Abstract
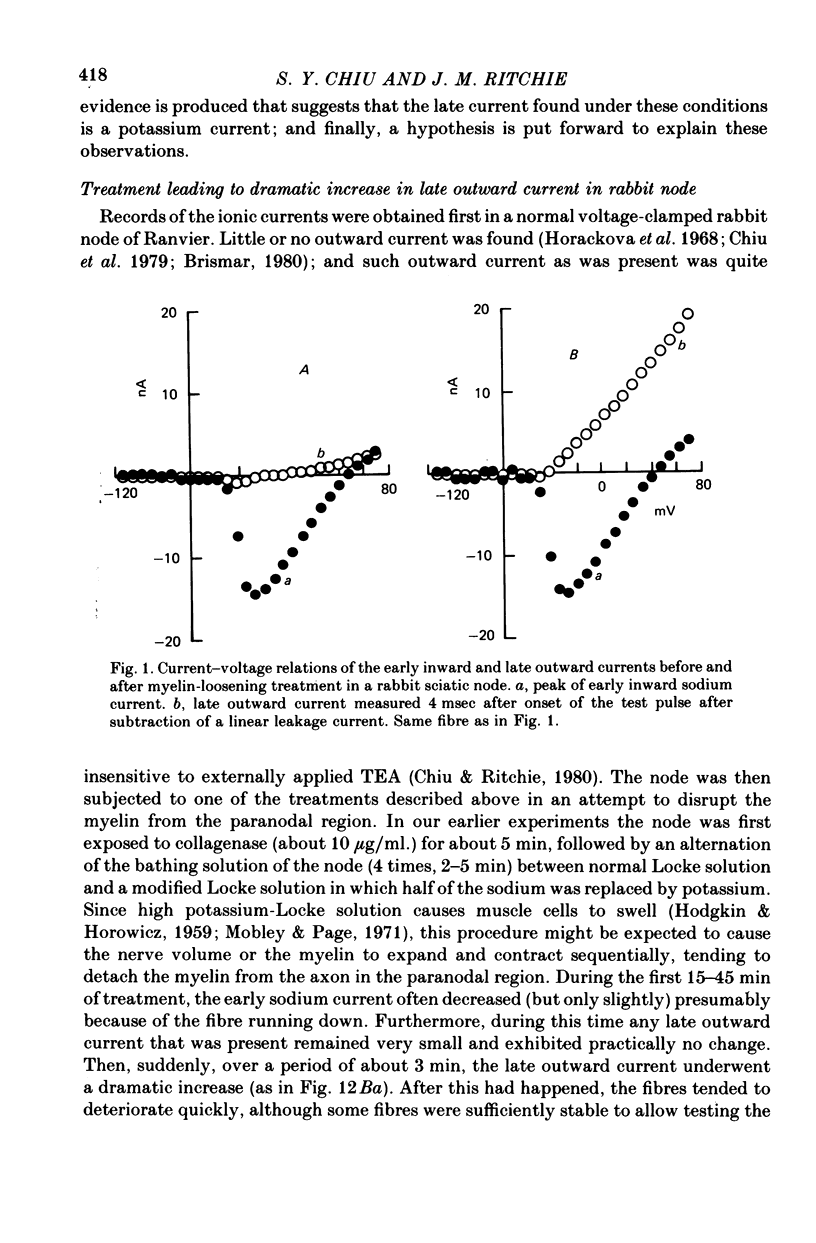
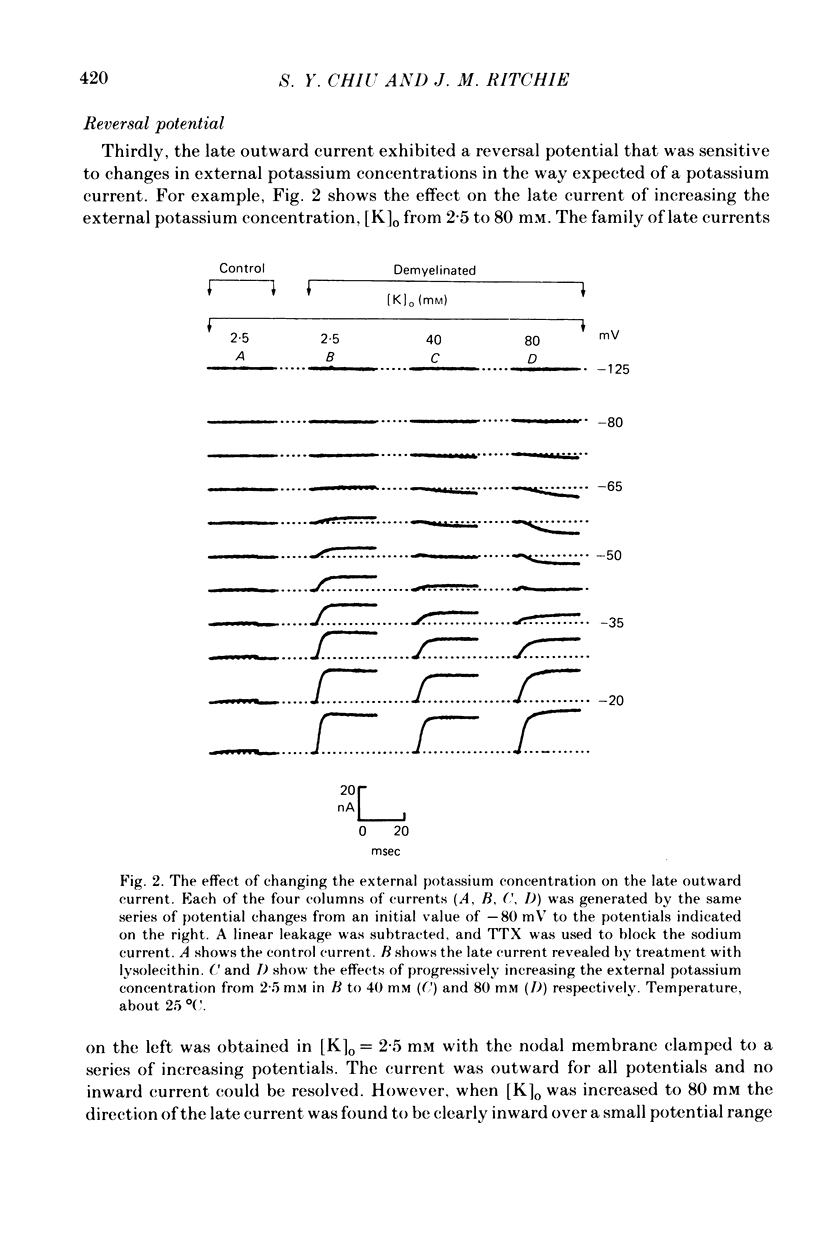
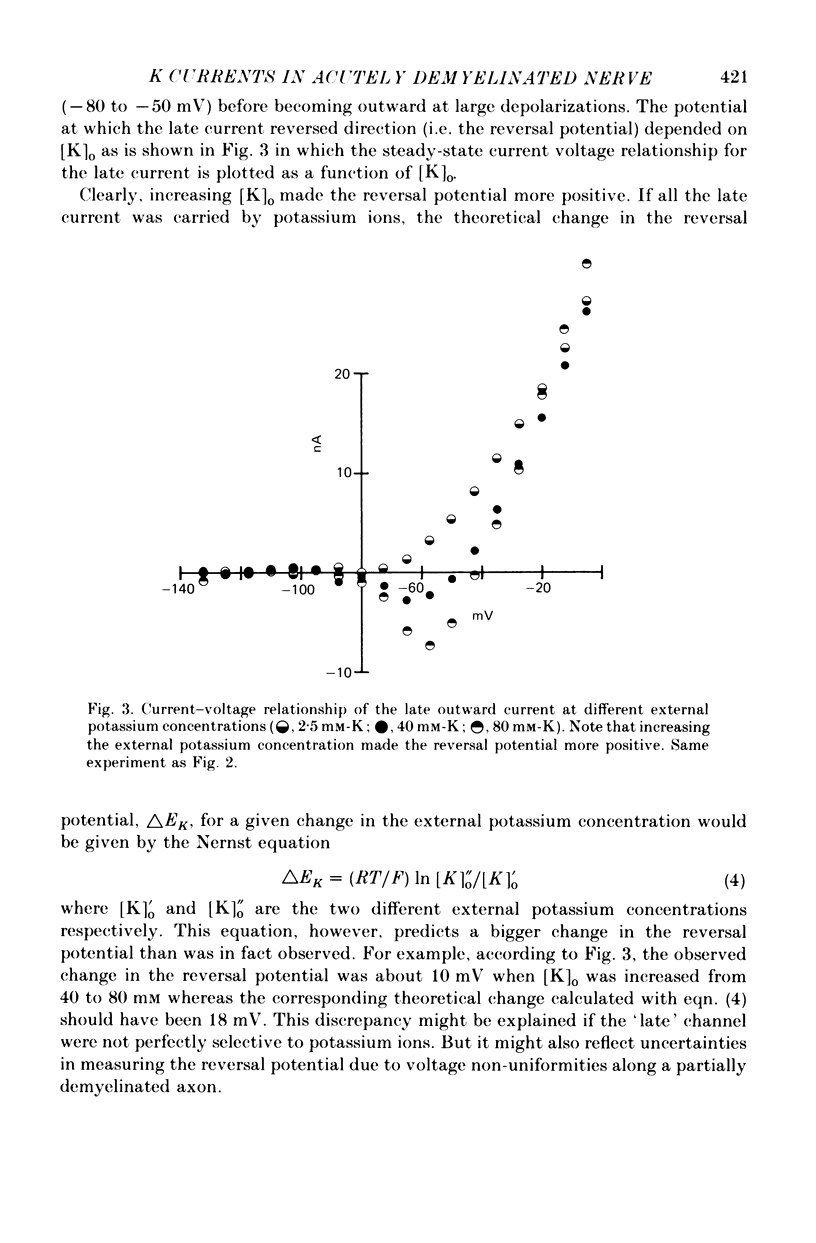
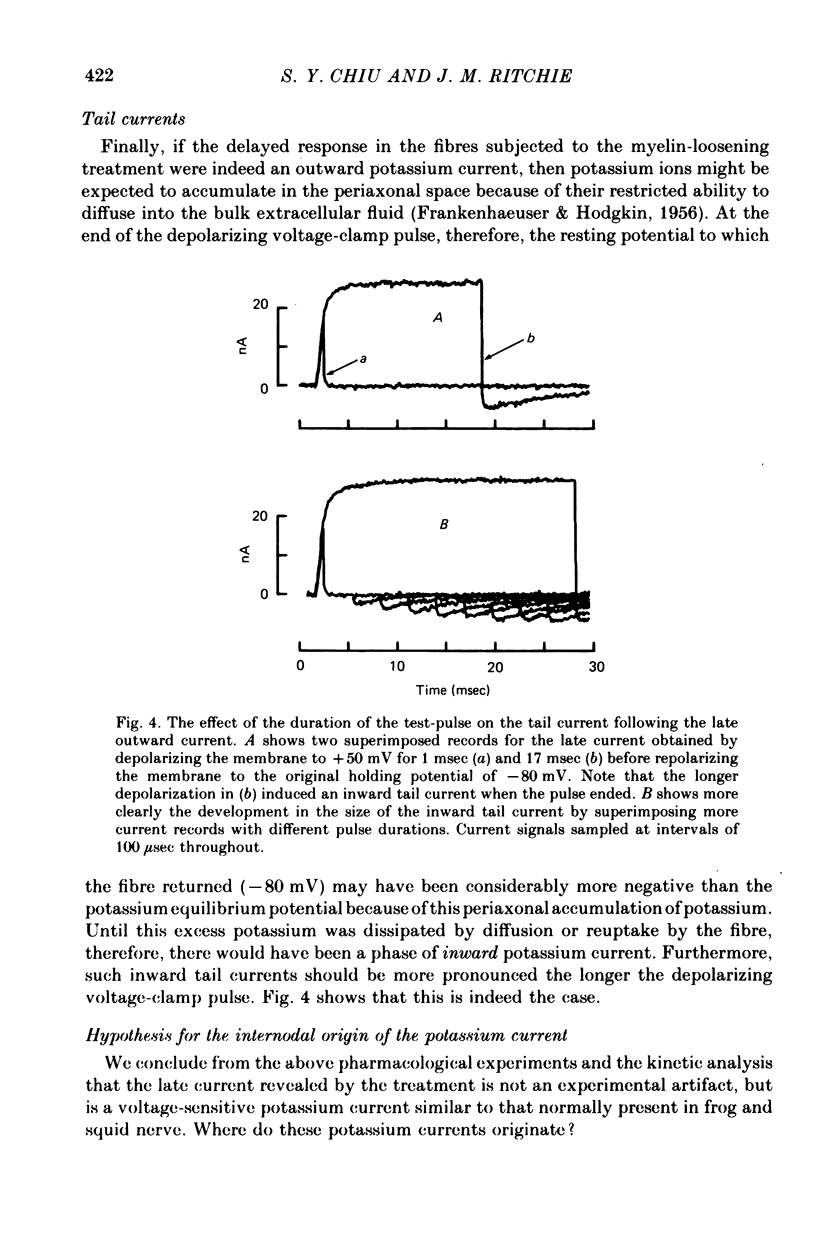
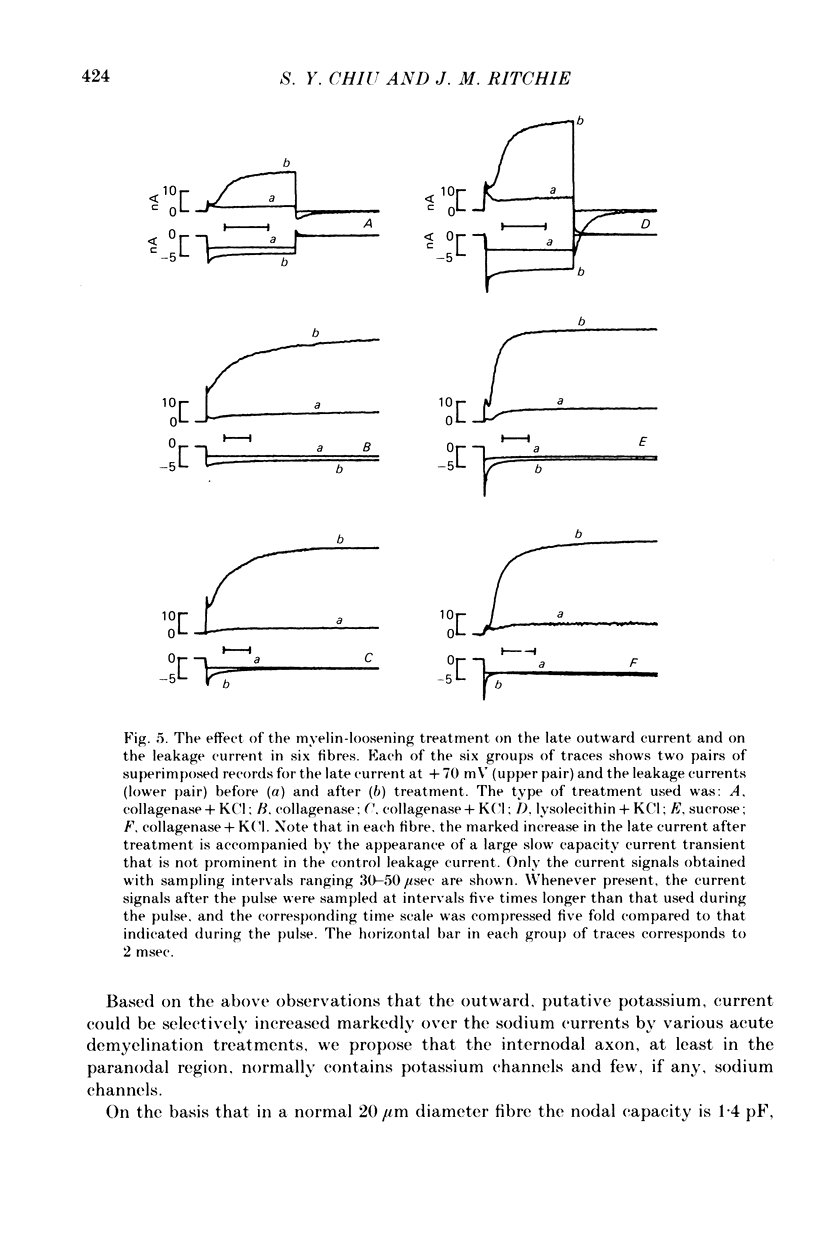
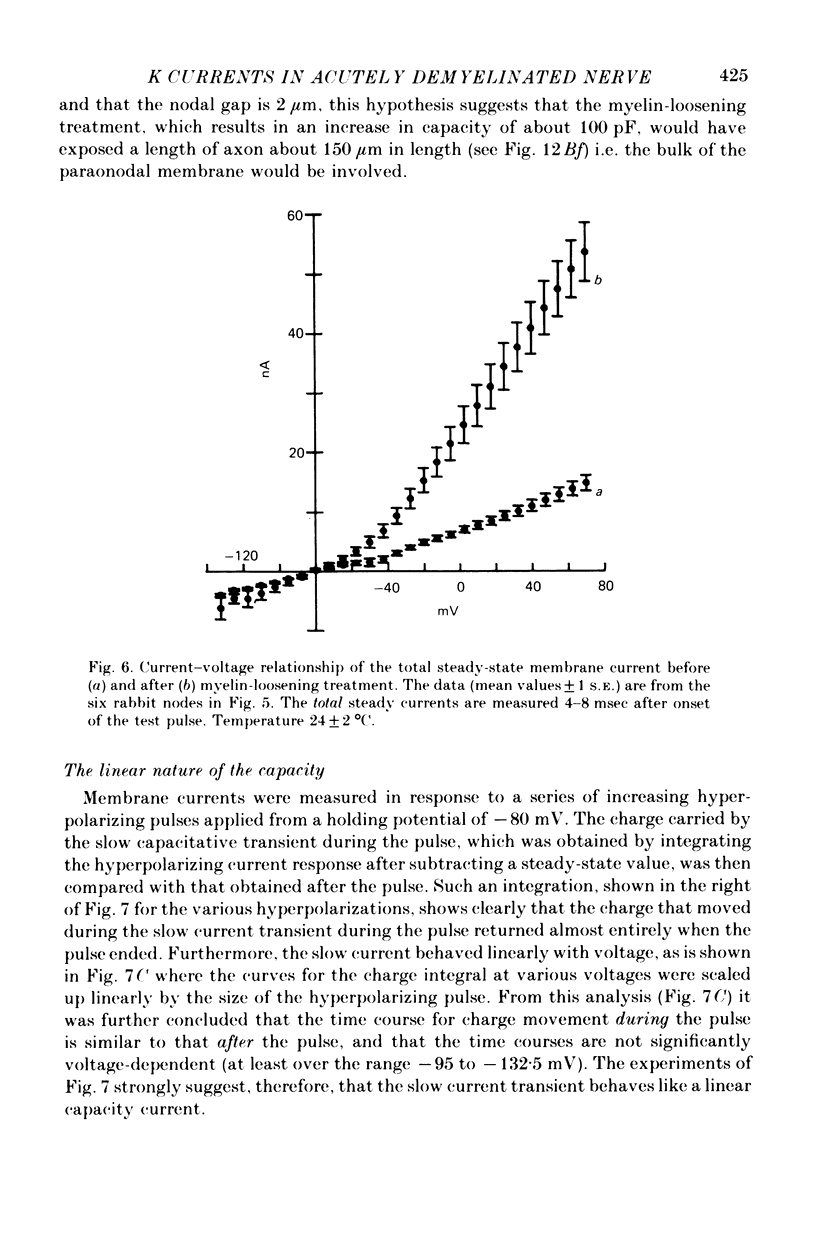
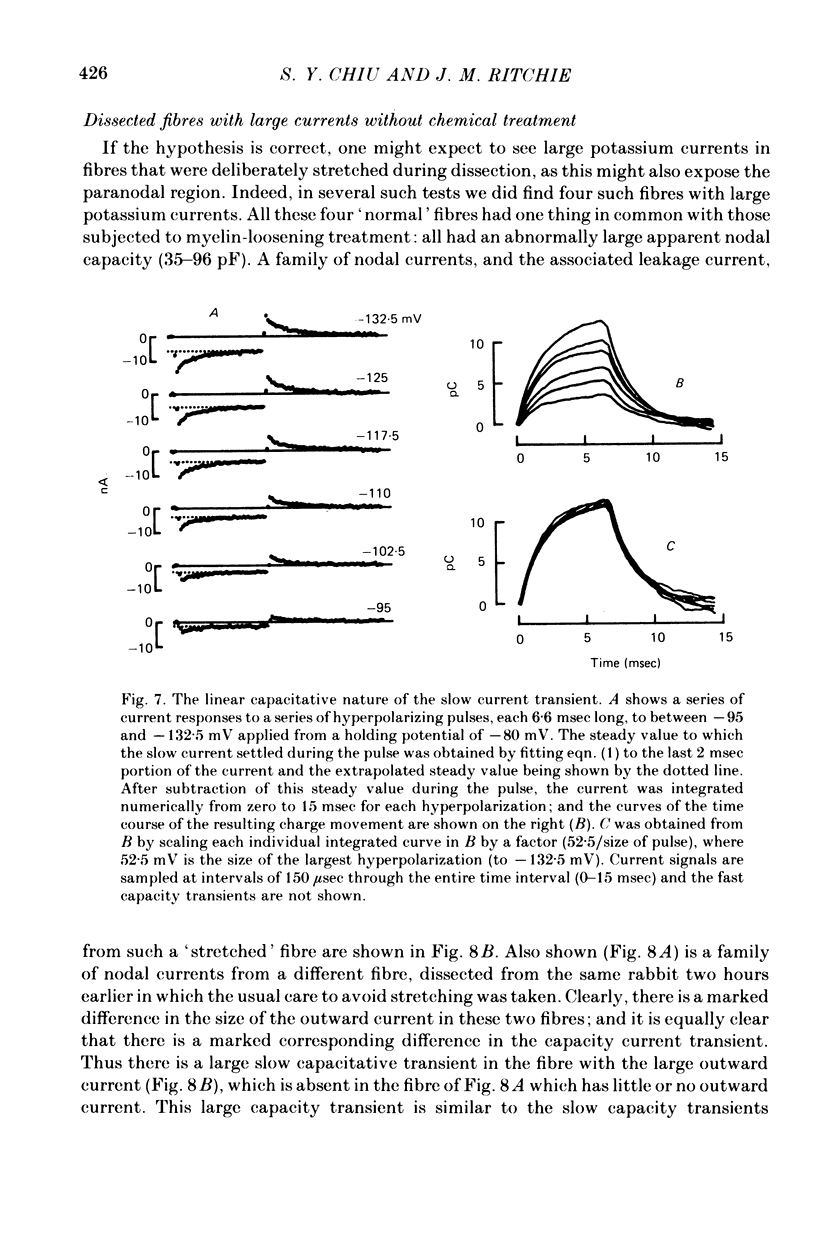
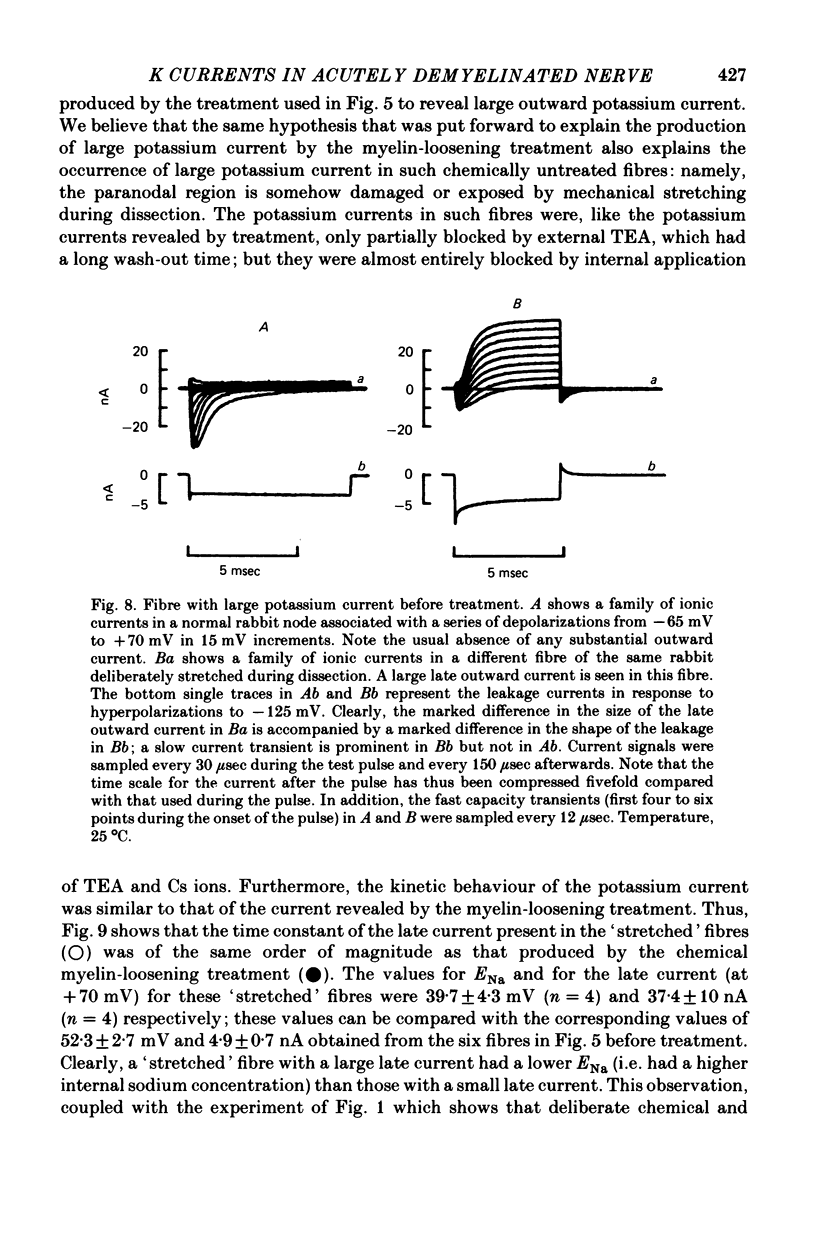
1. A study has been made of the ionic currents in voltage-clamped single rabbit nodes of Ranvier at 22-26 degrees C both under normal conditions, and after the nerve fibres had been acutely demyelinated by a variety of treatments designed to lossen the myelin from the axonal membrane. 2. The myelin-loosening treatments included application of various combinations of: lysolecithin (to dissolve the myelin); collagenase (to loosen the connective tissue in the nodal region); high-potassium Locke solution, hypertonic and hypotonic solutions (to induce axonal volume changes). 3. At a critical stage in such treatment (usually after 15-45 min) a large outward current suddenly appeared. 4. There was no substantial change in the size of the measured inward sodium current when measured at this critical stage. 5. The outward current was blocked by internal TEA and caesium ions, had a reversal potential that became more positive when the external potassium concentration was increased, was kinetically similar to the known potassium current in frog fibres, and was therefore assumed to be a potassium current. 6. The phase of large outward current, whenever it appeared, was always accompanied by the appearance of a slow transient capacitative component in the leakage current, which indicated a marked increase in the effective nodal capacity (of 10- to 60-fold). We suggest that the slow transient capacity current reflected charging of newly exposed axonal membrane, probably in the paranodal region, which was uncovered by the various acute demyelination treatments. This internodal membrane seems to contain mostly potassium channels and few, if any, sodium channels. 7. Newly dissected fibres occasionally showed large potassium currents before treatment, particularly if they were deliberately stretched during dissection; a marked slow capacity transient current was consistently present in these fibres. 8. The effects of acute paranodal demyelination on the sodium and potassium currents, and on the transient capacity currents, can be simulated by a model in which the node is coupled to a cable-like paranode which contains Hodgkin--Huxley type potassium channels and which has a much higher leakage resistance. 9. The functional significance of the presence of potassium channels in rhe internodal region (at least in the paranode) of mammalian fibres is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman C. Increase of sodium concentration near the inner surface of the nodal membrane. Pflugers Arch. 1970;317(4):287–302. doi: 10.1007/BF00586578. [DOI] [PubMed] [Google Scholar]
- Bergmann C., Nonner W., Stämpfli R. Sustained spontaneous activity of Ranvier nodes induced by the combined actions of TEA and lack of calcium. Pflugers Arch. 1968;302(1):24–37. doi: 10.1007/BF00586780. [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978 Jul;280:273–301. doi: 10.1113/jphysiol.1978.sp012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Potential clamp analysis of membrane currents in rat myelinated nerve fibres. J Physiol. 1980 Jan;298:171–184. doi: 10.1113/jphysiol.1980.sp013074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Potential clamp experiments on myelinated nerve fibres from alloxan diabetic rats. Acta Physiol Scand. 1979 Mar;105(3):384–386. doi: 10.1111/j.1748-1716.1979.tb06356.x. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Asymmetry currents in the mammalian myelinated nerve. J Physiol. 1980 Dec;309:499–519. doi: 10.1113/jphysiol.1980.sp013523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature. 1980 Mar 13;284(5752):170–171. doi: 10.1038/284170a0. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J Cell Sci. 1972 Mar;10(2):535–546. doi: 10.1242/jcs.10.2.535. [DOI] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. F., Calvin W. H., Loeser J. D. Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Res. 1976 Oct 29;116(1):139–144. doi: 10.1016/0006-8993(76)90255-9. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Krylov B. V., Makovsky V. S. Spike frequency adaptation in amphibian sensory fibres is probably due to slow K channels. Nature. 1978 Oct 12;275(5680):549–551. doi: 10.1038/275549a0. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The effect of potassium and chloride ions on the volume and membrane potential of single barnacle muscle cells. J Physiol. 1971 May;215(1):49–70. doi: 10.1113/jphysiol.1971.sp009457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A. 1977 Jan;74(1):211–215. doi: 10.1073/pnas.74.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt R. M., Bostock H., Sears T. A. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980 Feb 7;283(5747):570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]


