cells infected with HSV-2 strain 333 after a 2-h incubation period at 37°C, reduced in a concentration-dependent manner the virus-induced cytopathic effect. The concentrations of SLS required to reduce the virus-induced cytopathic effect by 50 and 90% were 77.61 ± 4.86 μM and 109.28 ± 20.42 μM, respectively, whereas the corresponding values for LS were 81.36 ± 11.26 μM and 114.81 ± 9.88 μM, respectively.
FIG. 6.
Effect of SLS (○) and LS (•) on HSV-2 strain 333-induced cytopathic effect on Vero cells. Virus was first adsorbed to confluent cell monolayers by a 2-h incubation period at 37°C. Unbound viruses were removed by aspiration. Cell sheets were then overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose (control) or with, in addition, increasing concentrations of SLS or LS and incubated for 48 h at 37°C. Results are expressed as the percentage of PFU compared with that of control cells. Values are the means ± standard deviations (error bars) of three independent experiments.
Inhibitory effect of combinations of surfactants.
Table 1 shows the inhibitory effects of various combinations of SLS and LS concentrations on the infectivity of HSV-2 strain 333 for Vero cells following a 1-h viral pretreatment period at 37°C. The inhibitory effects of combinations of both compounds on viral infectivity were analyzed by the isobologram method. Different effects were observed depending on the concentrations of surfactants mixed together. Combinations of SLS and LS acted synergistically to inactivate the virus infectivity when LS concentrations between 12.5 and 50 μM were added to increasing SLS concentrations. Combinations of LS and SLS acted synergistically when 12.5 μM SLS was added to increasing LS concentrations, whereas subsynergistic effects were obtained in the presence of 20 or 25 μM SLS.
TABLE 1.
Inhibitory effects of different combinations of SLS and LS concentrations on the infectivity of HSV-2 strain 333 on Vero cells after a 1-h pretreatment period at 37°C
| Compound(s) | Mean ED50 ± SD (μM)a | FICSLS + FICLSb | Inhibitory effect |
|---|---|---|---|
| SLS alone | 32.67 ± 2.18 | ||
| SLS + 12.50 μM LS | 10.34 ± 1.77 | 0.40 | Synergistic |
| SLS + 13.75 μM LS | 9.54 ± 1.42 | 0.39 | Synergistic |
| SLS + 15.00 μM LS | 3.12 ± 0.41 | 0.21 | Synergistic |
| SLS + 20.00 μM LS | 3.55 ± 0.28 | 0.25 | Synergistic |
| SLS + 25.00 μM LS | 3.50 ± 0.10 | 0.28 | Synergistic |
| SLS + 50.00 μM LS | 4.23 ± 0.25 | 0.48 | Synergistic |
| LS alone | 141.76 ± 4.10 | ||
| LS + 12.50 μM SLS | 3.32 ± 0.68 | 0.40 | Synergistic |
| LS + 20.00 μM SLS | 3.46 ± 0.70 | 0.75 | Subsynergistic |
| LS + 25.00 μM SLS | 2.73 ± 0.21 | 0.79 | Subsynergistic |
Results based on three independent experiments.
FICSLS and FICLS are FICs of SLS and LS, respectively.
Cellular viability.
Table 2 shows the effects of different concentrations of SLS or LS, alone or combined, on the viability of exponentially growing Vero cells following a 72-h incubation period. SLS and LS alone decreased, in a concentration-dependent manner, the viabilities of these cells. The 50% cytotoxic concentrations (CC50) of SLS and LS to this cell line were 202.04 ± 13.07 μM and 265.61 ± 10.74 μM, respectively. The CC50 of SLS and LS on resting Vero cell cultures incubated with either surfactant for 24 h were 266.30 ± 29.09 μM and 345.84 ± 11.79 μM, respectively (data not shown). Combinations of SLS and LS concentrations similar to those tested for their inhibitory effects on viral infectivity induced a significant decrease of the viability of exponentially growing cells compared with each compound alone.
TABLE 2.
Effects of SLS and LS, alone or combined, on the viability of exponentionally growing Vero cells after a 72-h incubation period
| Compound(s) | Mean CC50 ± SD (μM)a | Pb |
|---|---|---|
| SLS alone | 202.04 ± 13.07 | |
| SLS + 12.5 μM LS | 178.26 ± 8.65 | 0.0583 |
| SLS + 15.0 μM LS | 182.14 ± 0.18 | 0.0578 |
| SLS + 20.0 μM LS | 175.06 ± 7.65 | 0.0367 |
| SLS + 25.0 μM LS | 157.30 ± 3.13 | 0.0045 |
| SLS + 50.0 μM LS | 132.09 ± 21.84 | 0.0089 |
| LS alone | 265.61 ± 10.74 | |
| LS + 12.5 μM SLS | 130.44 ± 30.73 | 0.002 |
| LS + 20.0 μM SLS | 130.41 ± 20.13 | 0.0005 |
| LS + 25.0 μM SLS | 118.46 ± 23.21 | 0.0006 |
Results are based on three independent experiments.
Compared with the toxicity induced by the compound alone.
Inhibitory effect of SLS and LS in the presence of proteins.
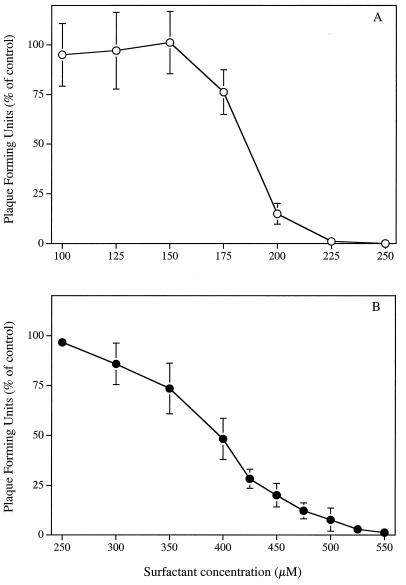
Figure 7 shows that the concentrations of SLS (Fig. 7A) and LS (Fig. 7B) required to inhibit the infectivity of HSV-2 strain 333 on Vero cells in complete culture medium (EMEM-5% FBS) were about 5.1 and 2.5 times higher, respectively, compared with viral pretreatment in PBS. The ED50 and ED90 values obtained following a viral pretreatment period of 1 h at 37°C with SLS in EMEM-5% FBS were 185.41 ± 3.52 μM and 207.63 ± 6.01 μM, respectively, whereas the corresponding values for LS were 384.86 ± 20.71 μM and 490.64 ± 23.08 μM.
FIG. 7.
Inhibitory effect of SLS (○) and LS (•) on HSV-2 strain 333 infectivity in complete culture medium (EMEM-5% FBS). Confluent Vero cells were inoculated with viruses pretreated with EMEM-5% FBS (control) or with EMEM-5% FBS containing increasing concentrations of SLS or LS for 1 h at 37°C. The plates were then immediately centrifuged (750 × g for 45 min at 20°C) to allow virus adsorption. Unbound viruses were removed by aspiration. Cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 48 h at 37°C. Infectivity was expressed as the percentage of PFU compared with that of control cells. Results are the means ± standard deviations (error bars) of three independent experiments.
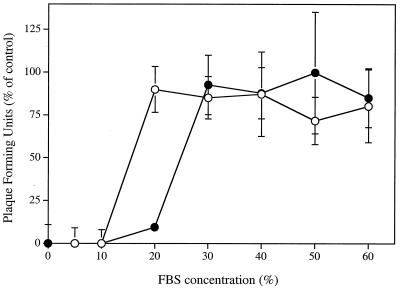
Figure 8 shows the effect of increasing FBS concentrations on the inhibitory potencies of SLS and LS on the infectivity of HSV-2 strain 333 on Vero cells. Pretreatment of HSV-2 strain 333 with 500 μM SLS for 1 h at 37°C inhibited viral infectivity at FBS concentrations of 10% and lower. About 90% of the viral infectivity was inhibited when the virus was pretreated with 1 mM LS in culture medium containing 20% FBS. At higher FBS concentrations, the viral infectivity was not affected by either surfactant treatment, probably because SLS and LS were bound to serum proteins.
FIG. 8.
Inhibitory effect of SLS (○) and LS (•) on HSV-2 strain 333 infectivity in the presence of increasing amounts of fetal bovine serum. Confluent cells were inoculated with viruses pretreated with EMEM containing increasing amounts of FBS (0 to 60%; control) or with, in addition, 500 μM SLS or 1 mM LS for 1 h at 37°C. The plates were immediately centrifuged (750 × g for 45 min at 20°C) to allow virus adsorption. Unbound viruses were removed by aspiration. Cell sheets were overlaid with EMEM-2% FBS containing 0.6% SeaPlaque agarose and incubated for 48 h at 37°C. Infectivity was expressed as the percentage of PFU compared with that of control cells. Results are the means ± standard deviations (error bars) of three independent experiments.
DISCUSSION
The development of safe and effective topical vaginal microbicides is a high priority for major health organizations. SLS and LS, two anionic surfactants with protein denaturant potency, are potential candidates for use as microbicides in topical vaginal formulations (reviewed in reference 29). In the present study, we have examined the mechanism(s) by which SLS and LS inactivate HSV infectivities in cultured cells. We have also evaluated the efficacies of several combinations of both surfactant concentrations to inhibit viral infectivity in relation to their effects on cell viability.
As we have previously shown (30, 34), SLS is a potent inhibitor of HSV-1 strain F and HSV-2 strain 333 infectivities for Vero cells. Howett et al. (15) have also reported that pretreatment of HSV-2 strain 333 with SLS at a concentration of 867 μM for 10 min at 37°C completely inactivated the infectivity of the virus for CV-1 cells. The difference between the doses of SLS required to completely inactivate the virus obtained by the two groups probably relates to the cell lines used (CV-1 versus Vero cells). Moreover, the use of complete culture medium containing 10% serum, compared to PBS in our studies, may decrease the surfactant availability due to the high affinity of SLS for the proteins present in the culture medium. In that respect, we showed that, under our experimental conditions, the use of complete culture medium (EMEM-5% FBS), rather than PBS, resulted in a fivefold-increased concentration of SLS required to inhibit viral infectivity.
LS, which is structurally close to SLS, is also a potent inactivator of HSV-1 strain F and HSV-2 strain 333 infectivities for Vero cells. The inhibitory potencies of SLS and LS against both strains of herpesviruses were directly related to the duration of the viral pretreatment period. In addition, no inactivation was observed when Vero cells were pretreated with either surfactant rather than the virus, suggesting that these compounds act mainly on viral particles. Compared with SLS, the concentration of LS required to completely inactivate the infectivity of HSV-2 strain 333 following a 1-h viral pretreatment period at 37°C was fivefold higher. In addition, at equipotent compound concentrations, LS completely inactivated viral infectivity following an eightfold-longer pretreatment period compared with SLS. The effects of molecules closely related to SLS or LS on the infectivities of herpesviruses have also been investigated. These data demonstrated that naturally occurring anionic surface-active agents present in bile salts, such as taurolithocholic acid-3-sulfate, alone or combined with glycocholic acid, were highly effective against HSV-1, HSV-2, HIV-1, C. trachomatis, and N. gonorrhoeae in vitro with little cytotoxicity (14).
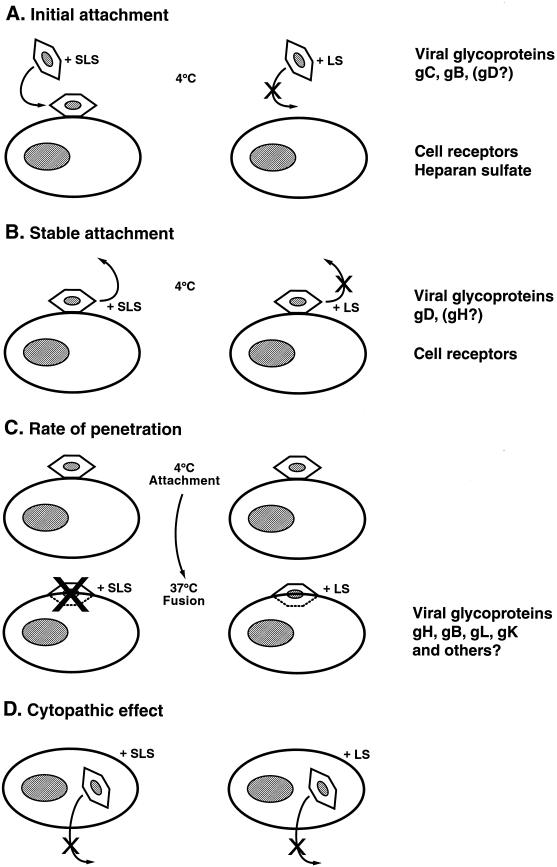
The different steps of HSV entry into cells are targets of choice for microbicides to prevent infection of susceptible cells. The attachment and penetration of HSV in target cells involve a three-step virus-cell interaction (24). These three stages correspond to (i) the initial attachment to cell surface heparan sulfate, which involves gC and gB (and possibly gD) and is resistant to PBS wash; (ii) the stable attachment to heparan sulfate or another unknown component which involves gD (and possibly gH) and is resistant to heparin wash; and (iii) the fusion followed by virus penetration which involves gH, gB, gL, and gK (and possibly others) and is resistant to low pH citrate buffer wash. Pretreatment of HSV-2 strain 333 with LS decreased the binding of [3H]methyl thymidine-labeled viral particles to Vero cells when assessed at 4°C. The good correlation between inhibition of both viral infectivity and binding to cell surface suggests that LS probably acts by abrogating the attachment step of the virus to target cells. In contrast, the viral attachment was not affected by SLS treatment. Previous studies from our laboratory showed that SLS did not prevent the binding of [35S]methionine-labeled HSV-1 strain F to Vero cells (30). In contrast, SLS, but not LS, added to cell cultures after the initial binding period at 4°C, inhibited the infectivity of HSV-2 strain 333 stably attached to cell surface. In addition, SLS decreased the rate of penetration of the virus into cells, probably by affecting the initial steps involved in the fusion process between the viral envelope and the cell surface. In contrast, the rate of penetration of the virus into cells was not affected following a treatment with LS. Both SLS and LS, when added after the infection of Vero cells, also inhibited the virus-induced cytopathic effect, probably by affecting newly synthesized virions that come into contact with the surfactant molecules present in culture medium. Based on the above demonstration, Fig. 9 summarizes our proposed hypothesis for the mechanisms of HSV inactivation by SLS and LS at the different steps of viral entry and infection of target cells.
FIG. 9.
Model of SLS- and LS-induced inhibition of herpesvirus infectivity in Vero cells. (A) Pretreatment of HSV-2 strain 333 with LS prevents the attachment of the virus to target cells at 4°C, whereas SLS has no effect. This step involves the binding of the viral gC and gB (and possibly gD) glycoproteins to cell surface heparan sulfate. (B) Treatment of herpesviruses stably attached to Vero cells at 4°C with SLS inhibits the infectivity of the virus, whereas LS exerts no effect. This step implicates the interaction between the viral gD (and possibly gH) glycoprotein to cell surface proteins. (C) SLS decreases the rate of penetration of HSV-2 strain 333 into cells, whereas LS does not interfere with the fusion process. This step involves several viral glycoproteins, such as gB, gH, gL, and gK (and possibly others). (D) Both SLS and LS inhibit the cytopathic effect induced by HSV-2 strain 333 in Vero cells. Both surfactants probably affect newly synthesized virions that come into contact with the compound present in culture medium.
The fact that the modes of herpesvirus inactivation by SLS and LS are different suggests that these surfactants affect different viral target proteins involved in the infectivity process. We have thus tested the efficacy of several combinations of SLS and LS concentrations to inhibit the infectivity of HSV-2 strain 333 on Vero cells following a 1-h viral pretreatment period at 37°C. Interestingly enough, some of these combinations acted synergistically to inactivate the infectivity of the virus. The improved efficacy of these combinations was accompanied by only a small increase in their toxicities for exponentially growing Vero cells, especially when fixed amounts of SLS were added to various LS concentrations. For instance, the addition of 15 μM LS to increasing SLS concentrations reduced by 10.5-fold the dose effective at inhibiting the infectivity of HSV-2 strain 333, whereas it only increases by 1.1 times the cytotoxicity following an incubation period of 72 h, suggesting that some combinations of both surfactant concentrations could be highly effective without causing a marked toxicity for the skin or mucosal surfaces.
Surfactants, like SLS and LS, first bind and then unfold and extract proteins before solubilizing membrane lipids. These effects greatly depend on the concentration of surfactants used and especially on their critical micellar concentrations. Kragh-Hansen et al. have reported that detergents with strongly hydrophilic heads like SLS only very slowly solubilize liposomal membranes (20). SLS specifically interacts with the protein component of Ca2+-ATPase membranes, leading to cooperative unfolding and extraction of the protein before solubilization of lipids. This effect results from combinations of hydrophobic and predominantly ionic interactions. LS, which is less hydrophilic than SLS, is thus a less effective solubilizing denaturant (8). Compared with the effect on proteins, the interaction of SLS with lipids is weaker and requires concentrations close to the critical micellar concentration. Using liposomes, it was shown that SLS extracts membrane lipids from the outer bilayer leaflet, which becomes partially depleted of phospholipids. Thereafter, a reorganization and a redistribution of phospholipid molecules from the inner to the outer leaflet occur. The vesicles slowly open up, leading to fragmentation and finally to solubilization into mixed micelles (20, 22). We thus propose that at the concentrations used here, which are well below the critical micellar concentration, SLS and LS probably inactivate HSVs by affecting envelope or capsid proteins (reviewed in reference 29). As suggested by the data presented in the present work, these proteins may play different roles in the viral replicative cycle (Fig. 9). Therefore, the use of these surfactants could represent a convenient tool to study the role of their target proteins in the pathogenesis of viral infection in cultured cells and in animal models.
We have demonstrated that SLS and LS are potent inhibitors of HIV-1 strain NL4-3 infectivity on 1G5 cells by eliminating the viral attachment and the fusion steps (4). In addition, SLS is a potent inactivator of the nonenveloped human papillomavirus infectivity and acts probably by denaturing capsid proteins (15). Of prime interest, the concentrations required to obtain a complete inactivation of HIV-1, HSV, and human papillomavirus infectivities are below toxic levels for cultured cell lines of different origins. Since the genital tract secretions are rich in proteins, we have evaluated the effect of increasing concentrations of FBS on the inhibitory potencies of SLS and LS against HSV-2 strain 333 infectivity for Vero cells. We showed that increasing the amount of proteins in the culture medium markedly decreased the inhibitory potencies of SLS and LS. However, by comparing the concentrations of surfactants required to completely inhibit the virus infectivity obtained at different concentrations of FBS, we estimated that approximately 50 μM SLS and 30 μM LS are neutralized by 1% FBS. Therefore, we may reasonably expect that under in vivo conditions, a gel formulation containing 2% SLS or LS (70 mM) could deliver sufficient concentrations of surfactants for inhibiting the infectivity of HSV and probably other pathogens.
We have previously demonstrated that gel formulations containing SLS or LS are effective in preventing the colonization of mouse genital mucosa by infectious viruses after intravaginal challenge with HSV-2 strain 333 (34). In addition, repeated administrations of these formulations were well tolerated by the vaginal mucosa in the rabbit susceptibility model. This observation is supported by the fact that toothpastes, mouthwashes, shampoos, and liquid soaps containing SLS, which are sold over the counter and used on a chronic basis are nontoxic for skin and/or mucosal surfaces. On the other hand, microorganism resistance to these compounds is unlikely to happen, contrary to resistance to more-specific targets such as antibodies. We thus believe that SLS and LS could be potential candidates for use as microbicides in topical vaginal formulations to prevent the transmission of HSV, HIV-1, and possibly other pathogens causing STDs. These active ingredient are cheap, and if proven effective in clinical trials, they will allow the production of topical vaginal microbicides affordable to people living in developing countries where HIV-1 epidemic is a major problem.
REFERENCES
- 1.Asculai, S. S., M. T. Weis, M. W. Rancourt, and A. B. Kupferberg. 1978. Inactivation of herpes simplex viruses by nonionic surfactants. Antimicrob. Agents Chemother. 13:686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfield, B. W., Y. Leduc, L. Esford, R. J. Visalli, C. R. Brandt, and F. Tufaro. 1995. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 208:531-539. [DOI] [PubMed] [Google Scholar]
- 3.Benes, S., and W. M. McCormack. 1985. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob. Agents Chemother. 27:724-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bestman-Smith, J., J. Piret, A. Désormeaux, R. F. Omar, M. J. Tremblay, and M. G. Bergeron. 2001. Sodium lauryl sulfate abrogates human immunodeficiency virus infectivity by affecting viral attachment step. Antimicrob. Agents Chemother. 45:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourinbaiar, A. S., and S. Lee-Huang. 1994. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9 and gossypol. Contraception 49:131-137. [DOI] [PubMed] [Google Scholar]
- 6.Brugha, R., K. Keersmaekers, A. Renton, and A. Meheus. 1997. Genital herpes infection: a review. Int. J. Epidemiol. 26:698-709. [DOI] [PubMed] [Google Scholar]
- 7.Buttke, T. M., J. A. McCubrey, and T. C. Owen. 1993. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphocyte-dependent cell lines. J. Immunol. Methods 157:223-240. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, J. A. A. 1993. Buffers, chelating agents and denaturants, p. 1-36. In J. A. A. Chambers and D. Rickwood (ed.), Biochemistry. Labfax. Academic Press, Inc., San Diego, Calif.
- 9.Elias, C. J., and L. L. Heise. 1994. Challenges for the development of female-controlled vaginal microbicides. AIDS 8:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Feldblum, P. J., C. S. Morrison, R. E. Roddy, and W. Cates. 1995. The effectiveness of barrier methods of contraception in preventing the spread of HIV. AIDS 9(Suppl A):S85-S93. [PubMed] [Google Scholar]
- 11.Feldblum, P. J., and S. S. Weir. 1994. The protective effect of nonoxynol-9 against HIV infection. Am. J. Public Health 84:1032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gessner, P. K. 1995. Isobolographic analysis of interactions: an update on applications and utility. Toxicology 105:161-179. [DOI] [PubMed] [Google Scholar]
- 13.Hermonat, P. L., R. W. Daniel, and K. V. Shah. 1992. The spermicide nonoxynol-9 does not inactivate papillomavirus. Sex. Transm. Dis. 19:203-205. [DOI] [PubMed] [Google Scholar]
- 14.Herold, B., R. Kirkpatrick, D. Marcellino, A. Travelstead, V. Pilipenko, H. Krasa, J. Breme, L. Dong, and M. Cooper. 1999. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob. Agents Chemother. 43:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howett, M. K., E. B. Neely, N. D. Christensen, B. Wigdahl, F. C. Krebs, D. Malamud, S. D. Patrick, M. D. Pickel, P. A. Welsh, C. A. Reed, M. G. Ward, L. R. Budgeon, and J. W. Kreider. 1999. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob. Agents Chemother. 43:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin, K., M. Scarlett, and R. Moseley. 1998. Observations from the CDC. The urgent need for new HIV/STD prevention options for women. J. Women's Health 7:1081-1086. [DOI] [PubMed] [Google Scholar]
- 17.Jennings, R., and A. Clegg. 1993. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in vitro. J. Antimicrob. Chemother. 32:71-82. [DOI] [PubMed] [Google Scholar]
- 18.Judson, F. N., J. M. Ehret, G. F. Bodin, M. J. Levin, and C. A. M. Rietmeijer. 1989. In vitro evaluations of condoms with and without nonoxynol-9 as physical and chemical barriers against Chlamydia trachomatis, herpes simplex virus type 2, and human immunodeficiency virus. Sex. Transm. Dis. 16:51-56. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff, S. J. 1992. Effects of the spermicidal agent nonoxynol-9 on vaginal microbial flora. J. Infect. Dis. 165:19-25. [DOI] [PubMed] [Google Scholar]
- 20.Kragh-Hansen, U., M. le Maire, and J. V. Moller. 1998. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys. J. 75:2932-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreiss, J., E. Ngugi, K. Holmes, J. Ndinya-Achola, P. Waiyaki, P. L. Roberts, I. Ruminjo, R. Sajabi, J. Kimata, T. R. Fleming, A. Anzala, D. Holton, and F. Plummer. 1992. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 268:477-482. [PubMed] [Google Scholar]
- 22.Lopez, O., M. Cocera, E. Wehrli, J. L. Parra, and A. de la Maza. 1999. Solubilization of liposomes by sodium dodecyl sulfate: New mechanism based on the direct formation of mixed micelles. Arch. Biochem. Biophys. 367:153-160. [DOI] [PubMed] [Google Scholar]
- 23.Malkovsky, M., A. Newell, and A. G. Dalgleish. 1988. Inactivation of HIV by nonoxynol-9. Lancet i:645. [DOI] [PubMed]
- 24.McClain, D. S., and A. O. Fuller. 1994. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology 198:690-702. [DOI] [PubMed] [Google Scholar]
- 25.Mertz, G. J., J. Benedetti, R. Ashley, S. A. Selke, and L. Corey. 1992. Risk factors for the sexual transmission of genital herpes. Ann. Intern. Med. 116:197-202. [DOI] [PubMed] [Google Scholar]
- 26.Mertz, G. J., R. W. Coombs, R. Ashley, J. Jourden, M. Remington, C. Winter, A. Fahlander, M. Guinan, H. Ducey, and L. Corey. 1988. Transmission of genital herps in couples with one symptomatic and one asymptomatic partner: a prospective study. J. Infect. Dis. 157:1169-1177. [DOI] [PubMed] [Google Scholar]
- 27.Mertz, G. J., O. Schimdt, J. L. Jourden, M. E. Guinan, M. L. Remongton, A. Fahlander, C. Winter, K. K. Holmes, and L. Corey. 1985. Frequency of acquisition of first episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex. Transm. Dis. 12:33-39. [DOI] [PubMed] [Google Scholar]
- 28.Niruthisard, S., R. E. Roddy, and S. Chutivongse. 1991. The effect of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 18:176-179. [DOI] [PubMed] [Google Scholar]
- 29.Piret, J., A. Désormeaux, and M. G. Bergeron. 2002. Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr. Drug Targets 3:17-30. [DOI] [PubMed] [Google Scholar]
- 30.Piret, J., J. Lamontagne, J. Bestman-Smith, S. Roy, P. Gourde, A. Désormeaux, R. F. Omar, J. Juhász, and M. G. Bergeron. 2000. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 38:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polsky, B., P. A. Baron, J. W. Gold, J. L. Smith, R. H. Jensen, and D. Armstrong. 1988. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet i:1456. [DOI] [PubMed]
- 32.Roddy, R. E., M. Cordero, C. Cordero, and J. A. Fortney. 1993. A dosing study of nonoxynol-9 and genital irritation. Int. J. STD AIDS 4:165-170. [DOI] [PubMed] [Google Scholar]
- 33.Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufé, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 339:504-510. [DOI] [PubMed] [Google Scholar]
- 34.Roy, S., P. Gourde, J. Piret, A. Désormeaux, J. Lamontagne, C. Haineault, R. F. Omar, and M. G. Bergeron. 2001. Thermoreversible gel formulations containing sodium lauryl sulfate and n-lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob. Agents Chemother. 45:1671-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short, R. V. 1994. Contraceptives of the future in the light of HIV infection. Aust. N. Z. J. Obstet. Gynaecol. 34:330-332. [DOI] [PubMed] [Google Scholar]
- 36.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 37.Stein, Z. A. 1990. HIV prevention: the need for methods women can use. Am. J. Public Health 80:460-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tallarida, R. J. 1992. Statistical analysis of drug combinations for synergism. Pain 49:93-97. [DOI] [PubMed] [Google Scholar]
- 39.Tallarida, R. J., F. Porreca, and A. Cowan. 1989. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 45:947-961. [DOI] [PubMed] [Google Scholar]
- 40.Wainberg, M. A. 1998. The need for vaginal microbicides with antiviral specificity. AIDS 12:4-6. [Google Scholar]
- 41.Whitley, R. 1993. Neonatal herpes simplex virus infections. J. Med. Virol. Suppl. 1:13-21. [DOI] [PubMed] [Google Scholar]