Abstract
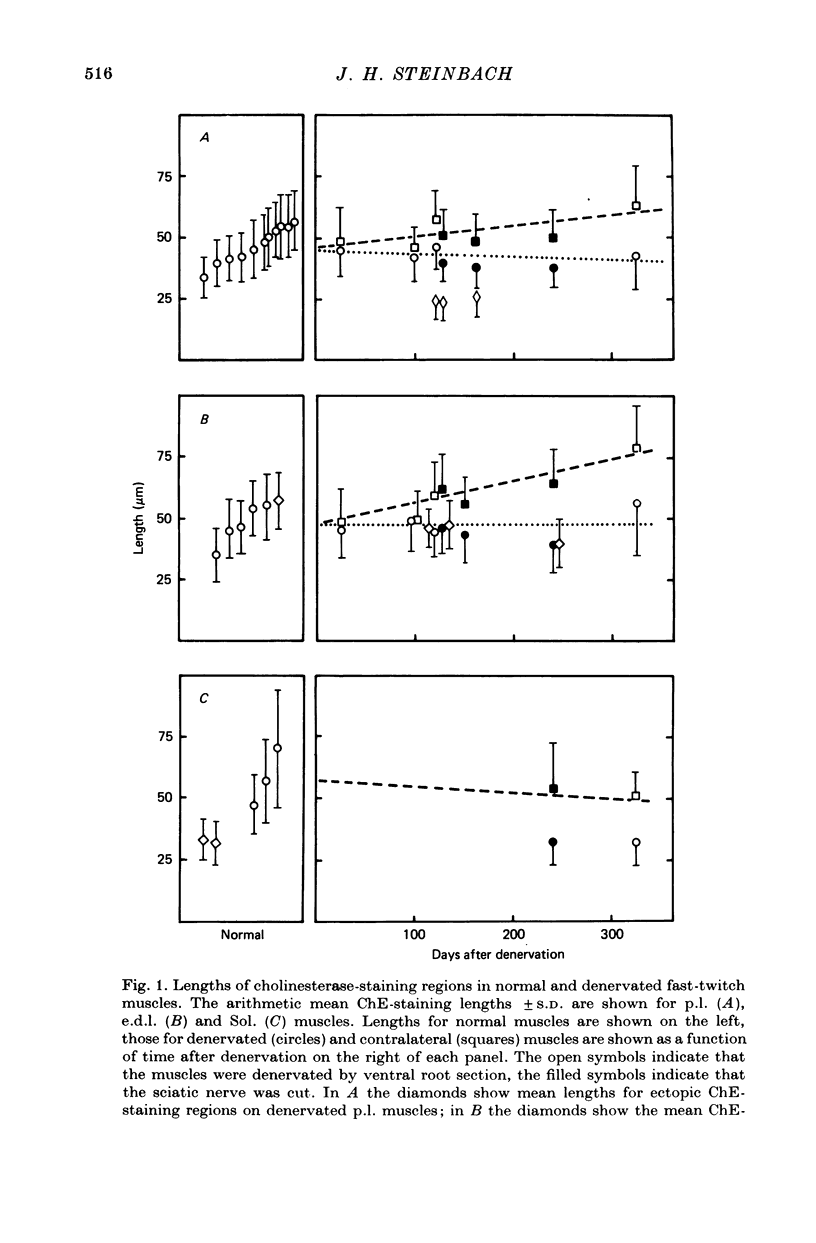
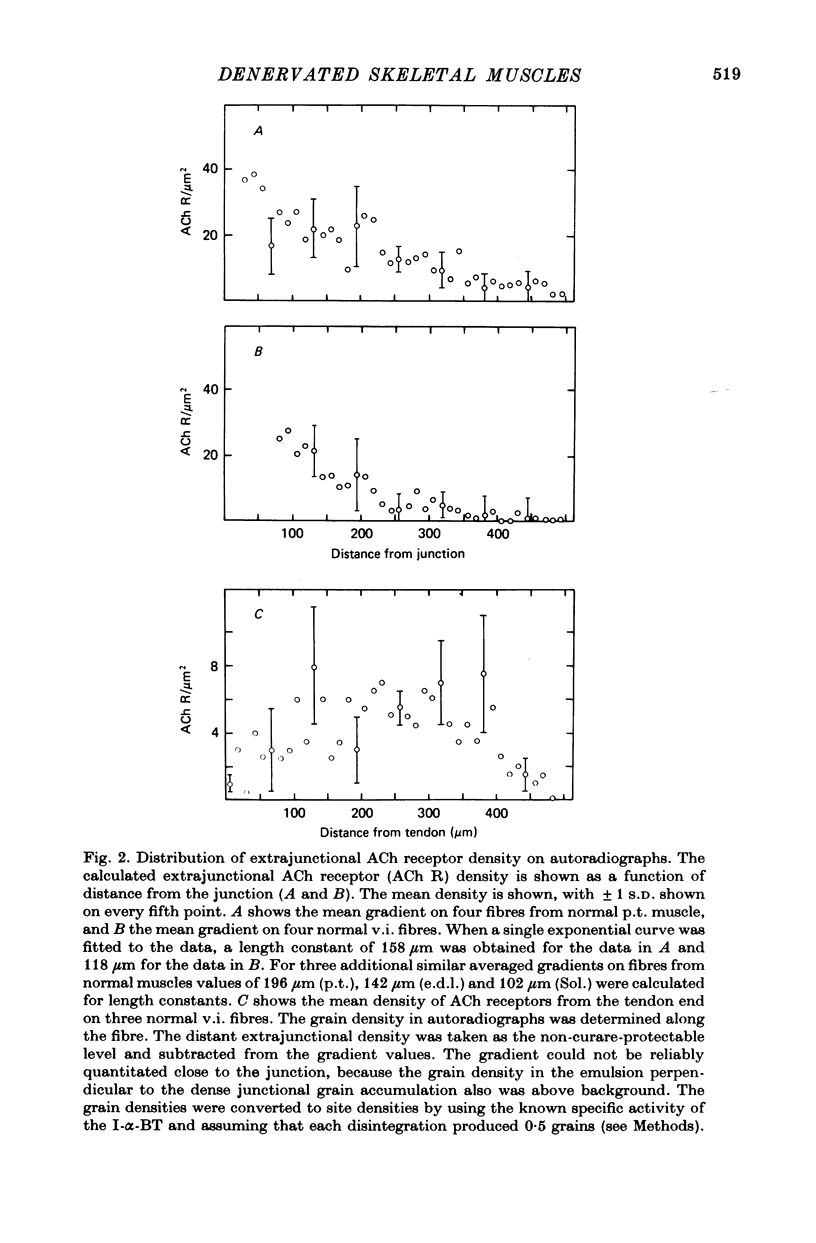
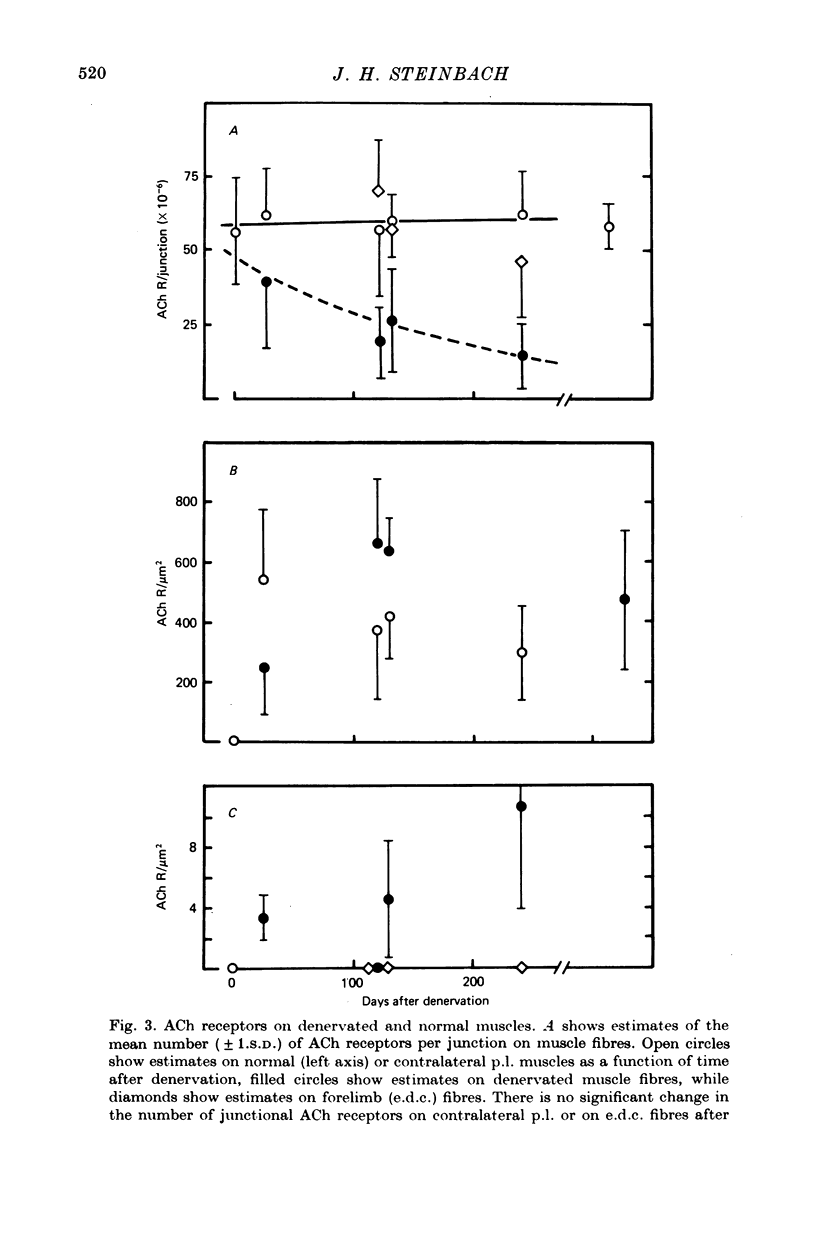
1. The distributions of cholinesterase (ChE) activity and acetylcholine (ACh) receptors were studied in normal and denervated cat hind-limb fast-twitch skeletal muscles and in muscles contralateral to denervated muscles. 2. On normal muscle fibres almost all receptors were confined to the immediately post-junctional membrane, although a perijunctional gradient of increased ACh receptor density was found on both fast- and slow-twitch fibres. After denervation, the extrajunctional ACh receptor density increased greatly and remained high for at least 10 months. Ectopic regions staining for ChE activity and having a high density of ACh receptors appeared in denervated muscle. The number of junctional ACh receptors decreased slowly after denervation, with a half-time of about 140 days. 3. Fast-twitch muscles contralateral to denervated muscles also showed changes, including an increase in junctional size and a small but significant increase in extra-junctional ACh receptor density.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANZENBACHER H., ZENKER W. UBER DIE GROESSENBEZIEHUNG DER MUSKELFASERN ZU IHREN MOTORISCHEN ENDPLATTEN UND NERVEN. Z Zellforsch Mikrosk Anat. 1963 Sep 18;60:860–871. [PubMed] [Google Scholar]
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ada G. L., Humphrey J. H., Askonas B. A., McDevitt H. O., Nossal G. J. Correlation of grain counts with radioactivity (125I and tritium) in autoradiography. Exp Cell Res. 1966 Mar;41(3):557–572. doi: 10.1016/s0014-4827(66)80106-4. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J Physiol. 1974 Mar;237(2):385–400. doi: 10.1113/jphysiol.1974.sp010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975 Nov;252(3):771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Patrick J. Purification of an acetylcholine receptor from a nonfusing muscle cell line. Biochemistry. 1977 Nov 1;16(22):4900–4908. doi: 10.1021/bi00641a025. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Suppression of motor nerve terminal sprouting in partially denervated mouse muscles [proceedings]. J Physiol. 1977 Oct;272(1):70P–71P. [PubMed] [Google Scholar]
- Burden S. J., Sargent P. B., McMahan U. J. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol. 1979 Aug;82(2):412–425. doi: 10.1083/jcb.82.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. Development of the neuromuscular junction in the chick embryo: the number, distribution, and stability of acetylcholine receptors. Dev Biol. 1977 Jun;57(2):317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., GUTMANN E. The effect of age on compensatory and "post-functional hypertrophy" in cross-straited muscle. Gerontologia. 1962;6:81–90. [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias P. L. Surface area of motor end plates in fast and slow twitch muscles of the rabbit. J Anat. 1974 Jul;117(Pt 3):453–462. [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edjtehadi G. D., Lewis D. M. Histochemical reactions of fibres in a fast twitch muscle of the cat. J Physiol. 1979 Feb;287:439–453. doi: 10.1113/jphysiol.1979.sp012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge L., Liebhold M., Steinbach J. H. Alterations in cat skeletal neuromuscular junctions following prolonged inactivity. J Physiol. 1981;313:529–545. doi: 10.1113/jphysiol.1981.sp013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Fernandez H. L., Duell M. J., Festoff B. W. Neurotrophic control of 16S acetylcholinesterase at the vertebrate neuromuscular junction. J Neurobiol. 1979 Sep;10(5):441–454. doi: 10.1002/neu.480100503. [DOI] [PubMed] [Google Scholar]
- Frank E., Gautvik K., Sommerschild H. Cholinergic receptors at denervated mammalian motor end-plates. Acta Physiol Scand. 1975 Sep;95(1):66–76. doi: 10.1111/j.1748-1716.1975.tb10026.x. [DOI] [PubMed] [Google Scholar]
- GUTH L., ALBERS R. W., BROWN W. C. QUANTITATIVE CHANGES IN CHOLINESTERASE ACTIVITY OF DENERVATED MUSCLE FIBERS AND SOLE PLATES. Exp Neurol. 1964 Sep;10:236–250. doi: 10.1016/0014-4886(64)90065-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian H. G. A rapid silver impregnation for central and peripheral nerve fibers in paraffin and frozen sections. Exp Neurol. 1977 Oct;57(1):296–301. doi: 10.1016/0014-4886(77)90065-6. [DOI] [PubMed] [Google Scholar]
- Harris A. J. Inductive functions of the nervous system. Annu Rev Physiol. 1974;36:251–305. doi: 10.1146/annurev.ph.36.030174.001343. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M. C., Vrbová G. Motor and sensory reinnervation of fast and slow mammalian muscles. Z Zellforsch Mikrosk Anat. 1973 Dec 31;146(2):261–279. doi: 10.1007/BF00307351. [DOI] [PubMed] [Google Scholar]
- Ironton R., Brown M. C., Holland R. L. Stimuli to intramuscular nerve growth. Brain Res. 1978 Nov 10;156(2):351–354. doi: 10.1016/0006-8993(78)90517-6. [DOI] [PubMed] [Google Scholar]
- JOHNS T. R., THESLEFF S. Effects of motor inactivation on the chemical sensitivity of skeletal muscle. Acta Physiol Scand. 1961 Feb-Mar;51:136–141. doi: 10.1111/j.1748-1716.1961.tb02121.x. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kean C. J., Lewis D. M., McGarrick J. D. Dynamic properties of denervated fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):103–113. doi: 10.1113/jphysiol.1974.sp010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko P. K., Anderson M. J., Cohen M. W. Denervated skeletal muscle fibers develop discrete patches of high acetylcholine receptor density. Science. 1977 Apr 29;196(4289):540–542. doi: 10.1126/science.850796. [DOI] [PubMed] [Google Scholar]
- Libelius R. Evidence for endocytotic uptake of cobra neurotoxin in mouse skeletal muscle. J Neural Transm. 1975;37(1):61–71. doi: 10.1007/BF01249766. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Fambrough D. M. Biosynthesis and degradation of acetylcholine receptors in rat skeletal muscles. Effects of electrical stimulation. Neuroscience. 1979;4(4):527–538. doi: 10.1016/0306-4522(79)90129-5. [DOI] [PubMed] [Google Scholar]
- Lubińska L., Zelená J. Formation of new sites of acetylcholinesterase activity in denervated muscles of young rats. Nature. 1966 Apr 2;210(5031):39–41. doi: 10.1038/210039a0. [DOI] [PubMed] [Google Scholar]
- Luttges M. W., Kelly P. T., Gerren R. A. Degenerative changes in mouse sciatic nerves: electrophoretic and electrophysiologic characterizations. Exp Neurol. 1976 Mar;50(3):706–733. doi: 10.1016/0014-4886(76)90039-x. [DOI] [PubMed] [Google Scholar]
- Mann W. S., Salafsky B. Development of the differential response to succinylcholine in the fast and slow-twitch skeletal muscle of the kitten. J Physiol. 1970 Oct;210(3):581–592. doi: 10.1113/jphysiol.1970.sp009228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Heinemann S., Lindstrom J. M. Acetylcholine receptor degradation in adult rat diaphragms in organ culture and the effect of anti-acetylcholine receptor antibodies. J Biol Chem. 1979 Jul 25;254(14):6320–6327. [PubMed] [Google Scholar]
- Miledi R., Stefani E., Zelená J. Neural control of acetylcholine-sensitivity in rat muscle fibers. Nature. 1968 Nov 2;220(5166):497–498. doi: 10.1038/220497a0. [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- Nyström B. Histochemical studies of end-plate bound esterases in "slow-red" and "fast-white" cat muscles during postnatal development. Acta Neurol Scand. 1968;44(3):295–317. doi: 10.1111/j.1600-0404.1968.tb05574.x. [DOI] [PubMed] [Google Scholar]
- Nyström B. Postnatal development of motor nerve terminals in "slow-red" and "fast-white" cat muscles. Acta Neurol Scand. 1968;44(3):363–383. doi: 10.1111/j.1600-0404.1968.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., McMahan U. J. Altered patterns of innervation in frog muscle after denervation. J Neurocytol. 1976 Dec;5(6):719–730. doi: 10.1007/BF01181583. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Sprouting of intact motor neurons induced by neuronal lesion in the absence of denervated muscle fibers and degenerating axons. Brain Res. 1978 Oct 27;155(2):354–356. doi: 10.1016/0006-8993(78)91029-6. [DOI] [PubMed] [Google Scholar]
- Syrový I., Gutmann E., Melichna J. The effect of denervation on contraction and myosin properties of fast and slow rabbit and cat muscles. Physiol Bohemoslov. 1972;21(4):353–359. [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]