Abstract
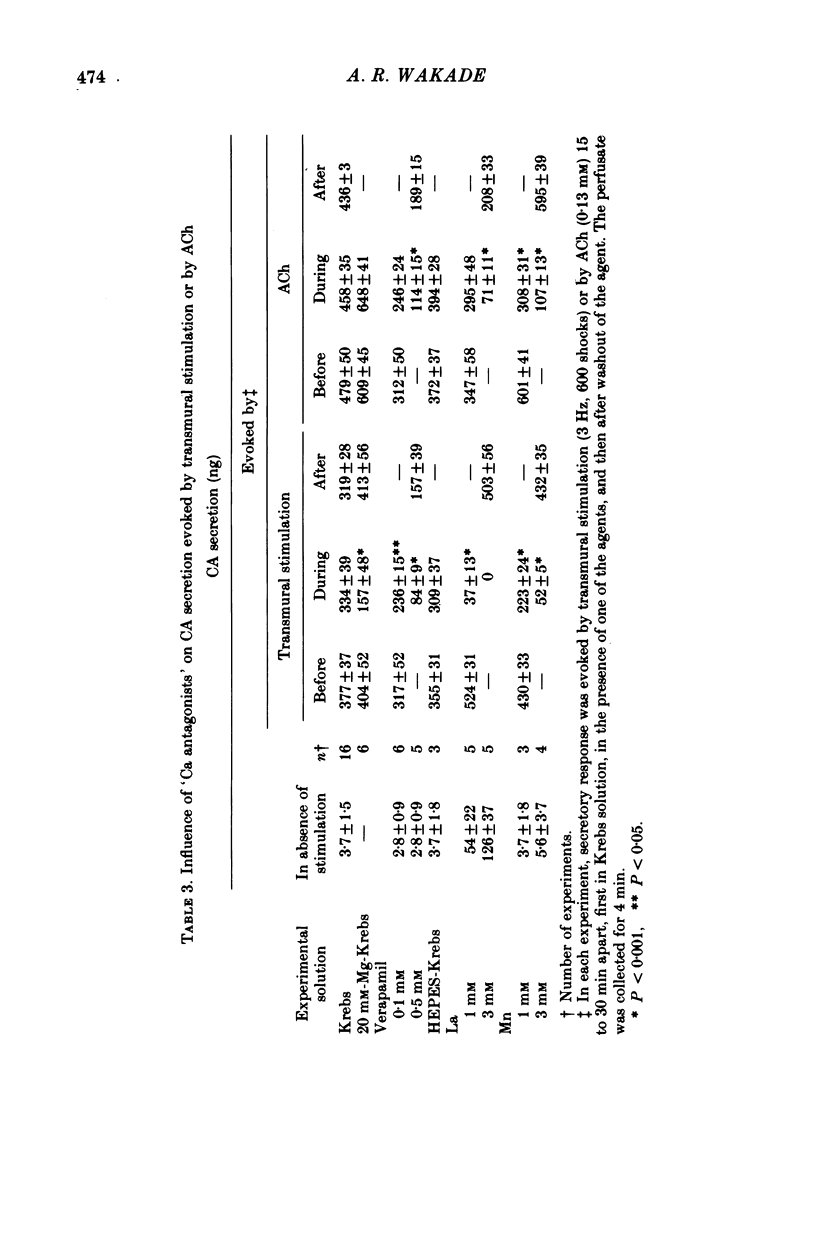
1. A method of studying the secretion of catecholamines (CA) in the isolated perfused rat adrenal gland by transmural stimulation or by application of acetylcholine (ACh) has been described. 2. Secretion of CA was practically linear in response to ACh administration, starting from 4.42 microM to 1.32 mM. Transmural stimulation enhanced secretion from a stimulation frequency of 0.5--3 Hz; the effect levelled at 10 Hz, and declined as frequency was raised to 30 Hz. The secretory response to transmural stimulation was maximal over 1 msec duration and 60 V. 3. Secretion evoked by transmural stimulation was blocked (70-95%) by 0.31 microM-tetrodotoxin (TTX) irrespective of stimulus duration, voltage and frequency of stimulation. Secretion evoked by ACh was depressed 43% by TTX. After mecamylamine (0.59 mM) treatment, secretory response evoked by either procedure was blocked by about 80%. 4. Adenosine (0.18 mM), adenosine monophosphate (0.28 mM), or adenosine triphosphate (0.19 mM) lowered CA secretion evoked by transmural stimulation by about 40%, but had no effect on secretion induced by ACh. 5. Isoprenaline (4.52 microM), propranolol (11.58 microM), clonidine (13.00 microM), phenoxybenzamine (3.30 microM), and 4-aminopyridine (3 mM) did not modify CA secretion evoked by transmural stimulation or by ACh. 6. Perfusion of the adrenal gland with 0.25 mM-Ca-Krebs solution completely abolished CA secretion evoked by transmural stimulation, but ACh-induced secretion was still 30-50% of the control value. 20 mM-Mg blocked electrically induced secretion by 60%, but that evoked by ACh was unaffected. 7. Perfusion with Ca-free Krebs solution for 2 hr did not completely abolish the response. However, treatment with EGTA (5 mM) for 30 min totally blocked ACh-induced secretion. 8. La or Mn were more effective in blocking transmurally evoked secretion than ACh-evoked secretion of CA. Verapamil (0.1 mM) had no significant effect on secretion evoked by either procedure. A 5-fold increase in its concentration caused about 75% blockade of secretion. 9. Differential effects of various ions and agents on CA secretion are explained on the basis that these compounds affect neurosecretory properties of the presynaptic splanchnic nerve terminals and of chromaffin cells differently.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Biales B., Dichter M., Tischler A. Electrical excitability of cultured adrenal chromaffin cells. J Physiol. 1976 Nov;262(3):743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaviroj P., Gutman Y. Inhibition by PGE2 and by phenylephrine of catecholamine release from human adrenal in vitro. Eur J Pharmacol. 1977 Jan 7;41(1):73–75. doi: 10.1016/0014-2999(77)90373-9. [DOI] [PubMed] [Google Scholar]
- Bozler E. Role of calcium in initiation of activity of smooth muscle. Am J Physiol. 1969 Mar;216(3):671–674. doi: 10.1152/ajplegacy.1969.216.3.671. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanachan A. S., Johns A., Paton D. M. Presynaptic inhibitory actions of adenine nucleotides and adenosine on neurotransmission in the rat vas deferens. Neuroscience. 1977;2(4):597–602. doi: 10.1016/0306-4522(77)90056-2. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Evidence that the secreting adrenal chromaffin cell releases catecholamines directly from ATP-rich granules. J Physiol. 1966 Mar;183(1):236–248. doi: 10.1113/jphysiol.1966.sp007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolysed) during release of catecholamines. J Physiol. 1966 Mar;183(1):249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enero M. A., Saidman B. Q. Possible feed-back inhibition of noradrenaline release by purine compounds. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):39–46. doi: 10.1007/BF00508808. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Hutter O. F. Proceedings: The actions of 4-aminopyridine on the delayed potassium current in skeletal muscle fibres. J Physiol. 1975 Nov;252(2):70P–71P. [PubMed] [Google Scholar]
- Gutman Y., Boonyaviroj P. Suppression by noradrenaline of catecholamine secretion from adrenal medulla. Eur J Pharmacol. 1974 Oct;28(2):384–386. doi: 10.1016/0014-2999(74)90294-5. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Fredholm B. B. Effects of adenosine on adrenergic neurotransmission; prejunctional inhibition and postjunctional enhancement. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jun;293(3):217–223. doi: 10.1007/BF00507344. [DOI] [PubMed] [Google Scholar]
- Horn A. S., Lambert J. J., Marshall I. G. A comparison of the facilitatory actions of 4-aminopyridine methiodide and 4-aminopyridine on neuromuscular transmission. Br J Pharmacol. 1979 Jan;65(1):53–62. doi: 10.1111/j.1476-5381.1979.tb17333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Kanno T. Influences of extracellular calcium and potassium concentrations on adrenaline release and membrane potential in the perfused adrenal medulla of the rat. Jpn J Physiol. 1978;28(3):275–289. doi: 10.2170/jjphysiol.28.275. [DOI] [PubMed] [Google Scholar]
- Ito S., Nakazato Y., Ohga A. The effect of veratridine on the release of catecholamines from the perfused adrenal gland. Br J Pharmacol. 1979 Feb;65(2):319–330. doi: 10.1111/j.1476-5381.1979.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRPEKAR S. M., CERVONI P. EFFECT OF COCAINE, PHENOXYBENZAMINE AND PHENTOLAMINE ON THE CATECHOLAMINE OUTPUT FROM SPLEEN AND ADRENAL MEDULLA. J Pharmacol Exp Ther. 1963 Oct;142:59–70. [PubMed] [Google Scholar]
- Kajimoto N., Kirpekar S. M. Effect of manganese and lanthanum on spontaneous release of acetylcholine at frog motor nerve terminals. Nat New Biol. 1972 Jan 5;235(53):29–30. doi: 10.1038/newbio235029a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar M., Kirpekar S. M., Prat J. C. Effect of 4-aminopyridine on release of noradrenaline from the perfused cat spleen by nerve stimulation. J Physiol. 1977 Nov;272(3):517–528. doi: 10.1113/jphysiol.1977.sp012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C. Release of catecholamines from perfused cat adrenal gland by veratridine. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2081–2083. doi: 10.1073/pnas.76.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Leander S., Thesleff S. Antagonism of the paralysis produced by botulinum toxin in the rat. The effects of tetraethylammonium, guanidine and 4-aminopyridine. J Neurol Sci. 1977 May;32(1):29–43. doi: 10.1016/0022-510x(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Lundh H., Thesleff S. The mode of action of 4-aminopyridine and guanidine on transmitter release from motor nerve terminals. Eur J Pharmacol. 1977 Apr 21;42(4):411–412. doi: 10.1016/0014-2999(77)90176-5. [DOI] [PubMed] [Google Scholar]
- MATILE P. [Light inhibition of oxidation of Krebs' cycle acids by cauliflower mitochondria]. Experientia. 1962 Mar 15;18:133–134. doi: 10.1007/BF02153858. [DOI] [PubMed] [Google Scholar]
- Meves H., Pichon Y. Proceedings: Effects of 4-aminopyridine on the potassium current in internally perfused giant axons of the squid. J Physiol. 1975 Sep;251(1):60P–62P. [PubMed] [Google Scholar]
- Michaelis M. L., Michaelis E. K., Myers S. L. Adenosine modulation of synaptosomal dopamine release. Life Sci. 1979 May 28;24(22):2083–2092. doi: 10.1016/0024-3205(79)90082-1. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Oashi H., Takewaki T., Okada T. Calcium and the contractile effect of carbachol in the depolarized guinea-pig taenia caecum. Jpn J Pharmacol. 1974 Aug;24(4):601–611. doi: 10.1254/jjp.24.601. [DOI] [PubMed] [Google Scholar]
- Oka M., Ohuchi T., Yoshida H., Imaizumi R. The importance of calcium in the release of catecholamine from the adrenal medulla. Jpn J Pharmacol. 1965 Dec;15(4):348–356. doi: 10.1254/jjp.15.348. [DOI] [PubMed] [Google Scholar]
- Pelhate M., Pichon Y. Proceedings: Selective inhibition of potassium current in the giant axon of the cockroach. J Physiol. 1974 Oct;242(2):90P–91P. [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Starke K., Görlitz B. D., Montel H., Schümann H. J. Local alpha-adrenoceptor mediated feed-back inhibition of catecholamine release from the adrenal medulla? Experientia. 1974 Oct 15;30(10):1170–1172. doi: 10.1007/BF01923671. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic inhibition of adrenergic transmission in rabbit blood vessels. J Pharmacol Exp Ther. 1978 Feb;204(2):351–361. [PubMed] [Google Scholar]
- Verhaeghe R. H., Vanhoutte P. M., Shepherd J. T. Inhibition of sympathetic neurotransmission in canine blood vessels by adenosine and adenine nucleotides. Circ Res. 1977 Feb;40(2):208–215. doi: 10.1161/01.res.40.2.208. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Facilitation of secretion of catecholamines from rat and guinea-pig adrenal glands in potassium-free medium or after ouabain. J Physiol. 1981;313:481–498. doi: 10.1113/jphysiol.1981.sp013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]


