Abstract
Inhibitory effects of ethanolic extracts from 10 Chinese herbs on herpes simplex virus type 1 (HSV-1) replication were investigated. By a bioassay-guided fractionation procedure, samarangenin B (Sam B) was isolated from Limonium sinense; Sam B significantly suppressed HSV-1 multiplication in Vero cells without apparent cytotoxicity. Time-of-addition experiments suggested that the inhibitory action of Sam B on HSV-1 replication was not due to the blocking of virus adsorption. In an attempt to further localize the point in the HSV-1 replication cycle where arrest occurred, a set of key regulatory events leading to viral multiplication was examined, including viral immediate-early (α), early (β), and late (γ) gene expression and DNA replication. Results indicated that levels of glycoprotein B (gB), gC, gD, gG, and infected-cell protein 5 (ICP5) expression and gB mRNA expression in Vero cells were impeded by Sam B. Data from PCR showed that replication of HSV-1 DNA in Vero cells was arrested by Sam B. Furthermore, Sam B decreased DNA polymerase, ICP0, and ICP4 gene expression in Vero cells. Results of an electrophoretic mobility shift assay demonstrated that Sam B interrupted the formation of an α-trans-induction factor/C1/Oct-1/GARAT multiprotein complex. The mechanisms of antiviral action of Sam B seem to be mediated, at least in part, by inhibiting HSV-1 α gene expression, including expression of the ICP0 and ICP4 genes, by blocking β transcripts such as DNA polymerase mRNA, and by arresting HSV-1 DNA synthesis and structural protein expression in Vero cells. These results show that Sam B is an antiviral agent against HSV-1 replication.
Herpes simplex virus type 1 (HSV-1) is an enveloped DNA virus which causes a variety of infections in humans. After primary infection, HSV-1 establishes latency in sensory and autonomic neurons innervating the mucosal tissues, where primary infection takes place, and is reactivated by the proper stimulus to cause recurrence (35). The period of recurrence is irregular (20). Immunocompromised individuals and those with cancer are in danger of recurrent HSV-1 infections (20, 21, 27). The recipients of organ transplantation are at high risk for increased severity of HSV-1 infection (33). Infection with HSV-1 can lead to life-threatening encephalitis and ocular infections that result in corneal inflammation and scarification. This scarification is a major cause of blindness in developing countries (8). In addition, HSV-1 has been shown to be a factor for spreading human immunodeficiency virus and causes severe diseases in AIDS patients (5, 29).
One successful replication cycle of HSV-1 is dependent on the completion of a number of steps, including virion entry, subsequent expression of viral immediate-early (α) genes such as infected-cell protein 0 (ICP0) and ICP4 genes, early (β1 and β2) genes including DNA polymerase and thymidine kinase genes, and late (γ1 and γ2) genes encoding glycoprotein B (gB), ICP5, and gC, and unpaired DNA replication (35). The initial expression of HSV-1 α genes depends on the binding of the α trans-induction factor (αTIF)/C1/Oct-1 multiprotein complex to the TAAGARAT (R, purine; GARAT) sequences of the cis-acting site (31). Inhibition of any of these stages blocks HSV-1 replication. Nucleoside analogues have been extensively investigated in the search for effective antiherpesvirus agents (9). Among these acyclovir is widely used for the systemic treatment of HSV infections. It is a highly selective antiviral agent because it is specifically phosphorylated by viral thymidine kinase in infected cells (10, 13). However, acyclovir-resistant HSV infection in immunocompromised patients such as transplant patients and patients with AIDS has recently been observed (6, 19). Therefore, it is desirable to develop new anti-HSV agents that substitute for or complement acyclovir.
Chinese herbs are potential sources of useful edible and medicinal plants. They are expected to find use as functional foods because of their various biological activities such as immunomodulatory and antitumor functions (22, 23). More and more people in developing countries utilize traditional medicine for their major primary health care needs (11, 17). However, ethnopharmacology also provides scientists with an alternative approach for the discovery of antiviral agents. The polysaccharides (30), anthraquinones (39), triterpenes (38), phloroglucinol (2), flavonoids (26), and catechin derivatives (12) isolated from medicinal plants have been found to have inhibitory activities against the replication of HSV-1. There has been a promising result for a naturally occurring antiherpetic agent, n-docosanol, which has recently completed extensive clinical evaluation and been approved by the U.S. Food and Drug Administration as a topical treatment for herpes labialis (1, 34, 36). These findings show that natural products are still potential sources in the search for new antiherpetic agents.
In the present study, 10 Chinese herbs which are widely known in folk medicine for the treatment of viral and bacterial infection were selected for an anti-HSV-1 replication assay. The herbs were Ventilago leiocarpa, Ecdysanthera rosea, Ecdysanthera utilis, Hippobroma longiflora, Ardrisia brevicaulis, Selaginella delicatula, Limonium sinense, Ardrisia japonica, Ardrisia violacea, and Andendron benthamianum. The ethanolic extracts that showed appreciable anti-HSV-1 activity were separated by a bioassay-guided fractionation procedure. The effect of active component samarangenin B (Sam B), isolated from L. sinense, on HSV-1 α, β, and γ gene expression and DNA replication in Vero cells was evaluated. The mechanisms of antiviral action of Sam B were elucidated in vitro.
MATERIALS AND METHODS
Preparation of crude extracts for Chinese herbs.
All 10 species of Chinese herbs were purchased from Chinese medicine shops in Taipei, Taiwan, and were identified by Lie-Chwen Lin. Each dried Chinese herb (600 g) was extracted with ethanol (three times in 5 liters). After solvent was removed, the crude extracts were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 100 mg/ml and stored at 4°C until use.
Sam B isolation from L. sinense.
The method followed procedures described previously (26). The ethanolic extract of the dried roots of L. sinense was partitioned in succession between H2O and n-hexane, followed by ethyl acetate and n-butanol (n-BuOH). The bioactive n-BuOH fraction was subjected to chromatography on Sephadex LH-20 with gradient methanol-H2O elution (from 0 to 100%) to produce 12 fractions. Sam B was identified from fraction 12 and analyzed with a high-performance liquid chromatography (HPLC) purity program. It was dissolved in DMSO to a concentration of 100 mM and stored at 4°C until use.
Cell culture and viruses.
Vero cells were cultured in minimal essential medium (MEM; GIBCO, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, Utah), 100 U of penicillin/ml, and 100 μg of streptomycin/ml and incubated at 37°C in a 5% CO2 incubator. The cells were free from mycoplasma contamination, which was checked by Mycotect kit (Life Technology, Gaithersburg, Md.). To prepare HSV-1 (KOS strain) stocks, Vero cells were infected by HSV-1 at a multiplicity of infection (MOI) of 3 and harvested at 24 h postinfection (p.i.) and centrifuged at 1,500 × g (CR312; Jouan, Nantes, France) at 4°C for 20 min. The supernatant was collected and stored at −70°C for use.
Plaque reduction assay.
The assay followed procedures described previously (24). Acyclovir was used as a positive control. Vero cells (3.5 × 105/dish) were incubated with 100 PFU of HSV-1, and the test extracts or acyclovir was added to the cells at various concentrations. The viruses were adsorbed for 1 h at 37°C, and 1% methylcellulose was added to each well. After 5 days, the virus plaques formed in Vero cells were counted by crystal violet staining. The activities of test extracts and acyclovir for inhibition of plaque formation were calculated.
Determination of cell viability.
Approximately 3.5 × 105 Vero cells were cultured in a 25-cm2 flask and incubated with 0.1% DMSO or 25 μM Sam B for 5 days. Total, viable, and nonviable cells were counted three times under the microscope with the help of a hemocytometer following staining by trypan blue. The percentage of viable cells was calculated. The cell viability was also evaluated as lactate dehydrogenase (LDH) release according to the manufacturer's instructions (Roche, Milan, Italy). LDH activity in milliunits per milliliter, where 1 mU is the amount of enzyme required to transform 0.0167 nM NAD per min, was determined.
Effect of Sam B on Vero cell growth.
The method was modified from that previously described (22). For the analysis of total cellular protein synthesis, Vero cells were preincubated with leucine-free MEM for 1 day and then cultured in MEM containing 2% fetal calf serum. The cells (5 × 103/well) were incubated with or without Sam B at various concentrations for 5 days. Then, for detection of DNA, RNA, and protein synthesis in Vero cells, tritiated thymidine, tritiated uridine, or tritiated leucine was added into each well (1 μCi/well; NEN), respectively. After a 16-h incubation, the cells were harvested on glass fiber filters by an automatic harvester (Multimash 2000; Dynatech, Billingshurst, United Kingdom). Radioactivity in the filters was measured by scintillation counting.
Extraction of total cellular DNA and RNA.
Cellular DNA and RNA were extracted from Vero cells by the method described previously (25, 24). Vero cells (5 × 106) were infected with HSV-1 at an MOI of 3 or were not infected in the presence or absence of Sam B (25 μM) and harvested at various times. For DNA extraction, the cells were lysed with 0.2 M Tris-HCl (pH 8.5) containing 100 mM EDTA, 100 mM NaCl, 0.5% NP-40, 1% sodium dodecyl sulfate (SDS), and 100 μg of proteinase K/ml. To extract cellular RNA, Vero cells were washed by cold Tris-saline (pH 7.4) and then suspended in NDD buffer (1% NP-40, 0.5% sodium deoxycholate, 1% dextran sulfate). The DNA and RNA solutions were extracted with phenol-chloroform and precipitated with 100% cold ethanol, and their concentrations were determined by measuring the optical density at 260 nm.
Synthesis of first-strand cDNA.
The method followed procedures described previously (24). Aliquots of 1 μg of RNA were reverse transcribed with the Advantage RT-for-PCR kit from Clontech according to the manufacturer's instructions. The reaction mixture was initially incubated at 42°C for 1 h and then at 94°C for 5 min to terminate the reaction. Diethyl pyrocarbonate-treated H2O (80 μl) was added to the tube, and the tube was then stored at −20°C for use in the PCR.
PCR.
The method has been described elsewhere (24, 37). Briefly, 10 μl of cDNA or total cellular DNA was mixed with 0.75 μM primers, 4 U of Taq polymerase, 10 μl of reaction buffer (2 mM Tris-HCl [pH 8.0], 0.01 mM EDTA, 0.1 mM dithiothreitol, 0.1% Triton X-100, 5% glycerol, 1.5 mM MgCl2), and 25 μl of water in a total volume of 50 μl. The oligonucleotide primer pairs were as follows: for gB (amplification fragment, 341 bp), 5′-CTGGTCAGCTTTCGGTACGA-3′ and 3′-CGTTTGTCGACGTGCTGGAC-5′; for HSV-1 DNA polymerase (600 bp), 5′-CGCACCATCTACGACGGCCAGC-3′ and 3′-CGCTTTCGTCTAGGCGAGCGCC-5′; for ICP0 (157 bp), 5′-TTCGGTCTCCGCCTGAGAGT-3′ and 3′-AGCATACGCCGACCTCCCAG-5′; for ICP4 (670 bp), 5′-CCCGCCGATGCTGCCCTAAAC-3′ and 3′-TTCGCCAGACACCTACTCAAG-5′; for β-actin (1,300 bp), 5′-TTGAGACCTTCAACACCC-3′ and 3′-CTCTACTGAAGCTTTTCGACT (7). The PCR was done at the following settings of the air thermocycler: denaturing temperature of 94°C for 1 min, annealing temperature of 53°C for 1 min, and elongation temperatures of 72°C for 2 min for the first 25 cycles and then 72°C for 10 min. The amplified products were quantitated with laser scanning densitometer SLR-2D/1D (Biomed Instruments Inc., Fullerton, Calif.).
Northern blot analysis.
The analysis was modified from that previously described (24). The HSV-1-infected (MOI, 3) Vero cells (5 × 106) were cultured with or without Sam B (25 μM) for 6 h. A 15-μg sample of total cellular RNA was resolved on a 6.66% formaldehyde-agarose gel and then transferred to a nitrocellulose filter (Schleicher & Schuell) with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The filter was prehybridized at 42°C for 4 h (prehybridization solution of 0.1% sodium pyrophosphate, 0.25 mg of denatured salmon sperm DNA/ml, and 50% deionized formamide). A digoxigenin-dUTP (Boehringer GmbH, Mannheim, Germany)-labeled DNA polymerase probe prepared by PCR was added to the same solution and incubated at 42°C for 12 h. The filter was washed with 0.1× SSC, detected by enzyme immunoassay using an antibody conjugated to alkaline phosphatase (Boehringer GmbH), and visualized by the chemiluminescence recorded on X-ray film (Kodak).
Western blot analysis.
The experiment followed procedures described previously (24). The 5 × 106 Vero cells were infected with HSV-1 at an MOI of 3 or were not infected in the presence or absence of Sam B (25 μM) for 16 h. Extracted cellular proteins (20 μg) were dissolved in the dissociation buffer (2% SDS, 5% β-mercaptoethanol, 0.05 M Tris-HCl, 20% glycerol, pH 7.6) and resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE) then transferred to nitrocellulose filters. After filters were blocked, they were incubated with mouse monoclonal antibodies (Virusys Corporation, East Coast Biologics, Inc., North Berwick, Maine) raised against HSV-1 γ proteins (gB, gC, gD, gG, or ICP5) and α proteins (ICP0 or ICP4). Specific reactive proteins were detected by an enhanced chemiluminescence method employing a rabbit anti-mouse immunoglobulin antibody linked to horseradish peroxidase (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.).
Electrophoretic mobility shift assay (EMSA).
Oligonucleotide sequences 5′-GATCCCGTGCATGCTAATGATATTCTTTGGG-3′ and 3′-GGCACGTACGATTACTATAAGAAACCCCTAG-5′, corresponding to consensus αTIF/C1/Oct-1 site (nucleotides −170 to −143) of the ICP0 gene were used as a probe. Nuclear extracts of HSV-1-infected Vero cells were prepared 5 h after infecting cells at an MOI of 20. Reaction mixtures (15 μl) contained, together with 3 μg of nuclear extracts, 5 × 104 dpm of 32P-end-labeled probe, 2 μg of poly(dI-dC), 5% glycerol, 1 mM EDTA, 100 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, and 10 mM Tris-HCl, pH 7.4. After incubation for 30 min at room temperature, reaction mixtures were applied to 4% polyacrylamide gels in 0.5% Tris-borate-EDTA buffer. When the effect of Sam B on the binding of the αTIF/C1/OTF-1 multiple-protein complex to the probes was determined, nuclear extracts from HSV-1-infected Vero cells were treated with 25 μM Sam B for 5 min prior to the addition of the probes.
Statistical analysis.
Data were presented as means ± standard deviations, and the differences between groups were assessed with Student's t test.
RESULTS
Effects of ethanolic extracts from 10 Chinese herbs on HSV-1 replication.
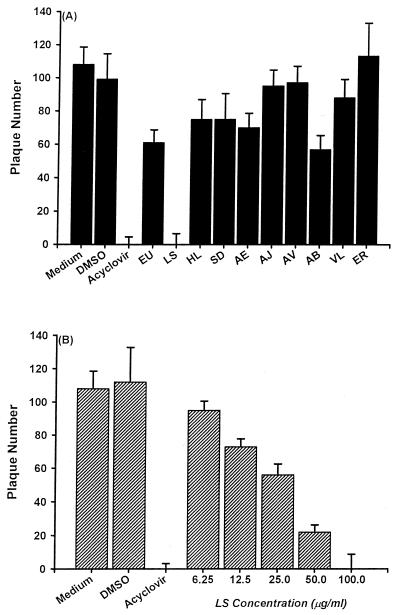
As shown in Fig. 1A, ethanolic extracts isolated from 10 Chinese herbs were evaluated for their activities in inhibiting HSV-1 plaque formation in Vero cells. HSV-1 replication was not affected by DMSO treatment. Acyclovir blocked HSV-1 plaque formation in Vero cells. While ethanolic extracts from Ventilago leiocarpa, Ecdysanthera rosea, Ecdysanthera utilis, Hippobroma longiflora, Ardrisia brevicaulis, Selaginella delicatula, Ardrisia japonica, Ardrisia violacea, and Andendron benthamianum had little effect on HSV-1 replication, 100 μg of L. sinense ethanolic extracts/ml significantly suppressed HSV-1 replication. The inhibitory action of L. sinense ethanolic extracts on HSV-1 replication was concentration dependent (Fig. 1B). The 50% inhibitory concentration (IC50) of L. sinense ethanolic extracts for HSV-1 replication was 25.0 ± 8.7 μg/ml.
FIG. 1.
Effects of ethanolic extracts from 10 Chinese herbs on HSV-1 replication in Vero cells determined by plaque reduction assay. Vero cells (3.5 × 105/dish) were incubated with 100 PFU of HSV-1 and 10 μM acyclovir, 100 μg of each extract/ml (A), or the indicated concentrations of L. sinense extracts (B). After adsorption for 1 h, 1% methylcellulose was added to each well. On the fifth day p.i., the virus plaques formed in Vero cells were counted by crystal violet staining. Each bar represents the mean of three independent experiments. VL, Ventilago leiocarpa, ER, Ecdysanthera rosea, EU, Ecdysanthera utilis, HL, Hippobroma longiflora, AB, Ardrisia brevicaulis, SD, Selaginella delicatula, LS, L. sinense, AJ, Ardrisia japonica, AV, Ardrisia violacea, AE, Andendron benthamianum.
Sam B identified from L. sinense inhibits HSV-1 replication.
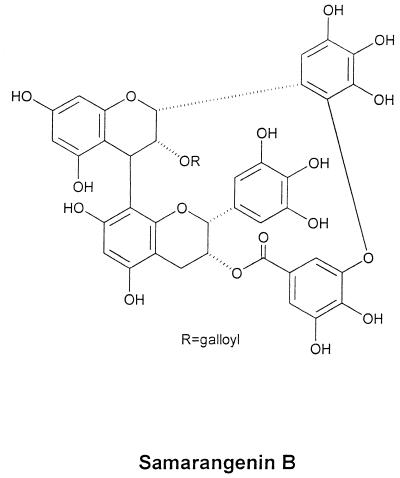
To extract pure active compounds from L. sinense, we used the complete isolation process in each chromatographic cycle and finally HPLC. The compound with the strongest activity was a brown amorphous powder. Nuclear magnetic resonance (NMR) and mass spectrum analyses indicated the structure shown in Fig. 2. The chemical name of this bioactive component is galloyl-epigallocatechin ([4β→8, 2′→3"-O]-galloyl-epigallocatechin; C44H31O22; molecular weight, 911). Both mass and NMR spectrum data for the compound were compatible with those previously reported for Sam B by Nonaka et al. (32). The common name given to this component at that time was Sam B. The purity of Sam B was assessed by an HPLC purity program (column 5C18-MS). The column was eluted with a 35:65 (vol/vol) mixture of methanol and 0.1% H3PO4 and analyzed by a UV detector at 254 nm. Sam B appeared as a single peak at a 3.973-min retention time, and its purity was 99.0%.
FIG. 2.
The structure of Sam B purified from L. sinense.
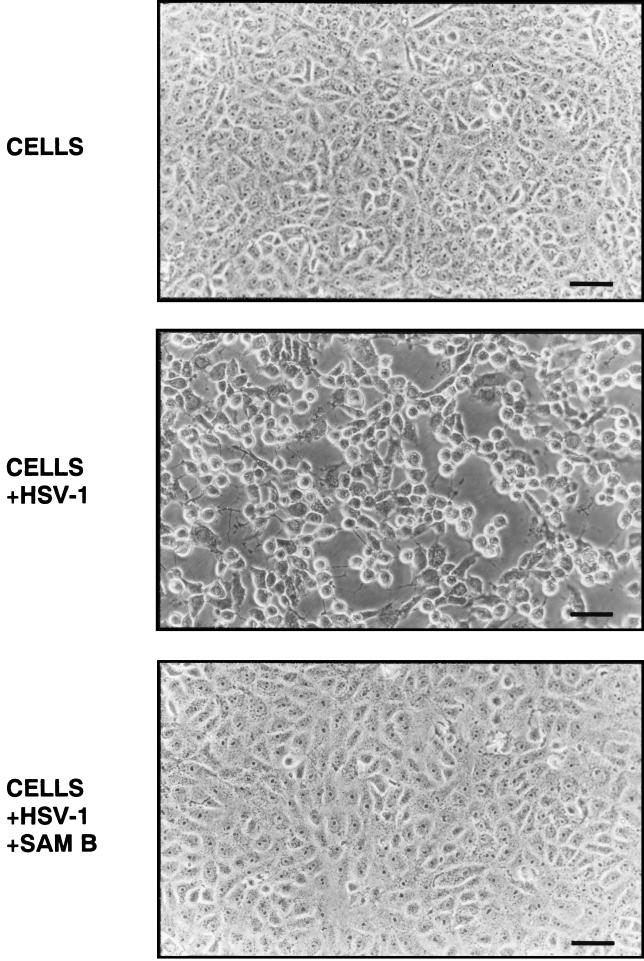
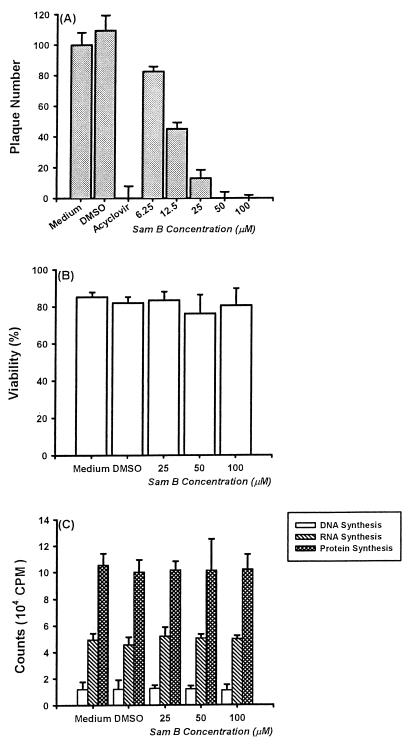
As shown in Fig. 3, Sam B blocked virus amplification, and thus its presence resulted in the formation of a cytopathic effect significant lower than that in control infections. The results of plaque reduction assay indicated that the inhibitory effect of Sam B on HSV-1 replication was concentration dependent. At 6.25 μM, Sam B suppressed HSV-1 replication by 24.6% ± 4.2%. The corresponding degrees of inhibition for 12.5, 25, 50, and 100 μM were 58.3 ± 3.0, 88.5 ± 7.3, 100 ± 5.2, and 100% ± 2.2%, respectively, with an IC50 value of 11.4 ± 0.9 μM (Fig. 4A).
FIG. 3.
Cytopathic effect of HSV-1 on Vero cells treated with or without Sam B. HSV-1-infected (MOI, 3) and uninfected Vero cells were treated with or without Sam B (25 μM). At 22 h p.i., cells were examined by microscopy (magnification, ×180). Bar, 50 μm.
FIG. 4.
Effects of Sam B on HSV-1 replication and Vero cell viability and growth. (A) Inhibitory effects of 10 μM acyclovir and indicated concentration of Sam B on HSV-1 replication were determined by plaque reduction assay. Each bar represents the mean of three independent experiments. (B) Vero cells (3.5 × 105 in 25-cm2 flasks) were treated with medium, 0.1% DMSO, or 25, 50, or 100 μM Sam B for 5 days. Then total, viable, and nonviable cells were counted after staining with trypan blue. Each bar represents the mean of three independent experiments. (C) Vero cells (5 × 103/well of a 96-well plate) were treated with Sam B at the indicated concentrations or were treated without Sam B for 5 days. Then tritiated thymidine, tritiated uridine, or tritiated leucine was added to each well (1 μCi/well) to detect cell growth. After a 16-h incubation, the cells were harvested by an automatic harvester and then radioactivity was measured by scintillation counting. Each bar represents the mean of three independent experiments.
Effects of Sam B on viability and growth of Vero cells.
To delineate whether the suppressive effect of Sam B on HSV-1 replication was related to cytotoxicity, we examined the viability of Vero cells after treatment with Sam B for 5 days. DMSO did not affect cell viability. Compared with that of control groups, the viability of Vero cells treated with 25, 50, and 100 μM Sam B was not significantly decreased (Fig. 4B). Moreover, an evaluation of the cytotoxic effect of Sam B on Vero cells in terms of LDH release indicated that LDH release from Vero cells in the presence of 100 μM Sam B for 5 days was not significantly different from that in the absence of Sam B (3.23 ± 2.2 versus 4.05 ± 1.7 mU/ml). Results indicated that, even at 200 μM, Sam B had no direct cytotoxicity on Vero cells (data not shown). The therapeutic index (50% effective concentration/IC50) for Sam B is higher than 18. Additionally, effects of Sam B on Vero cell growth were determined by tritiated thymidine, tritiated uridine, and tritiated leucine uptake methods. As shown in Fig. 4C, Sam B did not affect DNA, RNA, and protein synthesis in Vero cells. These results demonstrated that inhibitory mechanisms of Sam B on HSV-1 replication were not due to cytotoxicity and did not arrest Vero cell growth.
Effect of Sam B on HSV-1 adsorption.
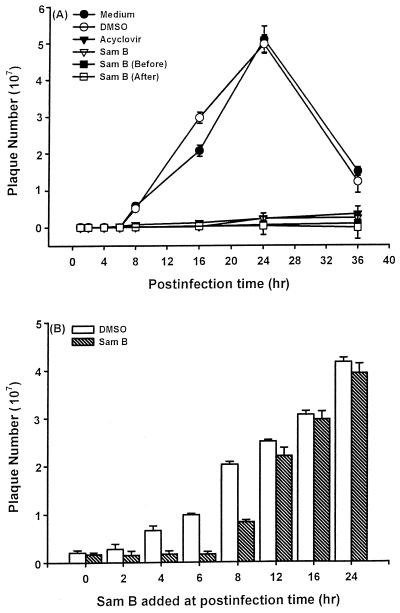
To further elucidate whether Sam B inhibition of HSV-1 replication was related to blocking viral adsorption, we examined the effect of its addition at various times. Cell supernatants were collected at 0, 2, 4, 6, 8, 16, 24, and 36 h p.i., and HSV-1 titers were determined by a plaque forming assay. As shown in Fig. 5A, the virus titers in cell supernatants gradually increased at 8 h p.i. and the highest titer was obtained at 24 h p.i. DMSO did not affect HSV-1 amplification. By contrast, whether 25 μM Sam B was added at the same time as HSV-1 or after HSV-1 adsorption for 1 h, the virus titers in cell supernatants were decreased. The same results were obtained in acyclovir-treated cells. In addition, the pretreatment of Vero cells with 25 μM Sam B for 2.5 h before infection still inhibited virus yield. These results demonstrated that Sam B did not affect HSV-1 adsorption to host cells.
FIG. 5.
Kinetics of inhibition of HSV-1 replication by Sam B. (A) Vero cells (5 × 106) were infected with HSV-1 at an MOI of 3 in the presence (▾) or absence of acyclovir (10 μM) or Sam B (25 μM). Treatments were as follows: Sam B was added at the same time as HSV-1 (▿), Sam B was present after the adsorption period (□), and Sam B was present for 2.5 h and removed before the adsorption phase by washing the cells with medium three times (▪). Then cell supernatants were collected at 0, 2, 4, 6, 8, 16, 24, and 36 h p.i. and the viral titers were determined by a plaque forming assay. Each point represents the mean of three independent experiments. (B) Vero cells (5 × 106) were infected with HSV-1 (MOI, 3), and Sam B (25 μM) was added at the indicated times. Cell supernatants were collected at 25 h p.i., and HSV-1 titers were determined as described in Materials and Methods. Each bar represents the mean of three independent experiments.
Time course analysis of the effect of Sam B on HSV-1 replication.
Time course experiments were performed to determine at what point in the replication process Sam B inhibited HSV-1 replication. Sam B (25 μM) was added at the same time as HSV-1 (0 h p.i.) or at 2, 4, 6, 8, 12, 16, and 24 h p.i. The cell supernatants were collected at 25 h p.i., and a plaque forming assay was performed. The results indicated that addition of Sam B between 0 and 8 h suppressed HSV-1 replication (Fig. 5B). Addition of Sam B at 12 to 24 h p.i. had only minimal inhibitory effects on HSV-1 replication. The fact that Sam B was inhibitory when added between 0 and 8 hr p.i. suggested that the suppressive effects of Sam B might be related to the blocking of biochemical events or gene expression such as that of the α, β, and γ genes, which are necessary for HSV-1 replication during this time frame.
Effects of Sam B on HSV-1 γ gene expression in Vero cells.
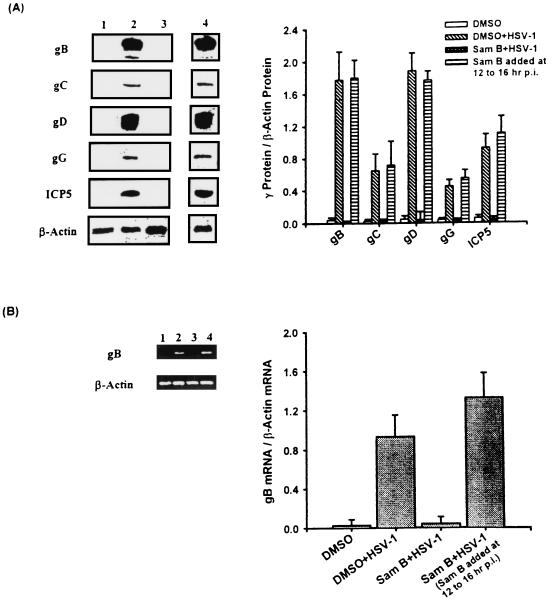
After adsorption, HSV-1 expresses γ genes about at 6 to 8 h p.i. or even earlier. The genes are needed for packaging viral particles (35). We analyzed whether Sam B suppression of HSV-1 replication was related to blocking the synthesis of γ proteins in Vero cells. After cells were treated with 25 μM Sam B for 16 h, the total cellular proteins were extracted and the expression of viral γ proteins including gB, gC, gD, gG, and ICP5 in Vero cells was determined by Western blot analysis. As shown in Fig. 6A, while uninfected cells expressed few of these γ proteins (lane 1), the gB (116 kDa), gC (78 kDa), gD (55 kDa), gG (45 kDa), and ICP5 (155 kDa) proteins were detectable in HSV-1-infected Vero cells (lane 2). However, Sam B suppressed the expression of these γ proteins in Vero cells (lane 3). When Sam B was added at 12 h to 16 h p.i., the inhibitory action of Sam B was interrupted and the expression of γ proteins in Vero cells was restored (lane 4). A graphical representation of the ratio of γ proteins to β-actin in Vero cells indicated that little signal was expressed in the cells treated with HSV-1 and Sam B at the same time.
FIG. 6.
Effects of Sam B on HSV-1 γ gene expression in Vero cells detected by Western blot analysis and RT-PCR. Vero cells (5 × 106) were infected with HSV-1 (MOI, 3) or were not infected in the presence or absence of Sam B (25 μM). (A) Lysates (20 μg of protein) were collected at 16 h p.i. and run on an SDS-10% PAGE gel and analyzed by immunoblotting with an anti-gB, -gC, -gD, -gG, or -ICP5 antibody. (B) The total cellular RNA was isolated from Vero cells at 16 h p.i. RT-PCR was done as described in Materials and Methods. Following the reaction, the amplified product was run on a 2% agarose gel. Lane 1, uninfected Vero cells; lane 2, HSV-1-infected cells; lane 3, cells treated with HSV-1 and Sam B at the same time; lane 4, infected cells treated with Sam B at 12 h to 16 h p.i. The graph indicates the ratio of γ protein or gB mRNA to β-actin mRNA. Each bar represents the mean of three independent experiments.
Because production of gB proteins in Vero cells was attenuated, we determined whether gB mRNA expression in the cells was affected by Sam B. At 16 h p.i., the total cellular RNA was collected from Vero cells in the presence or absence of Sam B (25 μM) and was available for reverse transcription-PCR (RT-PCR). Initially, we examined the dose-response relationship of the PCR amplification of cDNA (data not shown). The exponential phase of amplification was determined by performing 20, 25, and 30 cycles. We found that 25 cycles of PCR were optimal for the virion and β-actin mRNAs. As shown in Fig. 6B, neither DMSO (lanes 1 and 2) nor Sam B (lanes 3 and 4) affected the levels of β-actin mRNA in Vero cells. While little expression of gB mRNA was detectable in uninfected Vero cells (lane 1), the levels of gB mRNA were significantly increased in HSV-1-infected cells (lane 2). By contrast, the level of RT-PCR products for gB mRNA amplified from HSV-1-infected Vero cells was decreased by Sam B (lane 3). Laser densitometry analysis demonstrated that the ratio of gB to β-actin mRNAs was significantly decreased by Sam B. The inhibition of gB mRNA expression due to Sam B activity was blocked when Sam B was added in Vero cells between 12 and 16 h p.i. (lane 4). These results suggested that the decrease of gB proteins caused by Sam B was the result of a deficiency of gB mRNA expression in Vero cells.
Effect of Sam B on HSV-1 DNA synthesis.
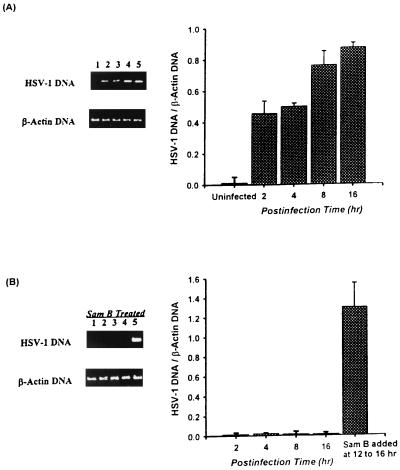
Because HSV-1 γ2 genes such as the gC gene are dependent on viral DNA synthesis for expression and because DNA synthesis plays important roles in HSV-1 replication (35), we further defined whether Sam B has any effect on HSV-1 DNA synthesis in Vero cells. After HSV-1 adsorption, the cellular DNA was harvested at 2, 4, 8, and 16 h p.i. and viral DNA was analyzed by PCR. As shown in Fig. 7A, although HSV-1 DNA could not be detected in uninfected Vero cells (lane 1), HSV-1 DNA synthesis was increased in HSV-1-infected cells (lanes 2 to 5). Graphical representation of the ratio of HSV-1 DNA to β-actin DNA showed that increasing the signal in HSV-1-infected cells corresponded to increasing the p.i. time. By contrast, 25 μM Sam B suppressed viral DNA synthesis in Vero cells (Fig. 7B, lanes 1 to 4). Additionally, HSV-1 DNA synthesis in Vero cells was restored when Sam B was added to cells at 12 h to 16 h p.i. (lane 5). These results suggested that the blocking of gC protein expression and HSV-1 replication by Sam B was related to impairment of HSV-1 DNA synthesis in Vero cells.
FIG. 7.
Effect of Sam B on HSV-1 DNA synthesis in Vero cells detected by PCR. Cells (5 × 106) were infected with HSV-1 (MOI, 3) or were not infected in the presence or absence of Sam B (25 μM). The cells were harvested at 2, 4, 8, and 16 h p.i., and total DNA was extracted with phenol-chloroform. The PCR was done as described in Materials and Methods. Following the reaction, the amplified product was run on a 2% agarose gel. (A) DNA was extracted from uninfected Vero cells at 16 h p.i. (lane 1) or from HSV-1-infected cells at 2, 4, 8, and 16 h p.i. (lanes 2 to 5). (B) Sam B and HSV-1 were added to the cells at the same time, and the total cellular DNA was extracted at 2 (lane1), 4 (lane 2), 8 (lane 3), and 16 h (lane 4) p.i. Lane 5, total cellular DNA extracted from infected cells treated with Sam B at 12 to 16 h p.i. Each band was quantitated by densitometer, and the ratio of HSV-1 DNA to β-actin DNA was calculated. Each bar represents the mean of three independent experiments.
DNA polymerase mRNA expression in Sam B-treated Vero cells detected by Northern blot analysis.
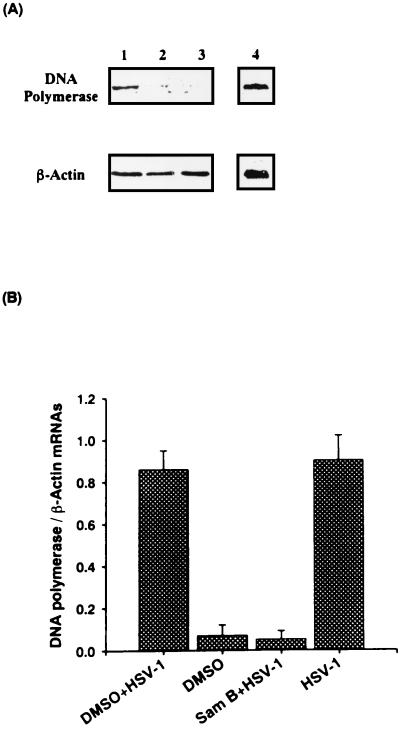
The appearance of HSV-1 β gene products such as DNA polymerase signals the onset of viral DNA synthesis (35). To elucidate whether Sam B-suppressed HSV-1 DNA synthesis was related to DNA polymerase gene expression, the cells were infected with HSV-1 or were not infected in the presence or absence of Sam B (25 μM) for 6 h and then DNA polymerase mRNA was determined by Northern blot analysis. As shown in Fig. 8A, 3.8 kb of DNA polymerase mRNA was expressed in HSV-1-infected Vero cells (lane 4) but was not expressed in uninfected cells (lane 2). DMSO did not affect DNA polymerase mRNA expression in Vero cells (lane 1). The level of DNA polymerase mRNA was impeded by Sam B treatment (lane 3). However, neither DMSO nor Sam B affected β-actin mRNA expression in Vero cells. The ratio of the absorbance value for DNA polymerase mRNA to that for the β-actin mRNA signal was calculated. Results indicated that Sam B significantly attenuated the ratio of HSV-1 DNA polymerase mRNA to β-actin mRNA (Fig. 8B). The data demonstrated that Sam B blockage of HSV-1 DNA synthesis was related to a deficiency of DNA polymerase.
FIG. 8.
Effects of Sam B on DNA polymerase gene transcription in Vero cells detected by Northern blot analysis. Vero cells (5 × 106) were infected with HSV-1 (MOI, 3) or were not infected in the presence or absence of Sam B (25 μM). (A) Total cellular RNA was isolated from the cells at 6 h p.i. and analyzed in 6.66% formaldehyde-agarose gel and hybridized with digoxigenin-labeled DNA polymerase cDNA or β-actin cDNA. Lanes 1 and 4, HSV-1-infected cells treated with or without DMSO; lane 2, HSV-1-infected cells treated with Sam B; lane 3, uninfected cells. (B) Each exposed band was quantitated by densitometer, and the ratio of DNA polymerase mRNA to β-actin mRNA was calculated. Each bar is the mean of three independent experiments.
Effects of Sam B on HSV-1 α gene expression in Vero cells.
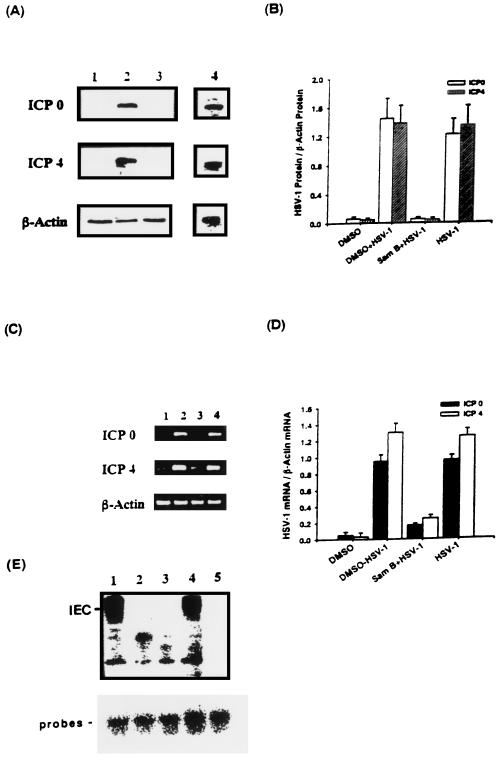
As we know, β genes of HSV-1 are not expressed in the absence of competent α proteins such as ICP0 and ICP4 (35). To confirm that Sam B inhibition of DNA polymerase mRNA was related to α gene expression, Vero cells were infected with HSV-1 or were not infected in the presence or absence of Sam B. The total cellular proteins was extracted at 4 h p.i., and ICP0 and ICP4 proteins were determined by Western blot analysis. As shown in Fig. 9A, while uninfected cells expressed few virus ICP0 and ICP4 proteins (lane 1), both 110-kDa ICP0 and 175-kDa ICP4 were synthesized in HSV-1-infected Vero cells (lane 4). DMSO did not affect the expression of either protein in Vero cells (lane 2). However, Sam B decreased levels of ICP0 and ICP4 proteins in HSV-1-infected Vero cells (lane 3). Graphical representation of the ratio of ICP0 or ICP4 to β-actin proteins in Vero cells indicated that little signal from infected Vero cells treated with Sam B was detectable. Furthermore, RT-PCR analysis was applied to determine whether decreasing ICP0 and ICP4 mRNA expression caused the impairment of protein production in Sam B-treated cells. As shown in Fig. 9C, while ICP0 and ICP4 mRNAs were expressed in HSV-1-infected Vero cells (lanes 2 and 4), Sam B decreased the levels of ICP0 and ICP4 mRNAs in the cells (lane 3).
FIG. 9.
Effects of Sam B on HSV-1 ICP0 and ICP4 gene expression and formation of IEC in Vero cells detected by Western blotting, RT-PCR, and EMSA, respectively. Vero cells (5 × 106) were infected with HSV-1 (MOI, 3) or were not infected in the presence or absence of Sam B (25 μM). (A) Lysates (20 μg of protein) were collected at 4 h p.i. and run on an SDS-10% PAGE gel and analyzed by immunoblotting with an anti-ICP0 or -ICP4 antibody. (C) Total cellular RNA was isolated from Vero cells at 4 h p.i. and analyzed by RT-PCR. Lane 1, uninfected Vero cells; lanes 2 and 4, HSV-1-infected cells treated with or without DMSO; lane 3, infected cells treated with Sam B. (B and D) Bar graphs indicating the ratio of ICP0 or ICP4 to β-actin proteins or mRNAs. Each bar represents the mean of three independent experiments. (E) EMSA was performed as described in Materials and Methods. Nuclear extracts from HSV-1-infected (lanes 1 and 4) or uninfected Vero cells (lane 3) were incubated with a 32P-end-labeled GARAT probe. The effects of Sam B on the formation of IEC in virus-infected nuclear extracts pretreated with 25 μM Sam B for 5 min and then mixed with the probes (lane 2) were detected. Lane 5, results of adding a 50-fold excess of unlabeled probe to the reaction mixture.
A novel virus-induced protein-DNA complex reduced by Sam B.
The formation of αTIF/C1/Oct-1/GARAT multiprotein complexes is responsible for induction of α genes (31). To determine whether Sam B suppression of ICP0 and ICP4 transcripts was related to this initial transcriptional trans activation event, the radiolabeled DNA fragment containing GARAT was incubated with Sam B-treated nuclear extracts from HSV-1-infected cells. As shown in Fig. 9E, with extracts from infected cells, a novel virus-induced protein-DNA complex (IEC) was detected (lanes 1 and 4). While DMSO did not influence protein-DNA complex formation (lane 1), a 50-fold excess of unlabeled probe blocked the formation of these complexes (lane 5). The level of IEC was reduced by Sam B treatment (lane 2). These results suggest that the reduction by Sam B of ICP0 and ICP4 mRNAs might be related to an interruption in the formation of αTIF/C1/Oct-1/GARAT multiprotein complexes.
DISCUSSION
The plaque reduction assay offers a popular system to evaluate the effect of antiviral agents against HSV-1 (3). In our study, 10 Chinese herbs were screened by this model and L. sinense was found to contain antiherpetic agents, supporting the validity of its use for pharmacological studies. Results shown here indicated that Sam B purified from L. sinense suppressed HSV-1 multiplication in Vero cells without significantly reducing cell viability and growth. The inhibitory effect of Sam B may be attributed to its interference with structural proteins and DNA synthesis, DNA polymerase mRNA transcription, and α gene expression of HSV-1. Hence, Sam B suppression of viral replication might have important implications for L. sinense therapeutic activity in microorganism infection. This is the first report of the antiviral action mechanisms of Sam B.
Sam B isolated from L. sinense is a polyphenol flavonoid compound (26, 32). Results showing that Sam B impaired HSV-1 replication in Vero cells were compatible with data reported by Vanden Berghe et al., which indicate that many polyphenols are known for their antiherpesvirus activities (40). Sam B blockage of HSV-1 replication was probably not related to DMSO because cell viability and growth and HSV-1 replication in Vero cells were not changed by DMSO. The morphology and characteristics of Vero cells treated with Sam B or other Chinese herb extracts were similar, suggesting that inhibitory effects of Sam B were not related to the pH, osmolarity, or other physiology variables in different preparations (data not shown). Although we did not determine whether Sam B bound to virus, results of electron microscopy observations indicated that the morphology of HSV-1 in the presence of Sam B is unchanged from that in its absence (data not shown). A comparison with control groups showed that the HSV-1 titer was not significantly decreased when viral particles were treated with 25 μM Sam B at 37°C for 1 h (data not shown). Moreover, pretreatment of cells with Sam B or addition of the drug after viral adsorption produced antiviral activity similar to that when HSV-1 and Sam B were added at the same time. These results suggest that the binding of Sam B to virion or host cells could not be a factor inhibiting virus replication. The preliminary data indicated that pretreatment of Vero cells with Sam B for 24 h and then its removal before infection still inhibit virus yield (data not shown). It is possible that the binding of Sam B to certain membrane molecules of the host cell different from the receptor, resulting in interference with virus penetration into the host cells, such as by interfering with virus-cell fusion, was not related to the inhibitory action of Sam B.
In the host cells, HSV-1 replication is coordinately regulated and sequentially ordered in a cascade and is believed to proceed as follows: (i) αTIF, a γ protein packaged in the virion, turns on the α genes to be transcribed; (ii) expression of α genes regulates the β genes to be expressed; (iii) both α and β gene expression initiates HSV-1 DNA replication; (iv) γ genes are synthesized, and then virions are assembled; and (v) HSV-1 is enveloped as it buds through the nuclear membrane (18, 28, 35). In the present study, we found that Sam B decreased ICP0 and ICP4 gene expression in Vero cells. We are currently attempting to further elucidate whether the mechanisms by which Sam B suppresses ICP0 and ICP4 gene expression are related to the initial transcriptional transactivation events, including formation of αTIF/C1/Oct-1/GARAT multiprotein complexes. Our initial experiments using EMSA indicated that retarded species IEC has been decreased in the GARAT probes incubated with Sam B-treated nuclear extracts infected by HSV-1. Thus, the possibility that Sam B inhibited HSV-1 replication through disturbance of αTIF/C1/Oct-1/GARAT stable complex formation cannot be excluded. ICP0 has been reported to perform several functions including selection of transcriptional termination sites and stimulation of DNA synthesis. Although ICP0 is not essential for HSV-1 replication in some cell cultures, defects in the associated gene delay the expression of β and γ genes and impair viral replication (35). ICP4 is the major transactivator of HSV-1 genes. Thus, ICP4 and ICP0 play important roles in regulation of β and γ gene expression and are essential for HSV-1 replication (14). Moreover, the present data indicated that Sam B impaired DNA polymerase transcripts and HSV-1 DNA synthesis in Vero cells. It is known that a large array of proteins including DNA polymerase are required for HSV-1 DNA synthesis. We suggest that the decrease in HSV-1 DNA synthesis due to Sam B is related to impairment of DNA polymerase. Recently, we detected thymidine kinase mRNA expression in Vero cells by RT-PCR, and preliminary results indicate that Sam B impeded thymidine kinase mRNA expression (data not shown). We also proved that the production of gB, gC, gD, gG, and ICP5 proteins in Vero cells was attenuated by Sam B. ICP5 is a major capsid protein and is made both early and late in infection. gB, gC, gD, and gG are all involved in the viral envelope structure and play important roles in viral attachment and penetration. The evidence demonstrates that ICP4 is required for expression of γ genes and that ICP4 binding sites enhance the transcription of the gD gene in vitro (15). Although effects of Sam B on gC, gD, gG, and ICP5 mRNA expression were not determined, it was found that Sam B decreased gB mRNA expression in Vero cells. HSV-1 DNA synthesis is required for gC mRNA expression. gD, gG, and ICP5 are similar to gB in that their mRNA expression requires ICP4 proteins (34). Thus, we predict that Sam B attenuates levels of gB, gC, gD, gG, and ICP5 proteins in Vero cells, which may be related to impairment of DNA synthesis and ICP4 and ICP0 production. The attenuation of DNA synthesis and ICP4 and ICP0 production may cause decreases of gB, gC, gD, gG, and ICP5 gene expression in Vero cells.
From the present results, we hypothesize that impairment of HSV-1 multiplication in Sam B-treated Vero cells, at least in part, was related to (i) decreases in HSV-1 ICP0 and ICP4 gene expression due to Sam B, which might be related to disturbance of the formation of αTIF/C1/Oct-1/GARAT multiprotein complexes, (ii) reduction in DNA polymerase transcriptions in the cells, (iii) inhibition of viral DNA synthesis, (iv) interference with gB protein synthesis due to blockage of gB mRNA synthesis, (v) impaired levels of viral capsid protein ICP5 and envelope proteins gC, gD, and gG, and (vi) no HSV-1 plaque formation in Vero cells. Unlike dextran sulfate isolated from Sargassum horneri, which inhibits HSV-1 replication at the adsorption step (16), Sam B blocked HSV-1 replication at the immediate-early and early steps. The expression of the immediate-early gene represents one stage of the HSV-1 replication cycle that could be targeted by a novel antiviral therapy to deliver a significant reduction in virus replication in both acute and latent infections. The expression of the immediate-early gene plays important roles in the regulation of all classes of viral genes during lytic infection and is the key initiating event in the process of reactivation of the latent HSV-1 genomes. Low levels of immediate-early transcripts can be identified in latently infected neuron cells. ICP0 mutant viruses that are defective in immediate-early transactivation have been shown to reactivate very poorly from latent infections (35). While nucleoside analogues have been successful in treating acute infections, they fail to modulate reactivation of latent virus. On the other hand, Sam B lacked the elevated cytotoxicity and antiproliferative properties of interferons (4). Because Vero cells are notoriously hardy and aneuploid, the effects of Sam B on the growth of human peripheral blood mononuclear cells (PBMC), which are normal diploid cells, were determined. Results indicated that Sam B did not affect DNA, RNA, and protein synthesis in PBMC (data not shown) and that the therapeutic index was about 13.2. Thus, small molecules identified from Chinese herbs such as Sam B which act as inhibitors of HSV-1 immediate-early gene expression may have the potential to impact clinical disease to a far greater extent than currently marketed nucleosides and cytokines. Future experiments with treatment of HSV-1-infected animals with Sam B will be necessary to define whether L. sinense can reduce experimental viral infection injury and prevent recurrent HSV-1 infection. Moreover, this study not only demonstrates that Chinese herbs are potential therapeutic drugs for the viral infection but also supports a model for future protocol design in preclinical studies.
Acknowledgments
This study was partially supported by a grant from the Department of Health, Taiwan, Republic of China (DOH 90-TD-1019).
We thank Wu-Tse Liu for giving us the KOS strain of HSV-1.
REFERENCES
- 1.Alrabiah, F. A., and S. L. Sacks. 1996. New antiherpes virus agents: their targets and therapeutic potential. Drugs 52:17-32. [DOI] [PubMed] [Google Scholar]
- 2.Arisawa, M., A. Fujita, T. Hayashi, K. Hayashi, H. Ochiai, and N. Morita. 1990. Cytotoxic and antiherpetic activity of phloroglucinol derivatives from Mallotus japonicus (Euphorbiaceae). Chem. Pharm. Bull. 38:1624-1626. [DOI] [PubMed] [Google Scholar]
- 3.Bacon, T. H., B. A. Howard, L. C. Spender, and M. R. Boyd. 1996. Activity of penciclovir in antiviral assays against herpes simplex virus. J. Antimicrob. Chemother. 37:303-313. [DOI] [PubMed] [Google Scholar]
- 4.Boulware, S. L., J. C. Bronstein, E. C. Nordby, and P. C. Weber. 2001. Identification and characterization of a benzothiophene inhibitor of herpes simplex virus type 1 replication which acts at the immediate early stage of infection. Antivir. Res. 51:111-125. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H., L. Teng, J. N. Li, R. Park, D. E. Mold, J. Gnabre, J. R. Hwu, W. N. Tseng, and R. C. Huang. 1998. Antiviral activities of methylated nordihydroguaiaretic acids. 2. Targeting herpes simplex virus replication by the mutation insensitive transcription inhibitor tetra-O-methyl-NDGA. J. Med. Chem. 41:3001-3007. [DOI] [PubMed] [Google Scholar]
- 6.Coen, D. M. 1996. Antiviral drug resistance in herpes simplex virus. Adv. Exp. Med. Biol. 394:49-57. [DOI] [PubMed] [Google Scholar]
- 7.Cone, R. W., A. C. Hobson, J. Palmer, M. Remington, and L. Corey. 1991. Extended duration of herpes simplex virus DNA in genital lesions detected by the polymerase chain reaction. J. Infect. Dis. 164:757-760. [DOI] [PubMed] [Google Scholar]
- 8.Corey, L., and P. G. Spear. 1986. Infections with herpes simplex viruses (1). N. Engl. J. Med. 314:686-691. [DOI] [PubMed] [Google Scholar]
- 9.Darby. G. 1994. A history of antiherpes research. Antivir. Chem. Chemother. 5(Suppl. 1):3-9. [Google Scholar]
- 10.Elion, G. B., P. A. Furman, J. A. Fyfe, P. de. Miranda, L. Beauchamp, and H. J. Schaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 74:5716-5720.202961 [Google Scholar]
- 11.Farnsworth, N. R. 1993. Ethnopharmacology and future drug development: the North American experience. J. Ethnopharmacol. 38:145-152. [DOI] [PubMed] [Google Scholar]
- 12.Ferrea, G., A. Canessa, F. Sampietro, M. Cruciani, G. Romussi, and D. Bassetti. 1993. In vitro activity of a Combretum micranthum extract against herpes simplex virus types 1 and 2. Antivir. Res. 21:317-325. [DOI] [PubMed] [Google Scholar]
- 13.Furman, P. A., M. H. St. Clair, J. A. Fyfe, J. L. Rideout, P. M. Keller, and G. B. Elion. 1979. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl) guanine and its triphosphate. J. Virol. 32:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis required functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino, T., T. Hayashi, K. Hayashi, J. Hamada, J. B. Lee, and U. Sankawa. 1998. An antivirally active sulfated polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol. Pharm. Bull. 21:730-734. [DOI] [PubMed] [Google Scholar]
- 17.Houghton, P. J. 1995. The role of plants in traditional medicine and current therapy. J. Altern. Complement. Med. 1:131-143. [DOI] [PubMed] [Google Scholar]
- 18.Jones, P. C., and B. Roizman. 1979. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J. Virol. 31:299-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimberlin, D. W., and R. J. Whitley. 1996. Antiviral resistance: mechanisms, clinical significance, and future implications. J. Antimicrob. Chemother. 37:403-421. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, Y. C., and C. Y. Lin. 1990. Recurrent herpes simplex virus type 1 infection precipitated by the impaired production of interleukin-2, alpha-interferon, and cell-mediated cytotoxicity. J. Med. Virol. 31:183-189. [DOI] [PubMed] [Google Scholar]
- 21.Kuo, Y. C., C. Y. Lin, S. F. Cheng, C. C. Lin, and W. T. Liu. 1987. Impaired natural killer cytotoxicity during recrudescence of recurrent herpes simplex virus type 1 infection. Cancer Detect. Prev. 1:51-55. [PubMed] [Google Scholar]
- 22.Kuo, Y. C., N. S. Yang, C. J. Chou, L. C. Lin, and W. J. Tsai. 2000. Regulation of cell proliferation, gene expression, production of cytokines, and cell cycle progression in primary human T lymphocytes by piperlactam S isolated from Piper kadsura. Mol. Pharmacol. 58:1057-1066. [DOI] [PubMed] [Google Scholar]
- 23.Kuo, Y. C., C. M. Sun, J. C. Ou, and W. J. Tsai. 1997. A tumor cell growth inhibitor from Polygonum hypoleucum Ohwi. Life Sci. 61:2335-2344. [DOI] [PubMed] [Google Scholar]
- 24.Kuo, Y. C., C. C. Chen, W. J. Tsai, and Y. H. Ho. 2001. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: relationship to gene expression, DNA replication, and protein synthesis. Antivir. Res. 51:95-109. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, Y. C., W. T. Liu, and C. Y. Lin. 1993. Herpes simplex virus type 1 genes in human mononuclear cells and affecting cell-mediated immunity. J. Med. Virol. 41:138-145. [DOI] [PubMed] [Google Scholar]
- 26.Lin, L. C., Y. C. Kuo, and C. J. Chou. 2000. Anti-herpes simplex virus type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Med. 66:333-336. [DOI] [PubMed] [Google Scholar]
- 27.Logan, W. S., J. P. Tindall, and M. L. Elson. 1971. Chronic cutaneous herpes simplex. Arch. Dermatol. 103:606-614. [PubMed] [Google Scholar]
- 28.Mackem, S., and B. Roizman. 1982. Structural features of the herpes simplex virus α gene 4, 0, and 27 promoter-regulatory sequences which confer α regulation on chimeric thymidine kinase genes. J. Virol. 44:939-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann, S. L., J. D. Meyers, K. L. Holmes, and L. Corey. 1984. Prevalence and incidence of herpesvirus infections among homosexually active men. J. Infect. Dis. 149:1026-1027. [DOI] [PubMed] [Google Scholar]
- 30.Marchetti, M., S. Pisani, V. Pietropaola, L. Seganti, R. Nicoletti, A. Degener, and N. Orsi. 1996. Antiviral effect of a polysaccharide from Sclerotium glucanicum towards herpes simplex virus type 1 infection. Planta Med. 62:303-307. [DOI] [PubMed] [Google Scholar]
- 31.McKnight, J. L. C., T. M. Kristie, and B. Roizman. 1987. Binding of the virion protein mediating α gene induction in herpes simplex virus 1-infected cells to its cis site required cellular proteins. Proc. Natl. Acad. Sci. USA 84:7061-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nonaka, G. I., Y. Aiko, K. Aritake, and I. Nishioka. 1992. Tannins and related compounds. CXIX. Samarangenins A and B, novel proanthocyanidins with doubly bonded structures, from Syzygium samaragens and S. aqueum. Chem. Pharm. Bull. 40:2671-2673. [Google Scholar]
- 33.Pass, R. F., W. K. Long, R. J. Whitley, S. J. Soong, A. G. Diethelm, D. W. Reynolds, and C. A. Alford, Jr. 1978. Productive infection with cytomegalovirus and herpes simplex virus in renal transplant recipients: role of source kidney. J. Infect. Dis. 137:556-563. [DOI] [PubMed] [Google Scholar]
- 34.Pope, L. E., J. F. Marceletti, L. R. Katz, and D. H. Katz. 1996. Anti-herpes simplex virus activity of n-docosanol correlates with intracellular metabolic conversion of the drug. J. Lipid Res. 37:2167-2178. [PubMed] [Google Scholar]
- 35.Roizman, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p. 2231-2295. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 36.Sacks, S. L., R. A. Thisted, T. M. Jones, R. A. Barbarash, D. J. Mikolich, G. E. Ruoff, J. L. Jorizzo, L. B. Gunnill, D. H. Katz, M. H. Khalil, P. R. Morrow, G. J. Yakatan, L. E. Pope, J. E. Berge, and The Docosanol 10% Cream Study Group. 2001. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 45:222-230. [DOI] [PubMed] [Google Scholar]
- 37.Saiki, P. K., S. Scharf, F. Faloona, K. B. Mullis, G. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 38.Simões, C. M. O., M. Amoros, and L. Girre. 1999. Mechanism of antiviral activity of triterpenoid saponins. Phytother. Res. 13:323-328. [DOI] [PubMed] [Google Scholar]
- 39.Sydiskis, R. J., D. G. Owen, J. L. Lohr, K. H. Rosler, and R. N. Blomster. 1991. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 35:2463-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanden Berghe, D. A., A. J. Vlietinck, and L. Van Hoof. 1986. Plant products as potential antiviral agents. Bull. Inst. Pasteur 84:101-147. [Google Scholar]