Abstract
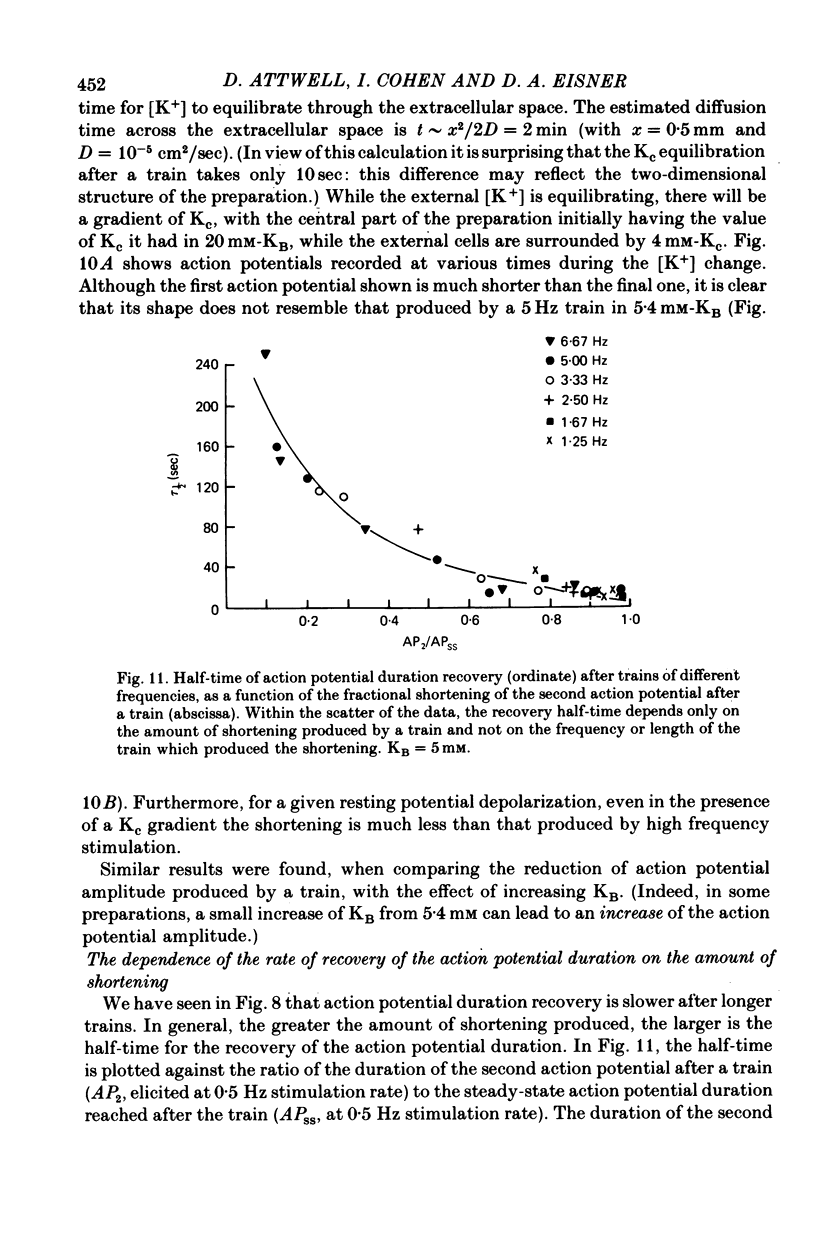
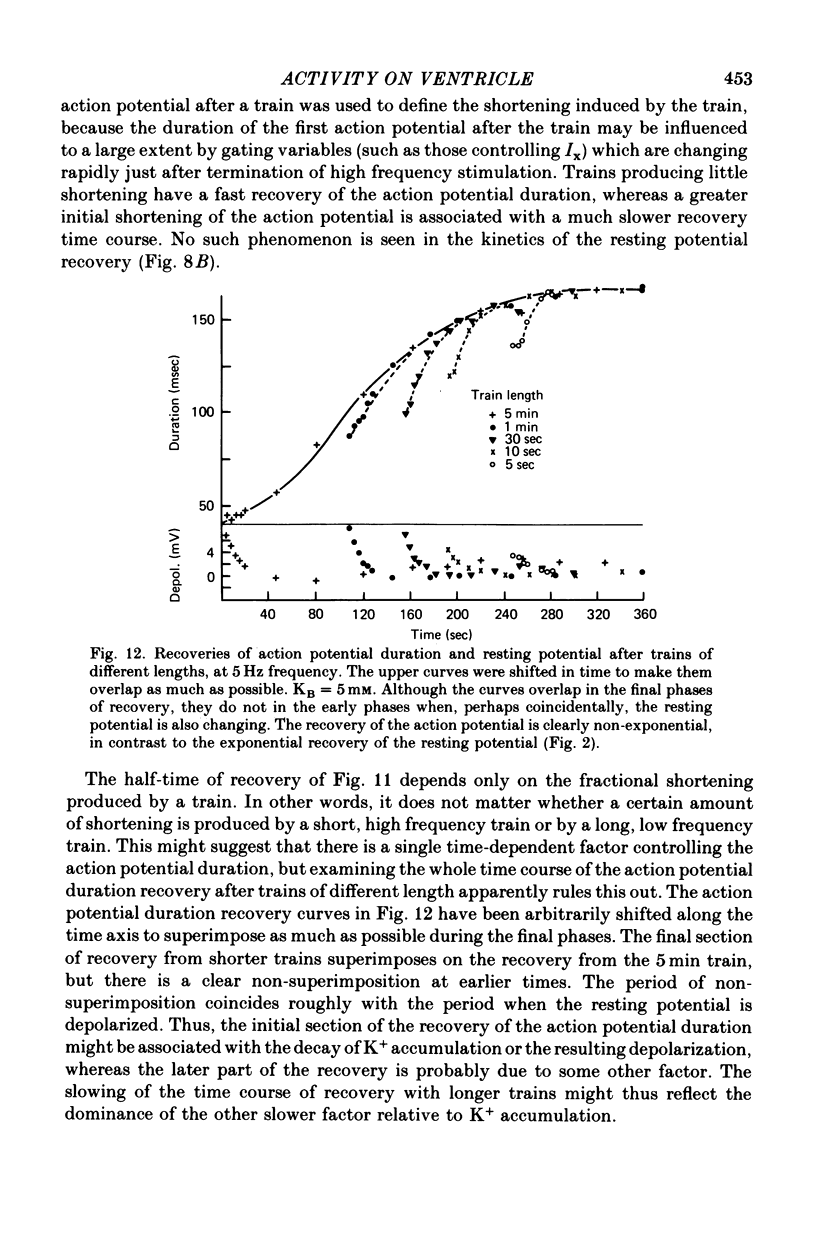
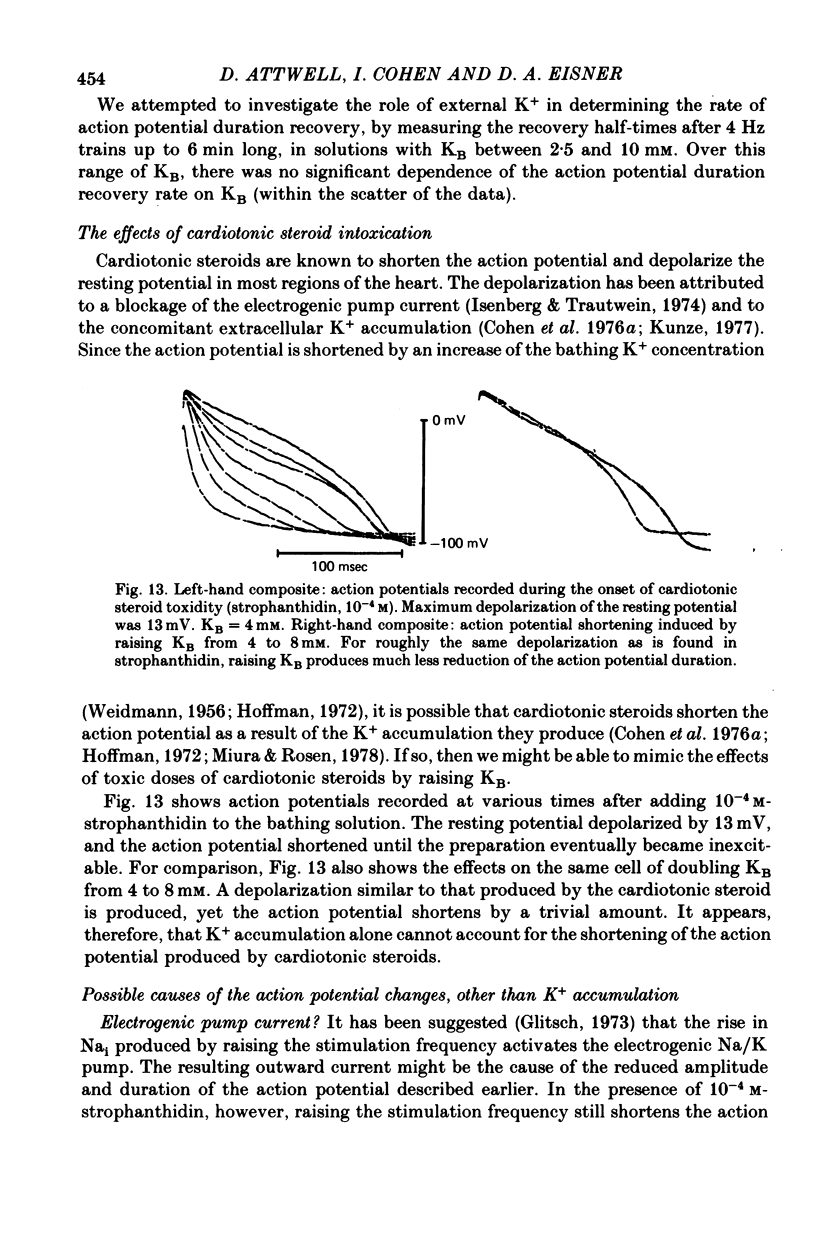
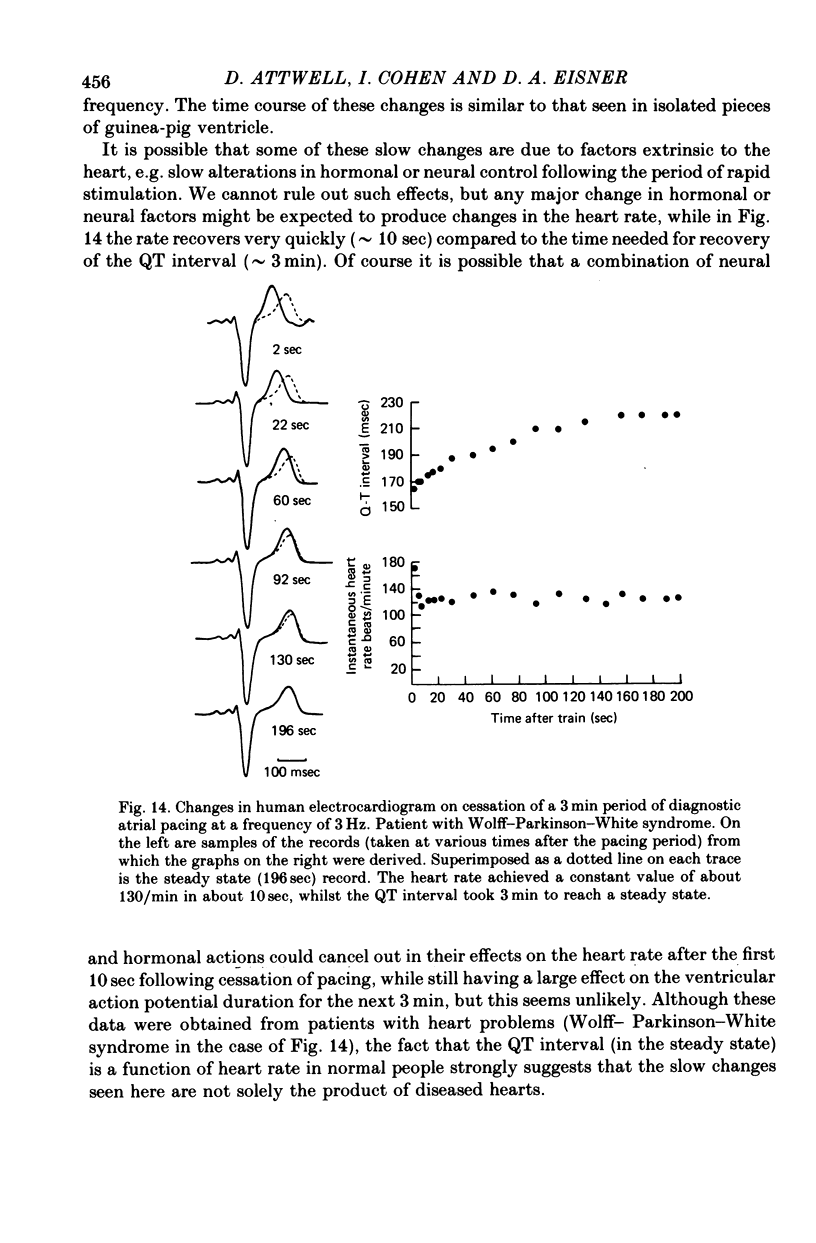
1. On increasing the stimulation frequency of isolated pieces of guinea-pig ventricular muscle, the resting potential depolarizes, and the action potential duration and amplitude are reduced. On termination of the high frequency train of action potentials, these changes are reversed. 2. The resting potential changes are roughly exponential, with a time constant of the order of 10 sec, and are attributable to K+ accumulation in the extracellular space. They are not explicable in terms of known gating variables. 3. The action potential duration and amplitude recover much more slowly than the resting potential, after a high frequency train (half-time approximately 5 min). The time course of these recoveries is not exponential, and is slower after trains which produce more shortening of the action potential. The slow time course suggests that K+ accumulation is not the main cause of the changes in action potential shape. Furthermore, when a certain depolarization of the resting potential is produced by a high frequency train, there is a greater reduction of the action potential duration than that which occurs when the bathing [K+] is raised to produce the same depolarization (Reiter & Stickel, 1968). This is so even when a gradient of extracellular [K+] is induced in the preparation, to mimic non-uniform K+ accumulation. 4. Similarly, the shortening of the action potential produced by toxic doses or cardiotonic steroids is probably not the result of K+ accumulation. 5. The slow changes of the action potential shape produced by a high frequency train are not attributable to the effects of gating variables, nor (solely) to a rise in the intracellular Na concentration stimulating the electrogenic Na/K pump. The dye 3,3'-diethylthiadicarbocyanine, which blocks the Ca2+-activated K conductance in the erythrocyte, has no significant effect on the shape changes. 6. After a sudden change in heart rate, the QT interval of the human electrocardiogram (e.c.g.) changes slowly to a new equilibrium value. The time course of this change is similar to that of the action potential duration in guinea-pig ventricle following a change in stimulation frequency. These changes of the e.c.g. are probably not due to slow alterations of neural or hormonal factors extrinsic to the heart. In the whole heart, the effects on the ventricular action potential duration of changes in sympathetic or vagal tone, or of circulating catecholamines, can be largely accounted for by the changes of atrial driving frequency they produce.
Full text
PDF



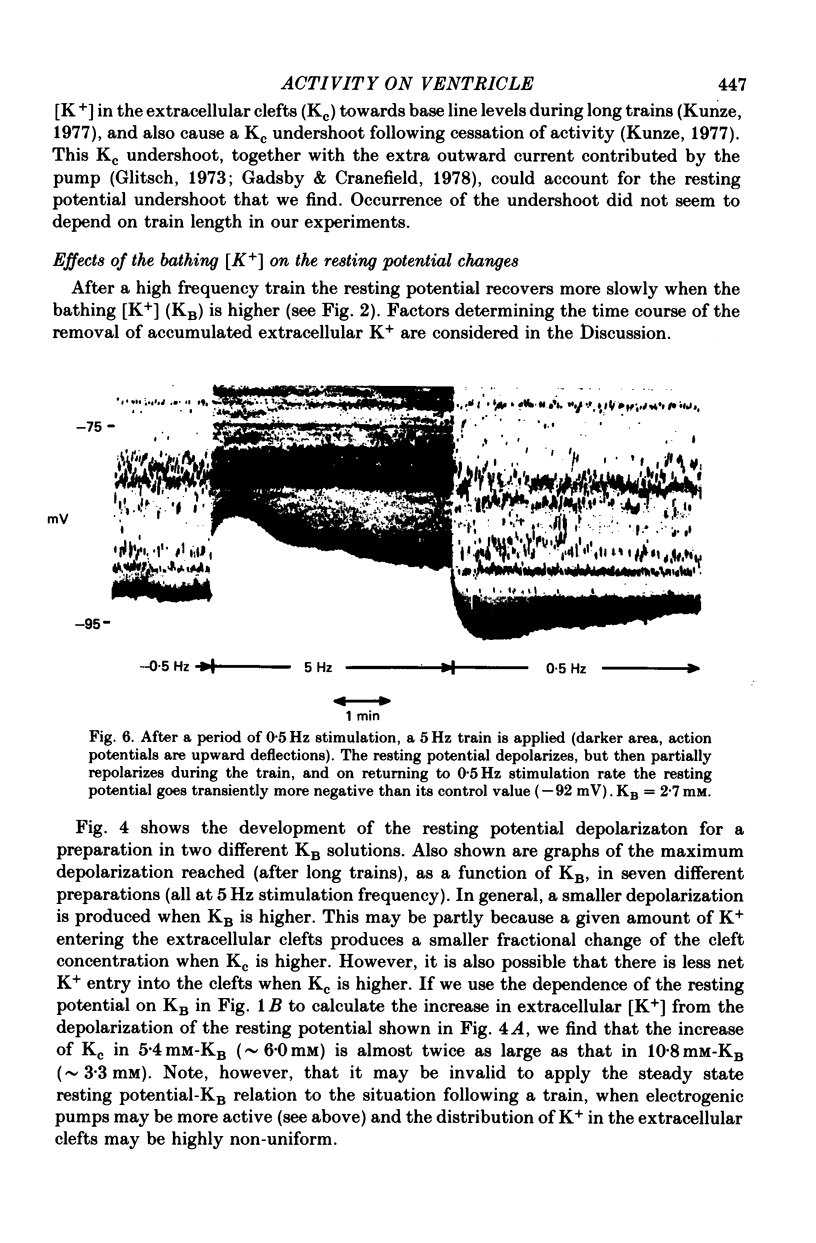
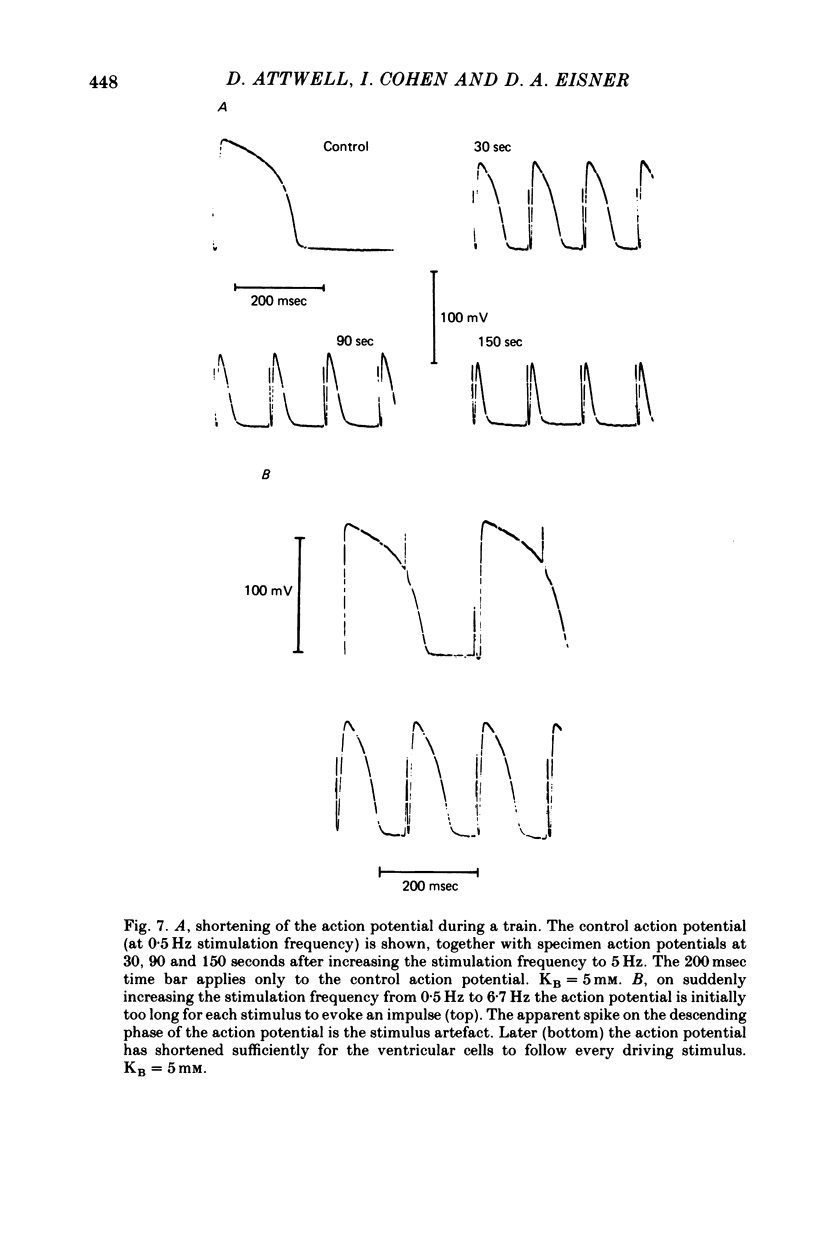

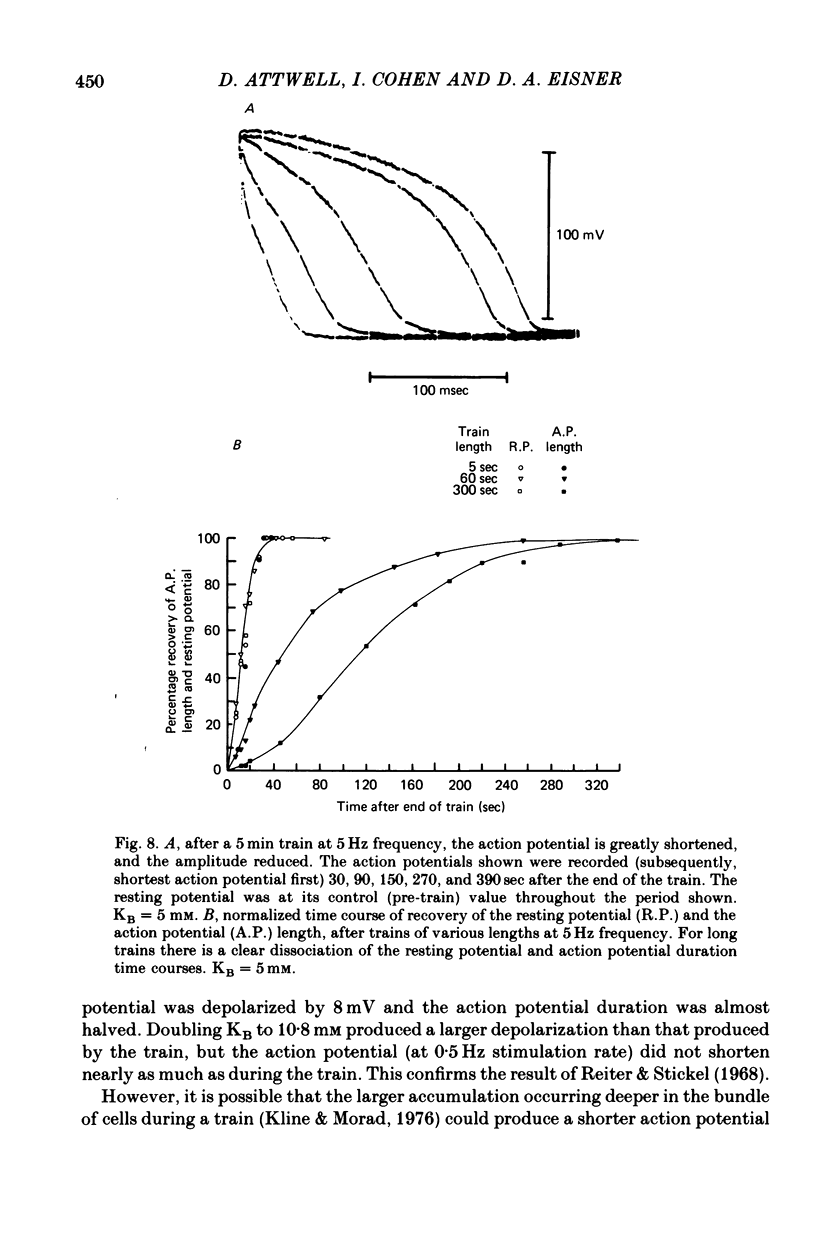
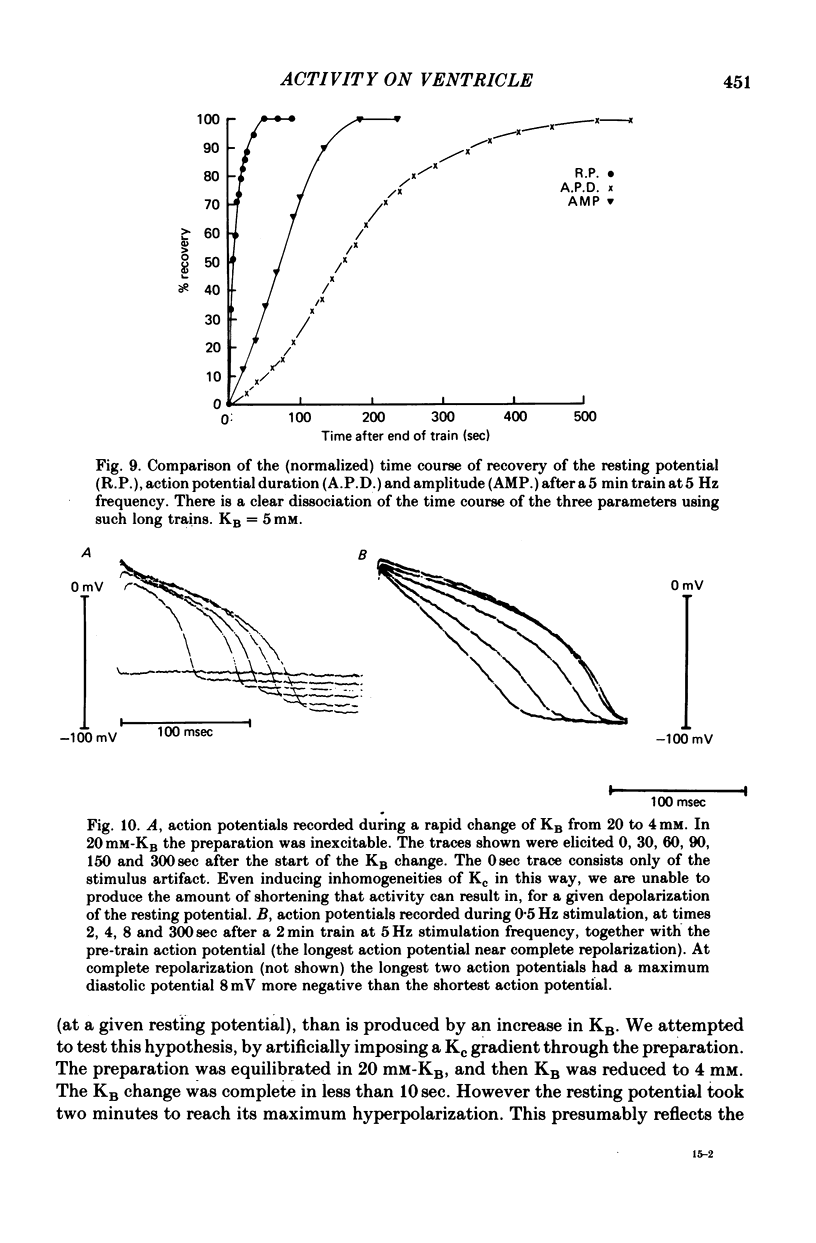






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Cohen I., Eisner D. A., Noble D. Activity dependent changes in mammalian ventricular muscle [proceedings]. J Physiol. 1977 Oct;271(2):17P–18P. [PubMed] [Google Scholar]
- Attwell D., Cohen I., Eisner D. Membrane potential and ion concentration stability conditions for a cell with a restricted extracellular space. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1163):145–161. doi: 10.1098/rspb.1979.0098. [DOI] [PubMed] [Google Scholar]
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978 Dec;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMELIET E. Modification de la durée du potentiel d'action cardiaque sous l'influence des excitants. J Physiol (Paris) 1958 Mar;50(2):204–207. [PubMed] [Google Scholar]
- Cleemann L., Morad M. Extracellular potassium accumulation and inward-going potassium rectification in voltage clamped ventricular muscle. Science. 1976 Jan 9;191(4222):90–92. doi: 10.1126/science.1246599. [DOI] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Giles W., Noble D. Cellular basis for the T wave of the electrocardiogram. Nature. 1976 Aug 19;262(5570):657–661. doi: 10.1038/262657a0. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Ojeda C. Arrhythmogenic effects of hypokalaemia on mammalian ventricular muscle [proceedings]. J Physiol. 1978 Jul;280:74P–75P. [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J. S., Langer G. A. The myocardial interstitium: its structure and its role in ionic exchange. J Cell Biol. 1974 Mar;60(3):586–601. doi: 10.1083/jcb.60.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS C. L., JOHNSON E. A. Effect of changes in frequency of stimulation upon rabbit ventricular action potential. Circ Res. 1961 Jan;9:165–170. doi: 10.1161/01.res.9.1.165. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G. An effect of the electrogenic sodium pump on the membrane potential in beating guinea-pig atria. Pflugers Arch. 1973 Nov 26;344(2):169–180. doi: 10.1007/BF00586550. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Cardiac cellular potentials; effect of vagal stimulation and acetylcholine. Am J Physiol. 1953 May;173(2):312–320. doi: 10.1152/ajplegacy.1953.173.2.312. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls the potassium permeability via the conductance components gK1 and gK2. Pflugers Arch. 1977 Oct 19;371(1-2):77–85. doi: 10.1007/BF00580775. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Morgenstern J. A. Effects of extracellular potassium on ventricular automaticity and evidence for a pacemaker current in mammalian ventricular myocardium. Circ Res. 1977 Jan;40(1):105–111. doi: 10.1161/01.res.40.1.105. [DOI] [PubMed] [Google Scholar]
- Kline R., Morad M. Potassium efflux and accumulation in heart muscle. Evidence from K +/- electrode experiments. Biophys J. 1976 Apr;16(4):367–372. doi: 10.1016/S0006-3495(76)85694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res. 1977 Jul;41(1):122–127. doi: 10.1161/01.res.41.1.122. [DOI] [PubMed] [Google Scholar]
- Maughan D. W. Some effects of prolonged polarization on membrane currents in bullfrog atrial muscle. J Membr Biol. 1973;11(4):331–352. doi: 10.1007/BF01869829. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D. S., Rosen M. R. The effects of ouabain on the transmembrane potentials and intracellular potassium activity of canine cardiac Purkinje fibers. Circ Res. 1978 Mar;42(3):333–338. doi: 10.1161/01.res.42.3.333. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Fox J. M., Drouin H., Schwarz W. Kinetics of the slow variation of peak sodium current in the membrane of myelinated nerve following changes of holding potential or extracellular pH. Biochim Biophys Acta. 1976 Mar 5;426(2):245–257. doi: 10.1016/0005-2736(76)90335-7. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Reconstruction of the repolarization process in cardiac Purkinje fibres based on voltage clamp measurements of membrane current. J Physiol. 1969 Jan;200(1):233–254. doi: 10.1113/jphysiol.1969.sp008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson S. B. Right ventricular monophasic action potentials during regular rhythm. A heart catheterization study in man. Acta Med Scand. 1972 Mar;191(3):145–157. [PubMed] [Google Scholar]
- Reiter M., Stickel F. J. Der Einfluss der Kontraktionsfrequenz auf das Aktionspotential des Meerschweinchem-Papillarmuskels. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(4):342–365. [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W., Kass R. S. Role of intracellular calcium in the transient outward current of calf Purkinje fibres. Nature. 1977 Oct 13;269(5629):611–613. doi: 10.1038/269611a0. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Carbocyanine dyes inhibit Ca-dependent K efflux from human red cell ghosts. Nature. 1976 Dec 2;264(5585):467–469. doi: 10.1038/264467a0. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G., NELSON R. M., HECHTHH Electrophysiological study of human heart muscle. Circ Res. 1962 Mar;10:306–312. doi: 10.1161/01.res.10.3.306. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Shortening of the cardiac action potential due to a brief injection of KCl following the onset of activity. J Physiol. 1956 Apr 27;132(1):157–163. doi: 10.1113/jphysiol.1956.sp005510. [DOI] [PMC free article] [PubMed] [Google Scholar]


